Climate Change and Refrigerants: Thermodynamic Properties of Low-GWP Fluids for Domestic Applications and Binary Systems for Low-Temperature Options
Abstract
1. Introduction
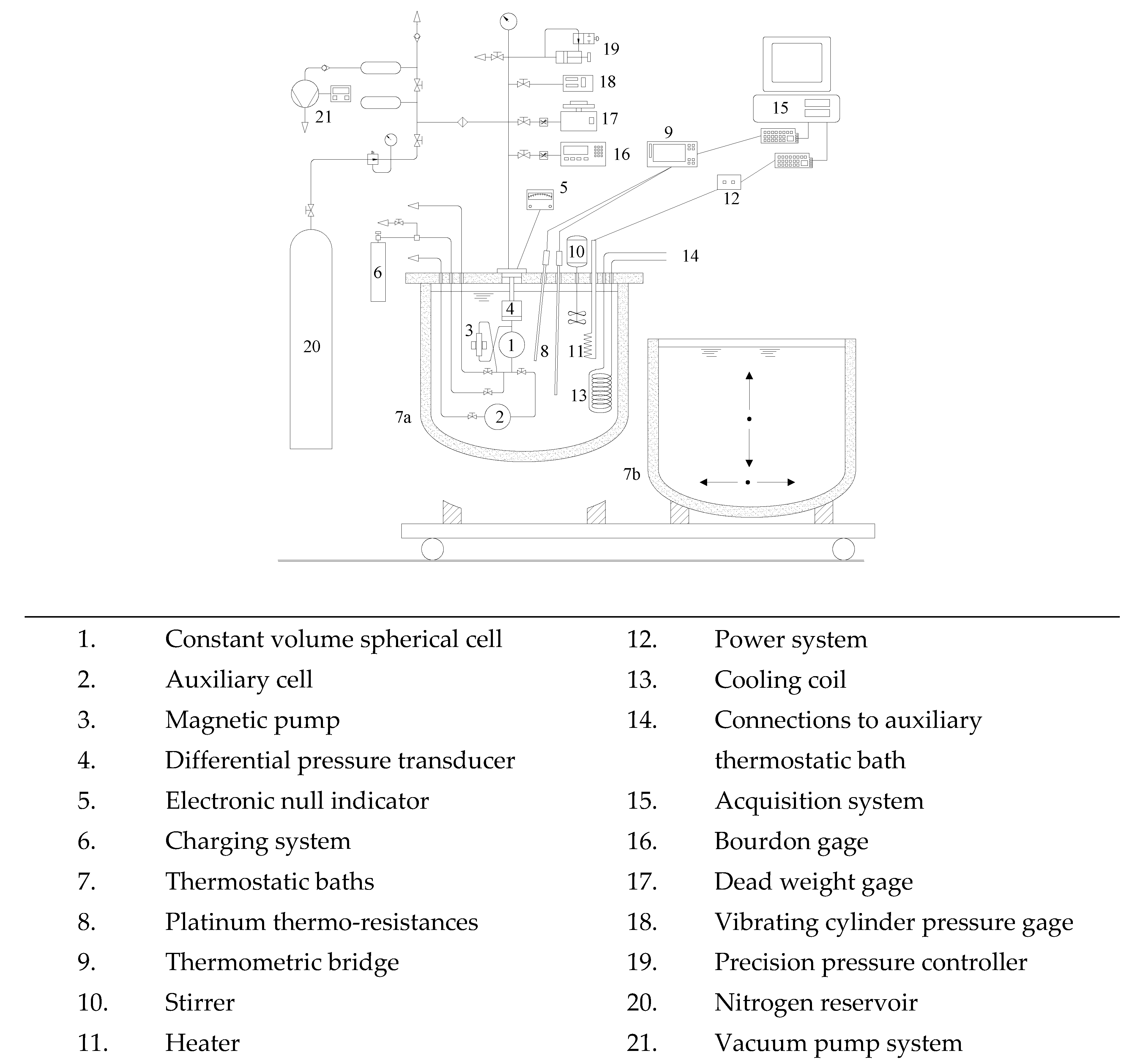
