Simulation Analysis and Experimental Validation of Cathode Tool in Electrochemical Mill-Grinding of Ti6Al4V
Abstract
:1. Introduction
2. Simulation Analysis
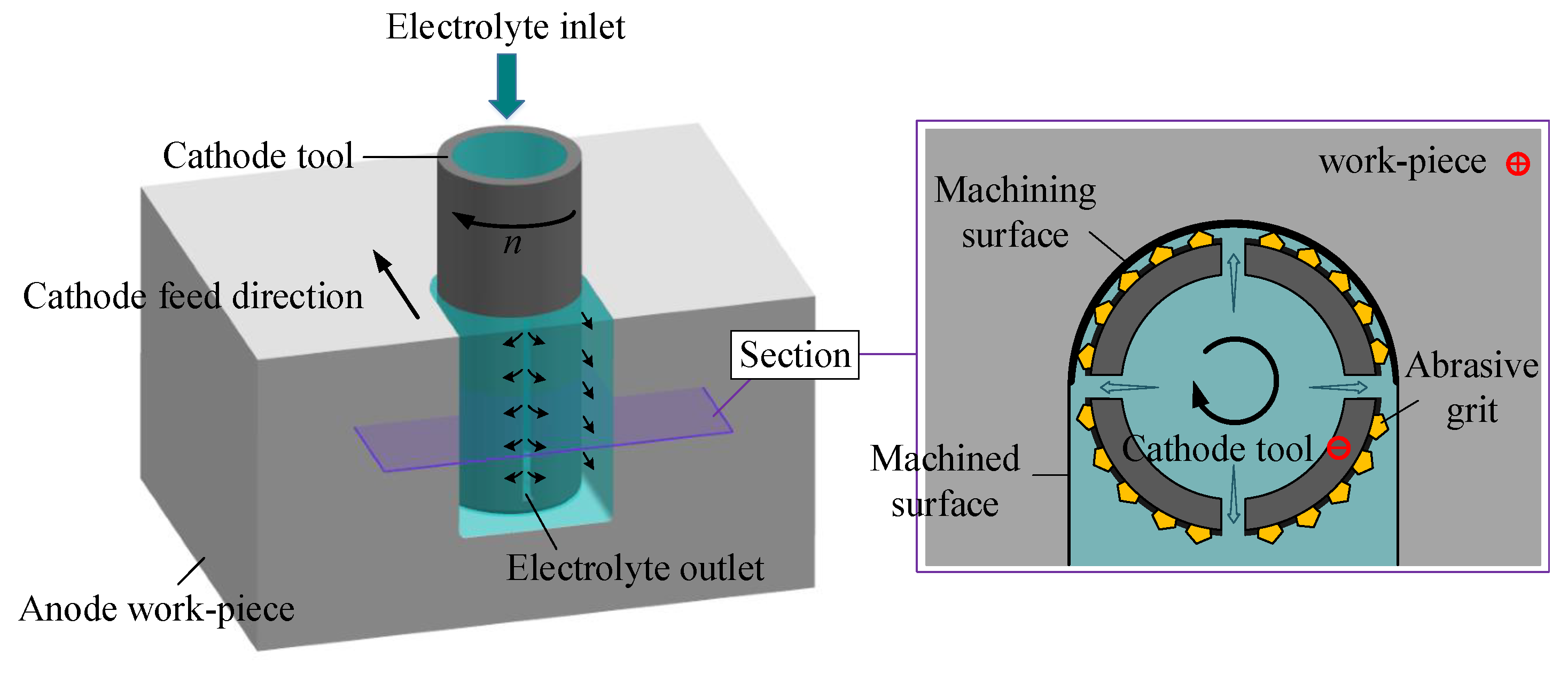
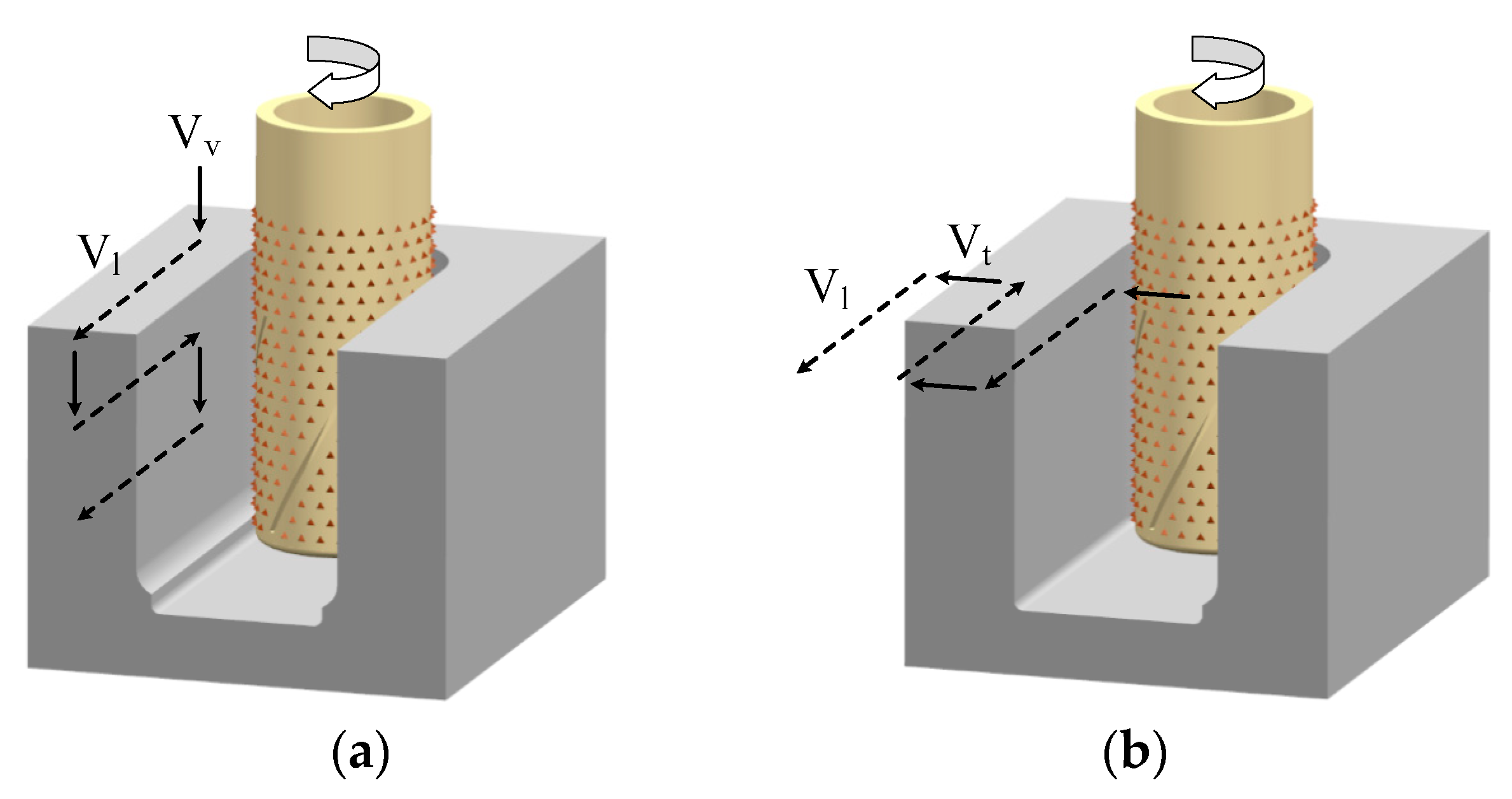