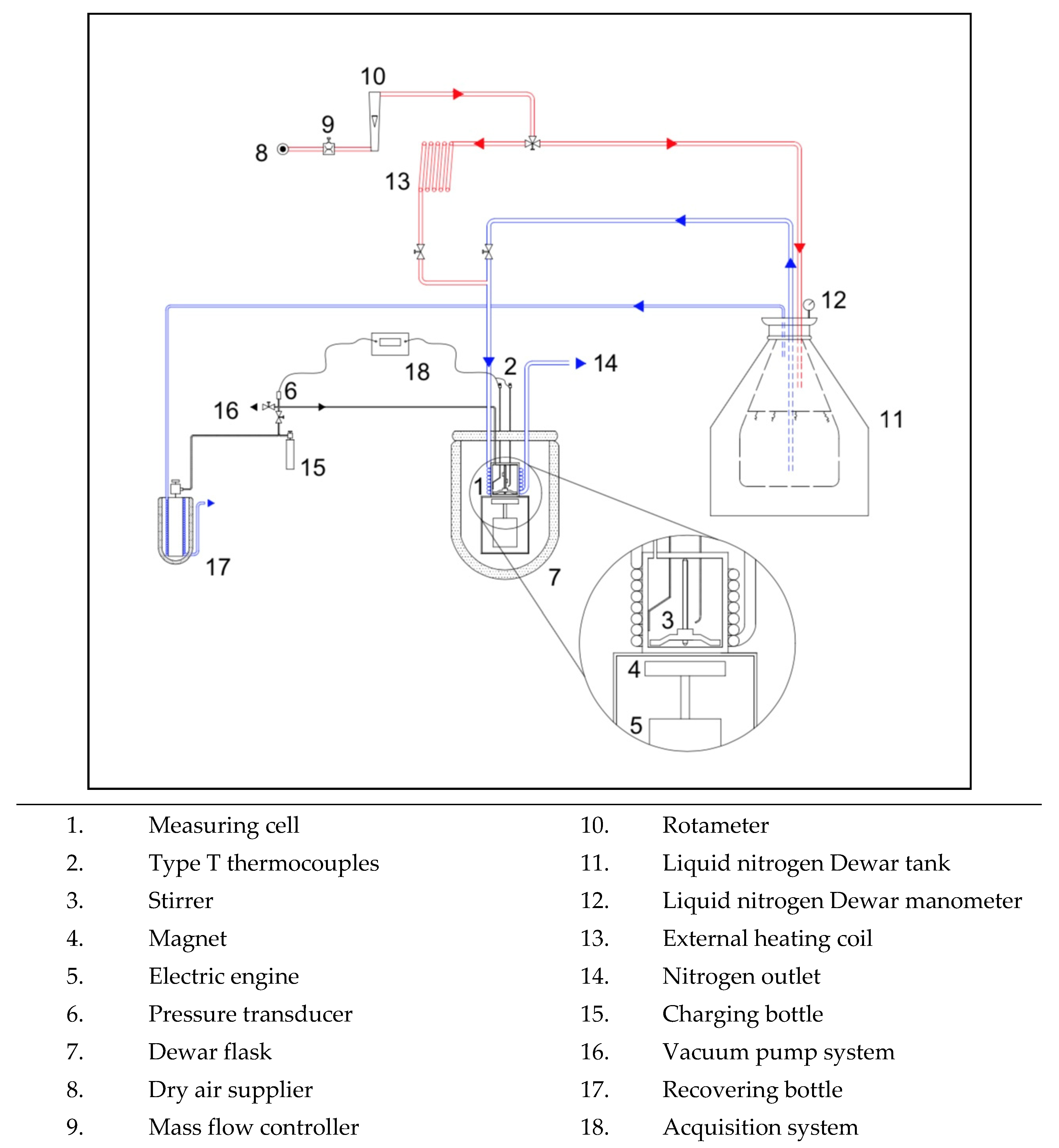
2. Materials and Methods
3. Results
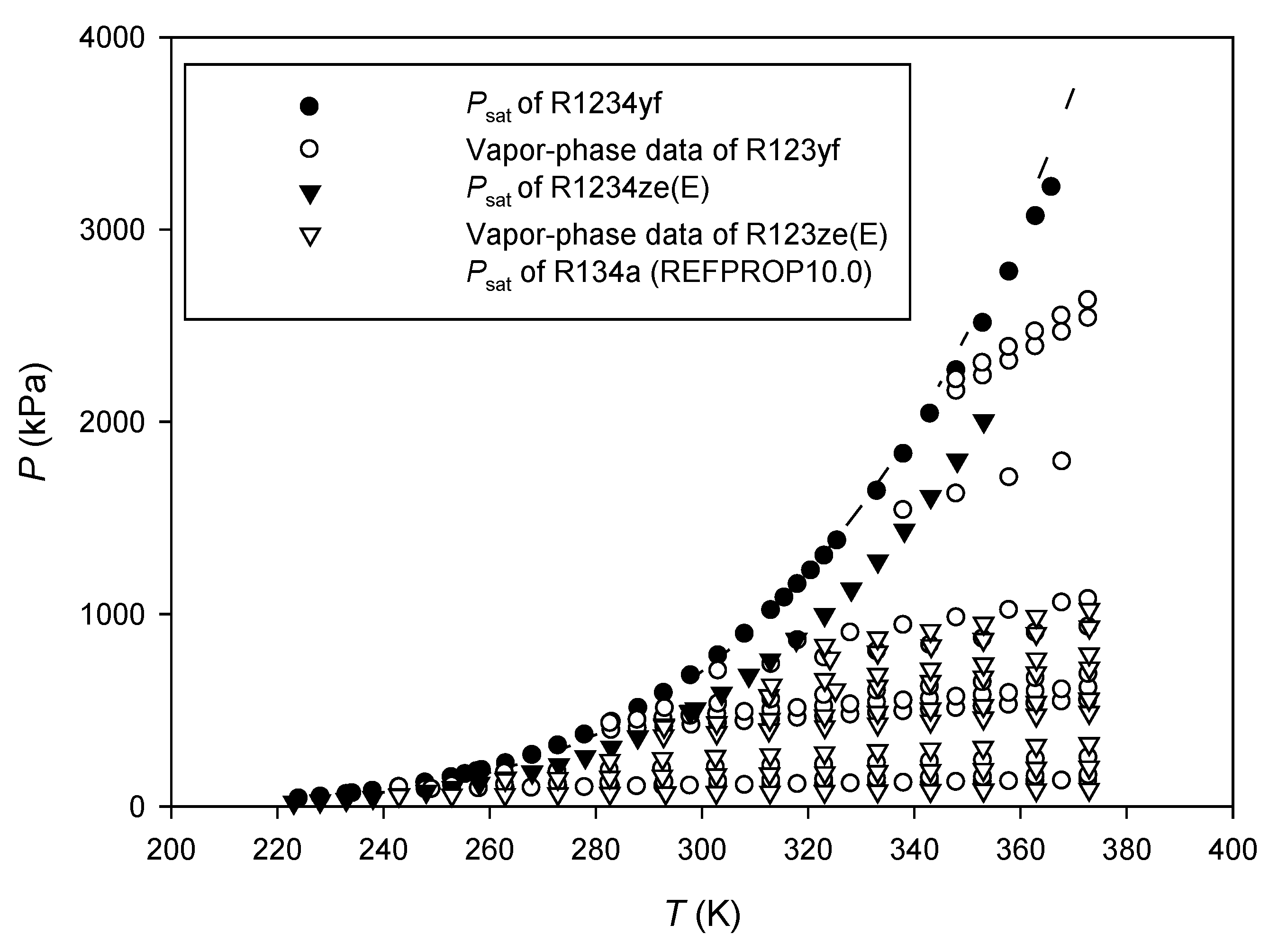
3.1. R1234yf
3.2. R1234ze(E)
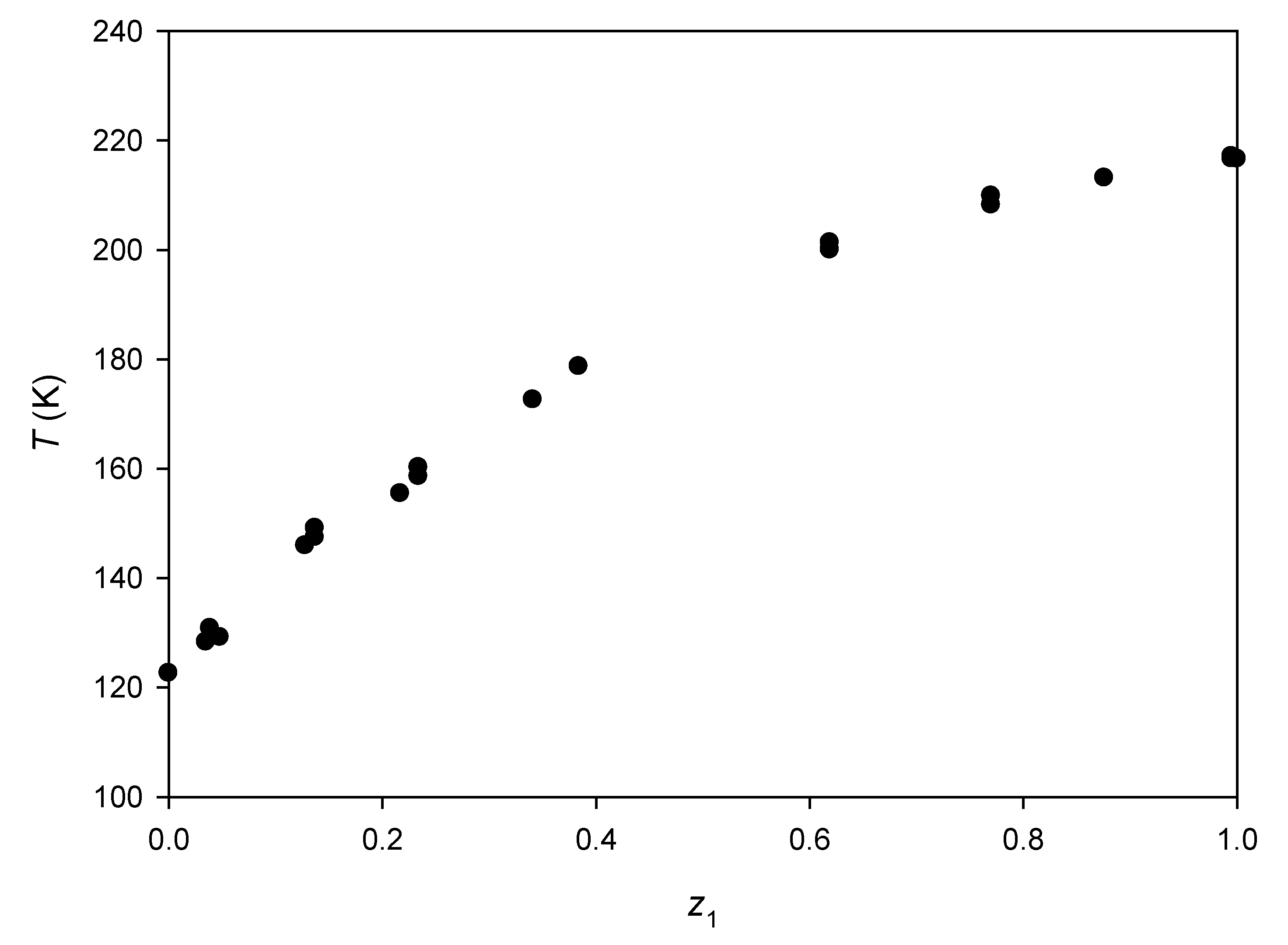