2.1. Principle of Electrochemical Mill-Grinding (ECMG)
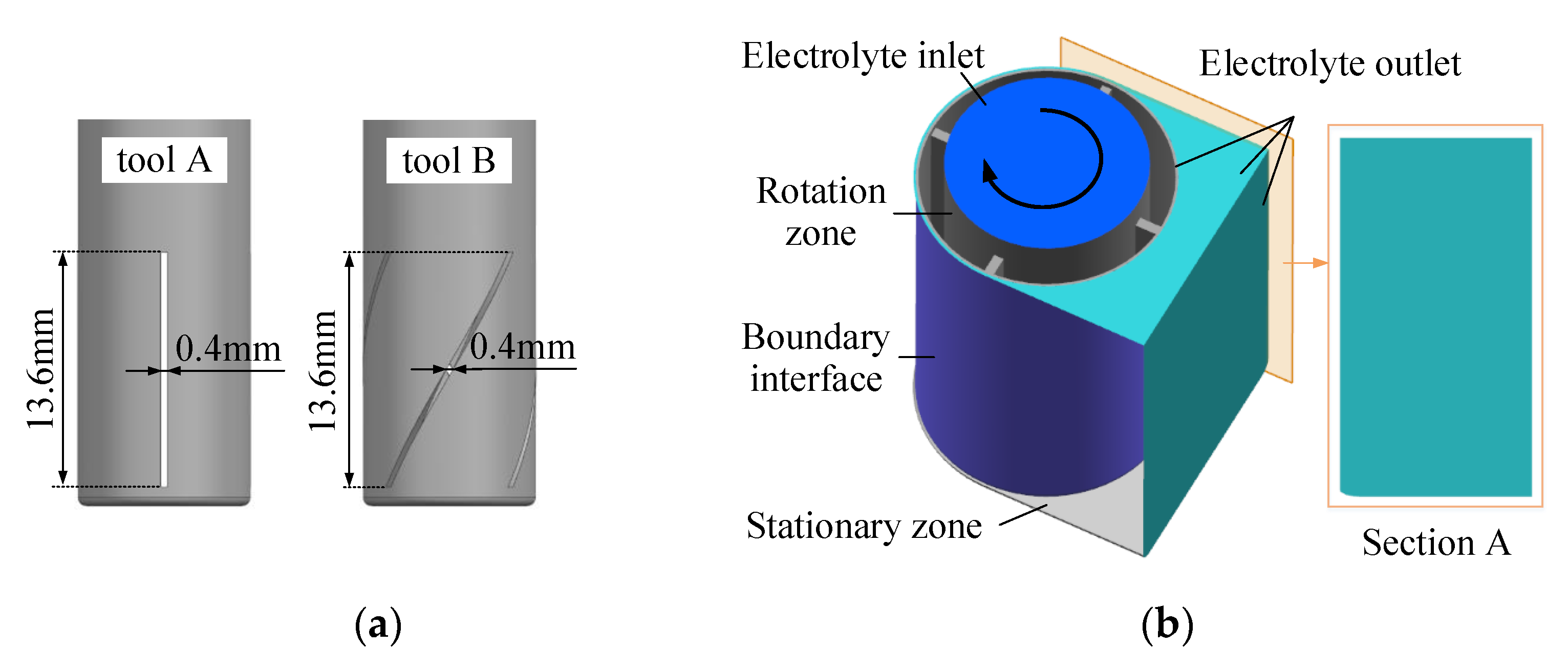
2.2. Flow-Field Simulation
3. Experimental
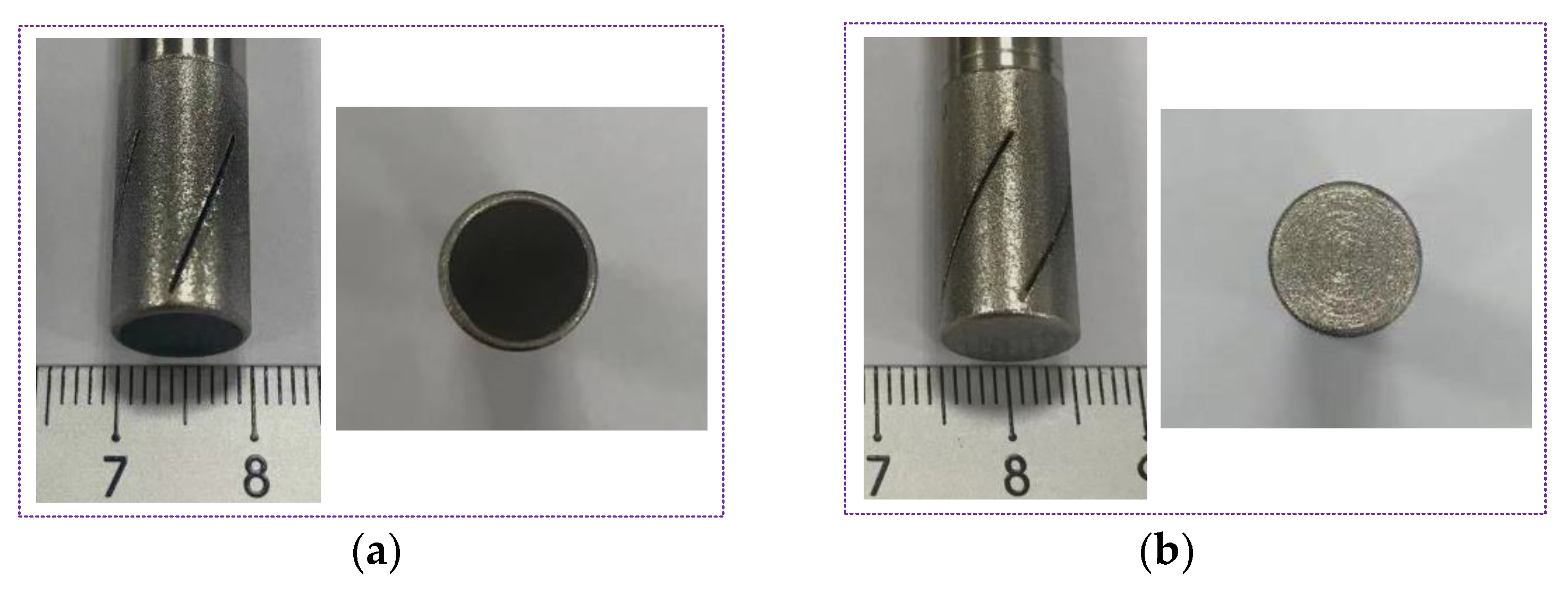
3.1. Experimental Set Up
3.2. Groove-Machining Experiment
4. Results and Discussion
5. Conclusions
- (1)
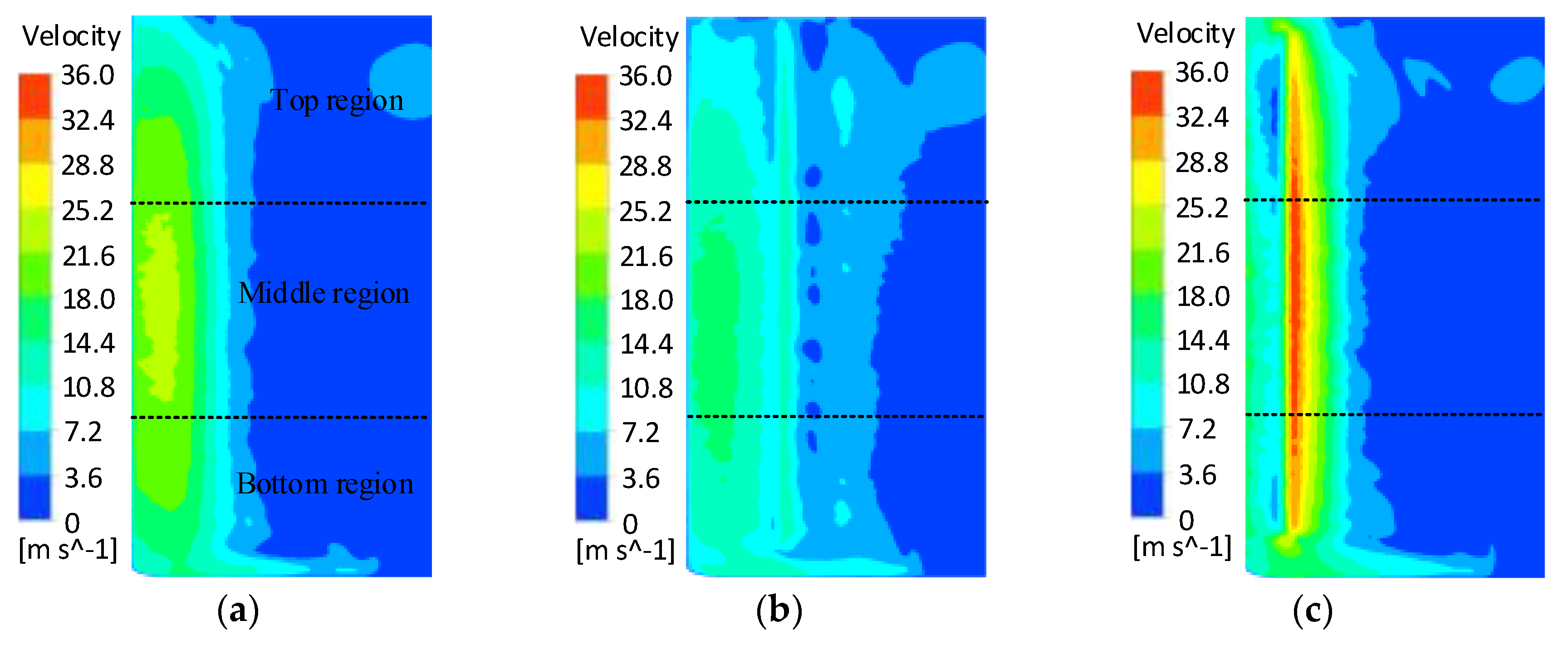
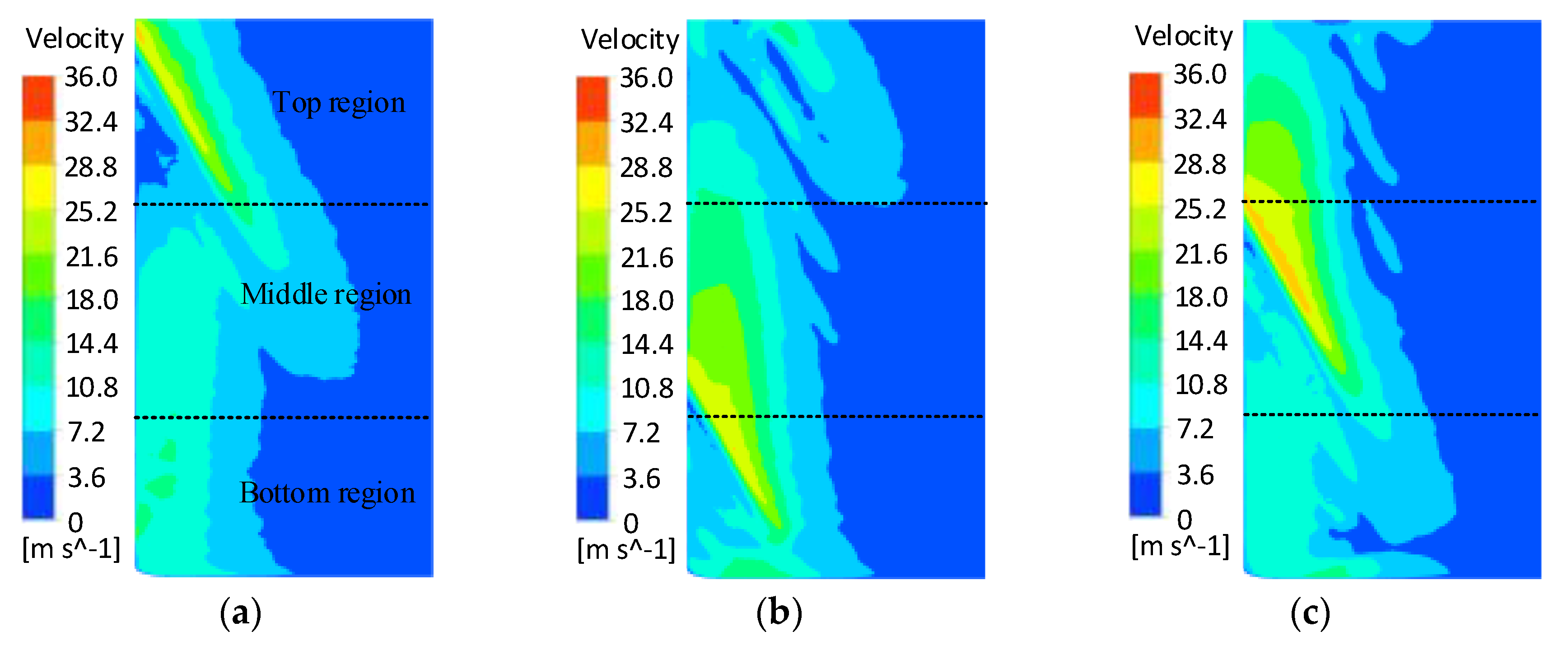
- The FLUENT 17.0 flow field simulation results showed that the flow field in the cathode tool machining gap was unevenly distributed with a linear electrolyte outlet slit, and the flow field did not improve as the cathode tool rotated. The cathode tool with a spiral outlet slit provided a uniformly changing flow field with its rotation, which reduced the area repeatedly subjected to high-speed electrolyte flow.
- (2)
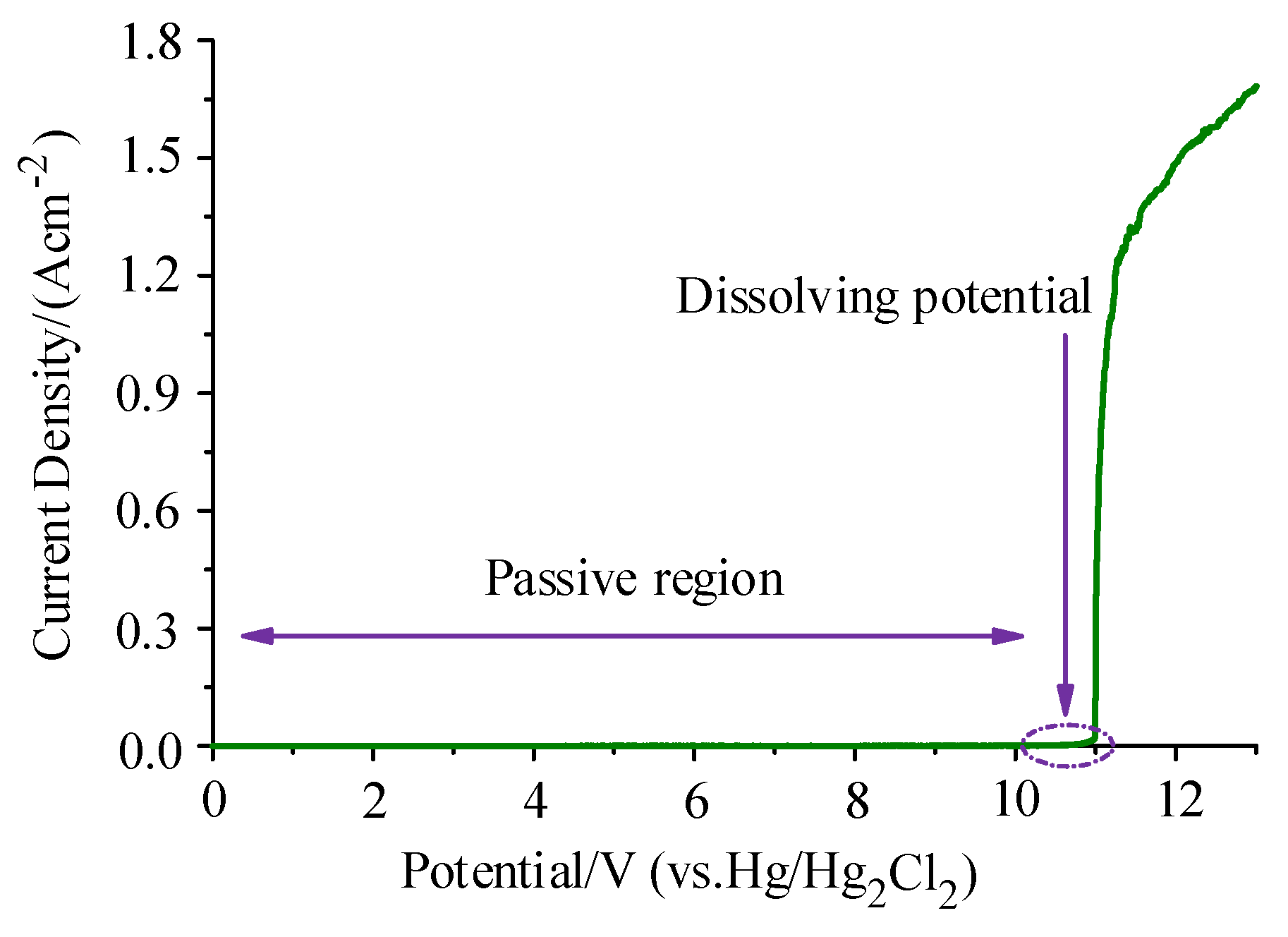
- According to the measurement results of the polarization curve, the dissolution potential of Ti6Al4V in a 10% NaNO3 solution at 30 °C was about 11 V. Thus, choosing a reasonable machining voltage can effectively improve the efficiency of rough machining and the accuracy of finish machining.
- (3)
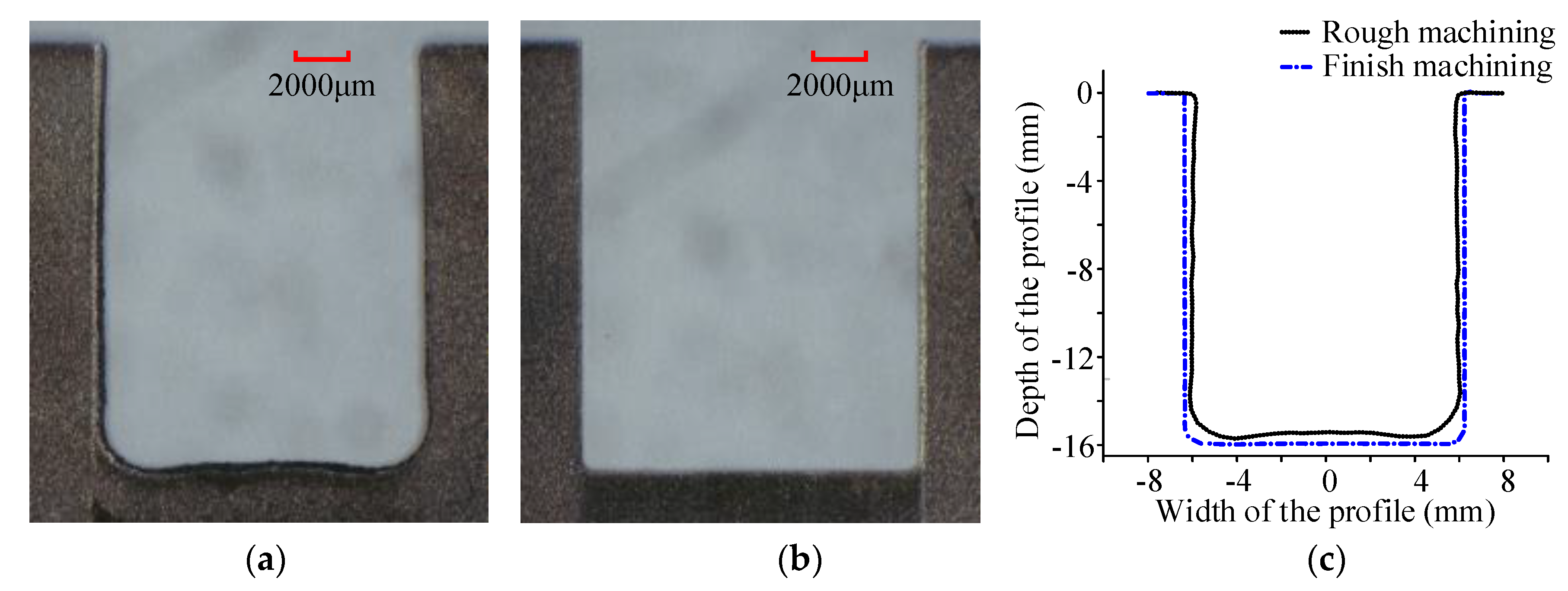
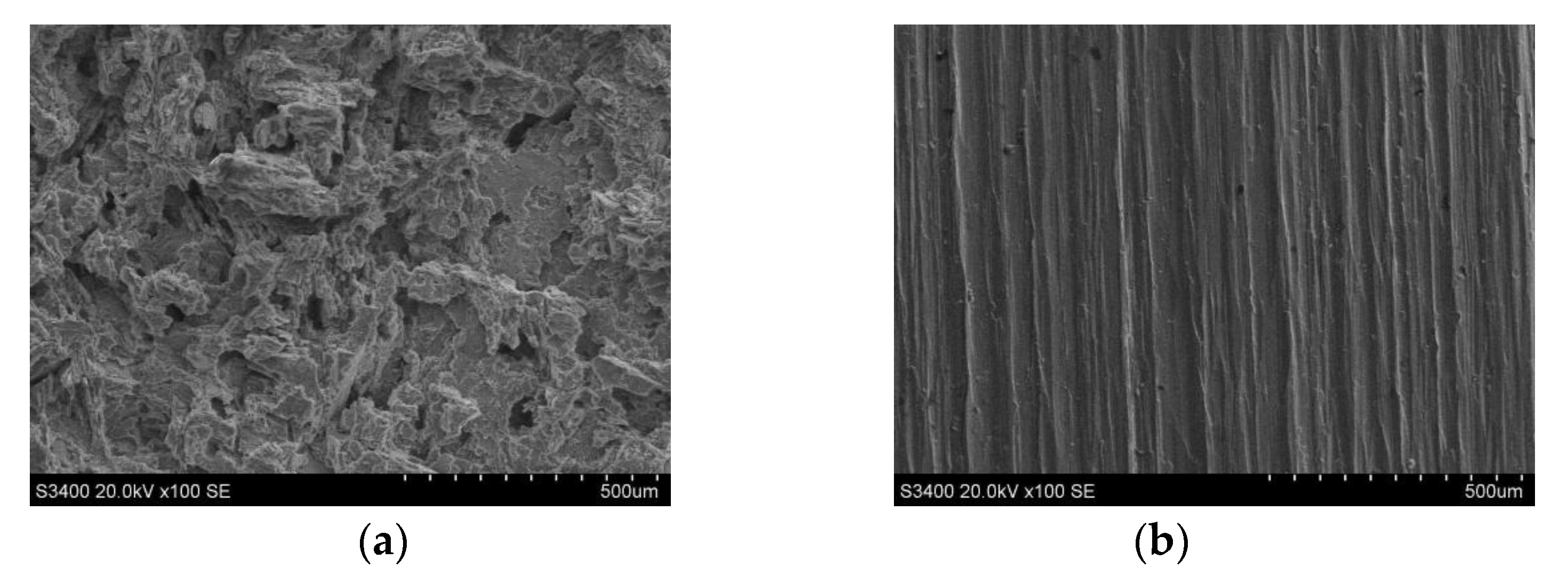
- The cathode tool with a cutting depth of 15 mm and a spiral electrolyte outlet slit could realize the ECMG rough machining of the Ti6Al4V titanium alloy. In addition, the flatness of the rough-processed sidewall obtained by using the proposed cathode tool was good, the processed surface had no flow marks, and the rough machining accounted for 92.37% of total removal, which realized the efficient processing of difficult-to-machine materials.
- (4)
- Under different processing parameters, the surface roughness of the titanium alloys varied greatly. At high voltage, a high amount of stray corrosion exhibited on the surface of the titanium alloy. At low voltage, the surface of the titanium alloy was not affected by stray corrosion. After finish machining, the surface roughness Ra was decreased from 5.182 μm to 0.760 μm.
- (5)
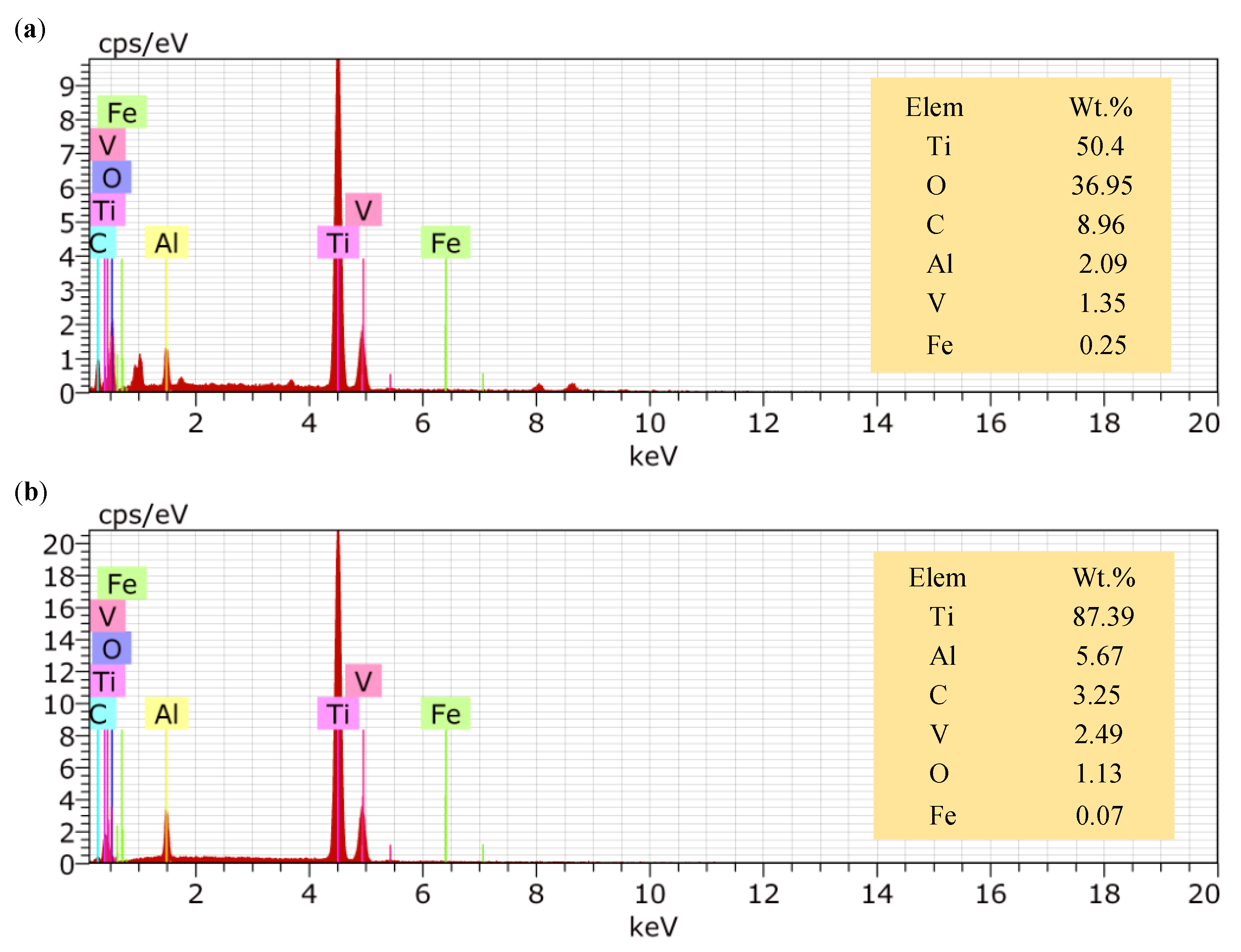
- The insoluble electrolytic products formed in rough machining were effectively eliminated by finish machining. The oxygen content decreased from 36.95 wt% to 1.13 wt%, and the carbon content decreased from 8.96 wt% to 3.25 wt%.
Author Contributions
Funding
Conflicts of Interest
References
- Ponce, M.B.; Vazquez-Martinez, J.M.; Davim, J.P.; Gomez, J.S. Analysis of secondary adhesion wear mechanism on hard machining of titanium aerospace alloy. Materials 2019, 12, 2015. [Google Scholar] [CrossRef] [Green Version]
- Babu, J.; Dutta, A. Low temperature superplasticity through grain refinement in Ti-6Al-4V by a novel route ofquench-roll-recrystallise. J. Mater. Res. Technol. 2015, 4, 348–352. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Pungotra, H.; Kalsi, N.S. On the characteristics of titanium alloys for the aircraftapplications. Mater. Today: Proc. 2017, 4, 8971–8982. [Google Scholar]
- Baila, D.I.; Tonoiu, S. Hydrides precipitation in Ti6Al4V titanium alloy used for airframemanufacturing. Bull. Pol. Acad. Sci. Tech. 2019, 67, 643–649. [Google Scholar]
- Cotton, J.D.; Briggs, R.D.; Boyer, R.R.; Tamirisakandala, S.; Russo, P.; Shchetnikov, N.; Fanning, J.C. State of the art in beta titanium alloys for airframe applications. JOM 2015, 67, 1281–1303. [Google Scholar] [CrossRef] [Green Version]
- Zoya, Z.A.; Krishnamurthy, R. The performance of CBN tools in the machining of titanium alloys. J. Mater. Process. Technol. 2000, 100, 80–86. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Kovalev, A.I.; Aguirre, M.H.; Beake, B.D.; Yamamoto, K.; Veldhuis, S.C.; Endrino, J.L.; Wainstein, D.L.; Rashkovskiy, A.Y. Design and performance of AlTiN and TiAlCrN PVD coatings for machining of hard to cut materials. Surf. Coati. Technol. 2009, 204, 489–496. [Google Scholar] [CrossRef]
- Wang, T.; Zha, J.; Jia, Q.; Chen, Y.L. Application of low-melting alloy in the fixture for machining aeronauticalthin-walled component. Int. J. Adv. Manuf. Tech. 2016, 87, 2797–2807. [Google Scholar] [CrossRef]
- Lunarska, E.; Zaborski, S. Hydrogen effects in anodic grinding of Ti alloy. Wear 2001, 249, 606–616. [Google Scholar] [CrossRef]
- Liu, G.Y.; Guo, Z.N.; Li, Y.B.; Huang, Z.G. An Investigation on PRMMC Polishing with Electrochemical Mechanical Machining Process. Adv. Mater. Res. 2010, 135, 226–231. [Google Scholar] [CrossRef]
- Van Camp, D.; Qian, J.; Vleugels, J.; Lauwers, B. Experimental investigation of the process behaviour in Mechano-Electrochemical Milling. CIRP Ann. 2018, 67, 217–220. [Google Scholar] [CrossRef]
- Kozak, J.; Oczos, K.E. Selected problems of abrasive hybrid machining. J. Mater. Process.. Technol. 2001, 109, 360–366. [Google Scholar] [CrossRef]
- Zaborski, S.; Lupak, M.; Poros, D. Wear of cathode in abrasive electrochemical grinding of hardly machined materials. J. of Mater. Process. Technol. 2004, 149, 414–418. [Google Scholar] [CrossRef]
- Lupak, M.; Zaborski, S. Simulation of energy consumption in electrochemical grinding of hard-to-machine materials. J. Appl. Electrochem 2009, 39, 101–106. [Google Scholar] [CrossRef]
- Lyubimov, V.V.; Yerokhin, A.L.; Tchmir, M.Y. Mechanisms of synthetic diamond wear in tools forelectrochemical grinding. Diamond Relat. Materials 1998, 7, 1267–1271. [Google Scholar]
- Goswami, R.N.; Mitra, S.; Sarkar, S. Experimental investigation on electrochemical grinding (ECG) of alumina-aluminum interpenetrating phase composite. Int. J. Adv. Manuf. Tech. 2009, 40, 729–741. [Google Scholar] [CrossRef]
- Qu, N.S.; Zhang, Q.L.; Fang, X.L.; Ye, E.K.; Zhu, D. Experimental investigation on electro-chemical grinding of Inconel 718. Procedia CIRP 2015, 35, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Li, H.S.; Fu, S.X.; Zhang, Q.L.; Niu, S.; Qu, N.S. Investigation of material removal in inner-jet electrochemical grinding of GH4169 alloy. Sci. Rep. 2017, 7, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Qu, N.S.; Yue, X.K. Effect of tool-sidewall outlet hole design on machining performancein electrochemical mill-grinding of Inconel 718. J. Manuf. Process. 2019, 41, 10–22. [Google Scholar] [CrossRef]
- Yue, X.K.; Qu, N.S.; Niu, S.; Li, H.S. Improving the machined bottom surface in electrochemical mill-grinding by adjusting the electrolyte flow field. J. Mater. Process. Technol. 2020, 276, 116413. [Google Scholar] [CrossRef]
- Li, H.S.; Fu, S.X.; Niu, S.; Qu, N.S. Simulation and experimental investigation of electrochemical mill-grinding of GH4169 alloy. Int. J. Electrochem. Sci. 2018, 13, 6608–6625. [Google Scholar] [CrossRef]
- Ge, Y.C.; Zhu, Z.W.; Ma, Z.; Wang, D.Y. Tool design and experimental study on electrochemical turning of nickel-based cast superalloy. J. Electrochem. Soci. 2018, 165, 162–170. [Google Scholar] [CrossRef]
- Riggs, J.B.; Muller, R.H.; Tobias, C.W. Prediction of work piece geometry in electrochemical cavity sinking. Electrochim. Acta 1981, 26, 961–969. [Google Scholar] [CrossRef]
- Qian, N.; Ding, W.F.; Zhu, Y.J. Comparative investigation on grindability of K4125 and Inconel718 nickel-based superalloys. Int. J. Adv. Manuf. Tech. 2018, 97, 1649–1661. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Inlet pressure (MPa) | 0.6 |
| Outlet pressure (MPa) | 0 |
| Side and bottom gap (mm) | 0.2 |
| Rotational speed (rpm) | 1000 |
| Step times (s) | 2.5 × 10−3 |
| Total simulation times (s) | 6.25 × 10−2 |
| Processing Parameter | Value |
|---|---|
| Processing voltage (V) | 25 |
| Electrolyte temperature (°C) | 30 |
| Electrolyte pressure (MPa) | 0.6 |
| Feed rate V (mm·min−1) | 1.2 |
| Cutting depth (mm) | 15 |
| Spindle speed (rpm) | 1000 |
| Processing Parameter | Value |
|---|---|
| Processing voltage (V) | 1 |
| Electrolyte temperature (°C) | 30 |
| Electrolyte pressure (Mpa) | 0.2 |
| Feed rates Vl/Vv/Vt (mm·min−1) | 60/1/1 |
| Cutting depth (mm) | 0.05 |
| Spindle speed (rpm) | 1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, H.; Hu, X.; Niu, S.; Xu, G. Simulation Analysis and Experimental Validation of Cathode Tool in Electrochemical Mill-Grinding of Ti6Al4V. Appl. Sci. 2020, 10, 1941. https://doi.org/10.3390/app10061941
Li J, Li H, Hu X, Niu S, Xu G. Simulation Analysis and Experimental Validation of Cathode Tool in Electrochemical Mill-Grinding of Ti6Al4V. Applied Sciences. 2020; 10(6):1941. https://doi.org/10.3390/app10061941
Chicago/Turabian StyleLi, Jie, Hansong Li, Xiaoyun Hu, Shen Niu, and Guoliang Xu. 2020. "Simulation Analysis and Experimental Validation of Cathode Tool in Electrochemical Mill-Grinding of Ti6Al4V" Applied Sciences 10, no. 6: 1941. https://doi.org/10.3390/app10061941