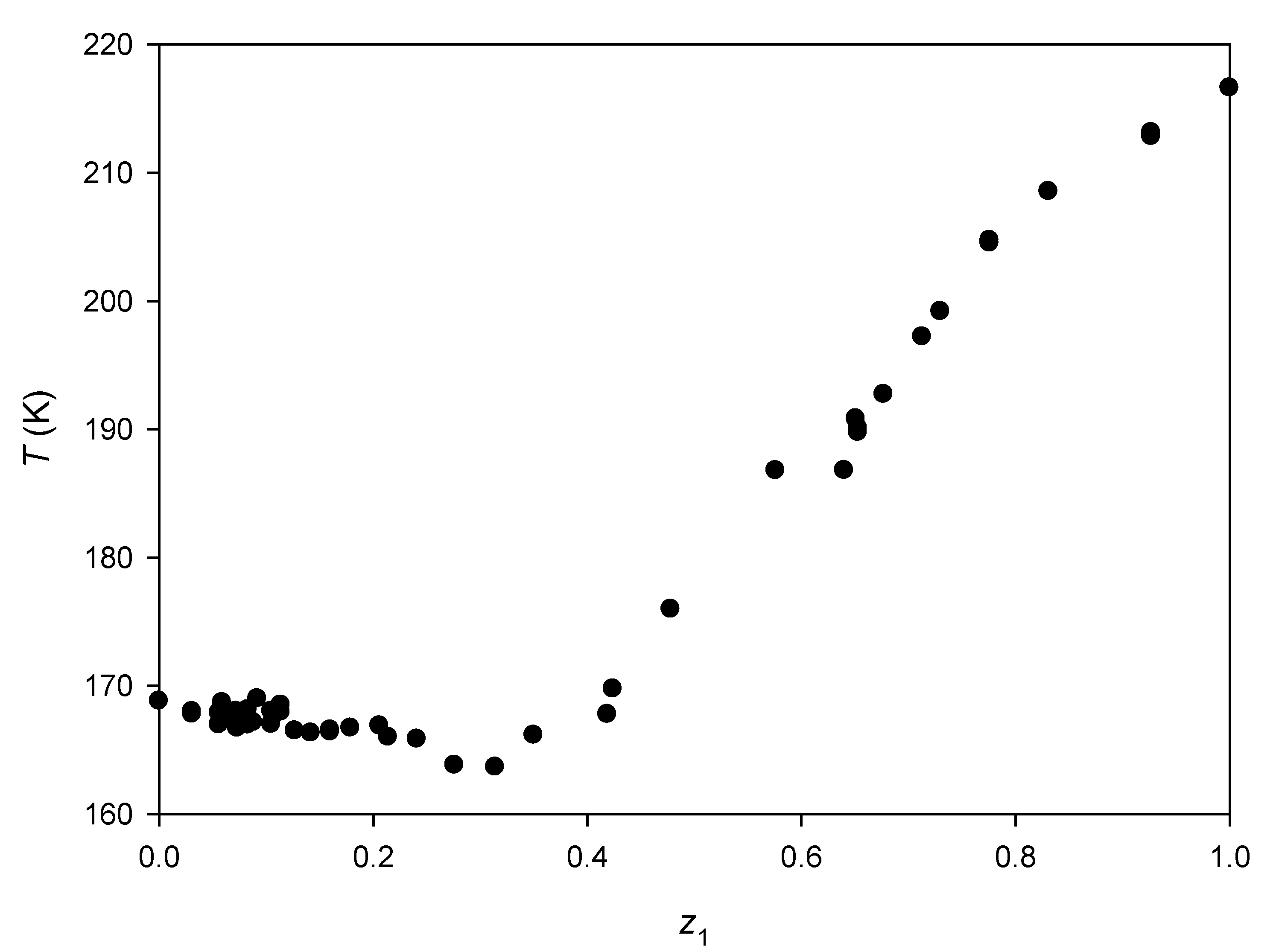
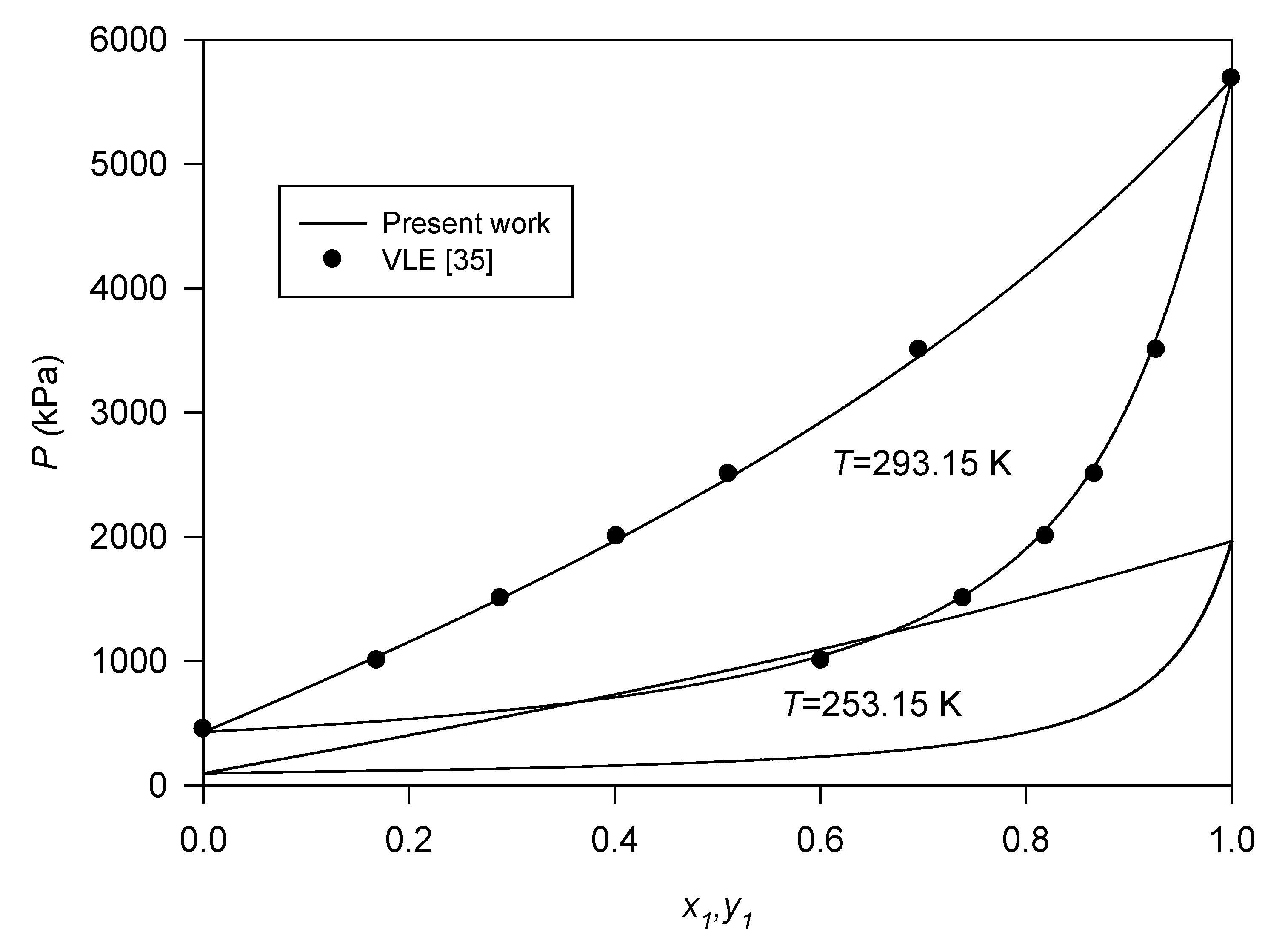
3.3. Binary Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- UNEP. Amendment to the Montreal Protocol on Substances that Deplete the Ozone Layer (Kigali Amendment). Int. Leg. Mater. 2016, 56, 193–205. [Google Scholar]
- Bell, I.H.; Domanski, P.A.; McLinden, M.O.; Linteris, G.T. The hunt for nonflammable refrigerant blends to replace R-134a. Int. J. Refrig. 2019, 104, 484–495. [Google Scholar] [CrossRef]
- Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing REGULATION (EC) No 842/2006 Text with EEA Relevance. 2014. Available online: https://www.eea.europa.eu/policy-documents/regulation-eu-no-517-2014 (accessed on 3 February 2020).
- European Commission Proposal for a Council. Decision on the Conclusion of the Agreement to Amend the Montreal Protocol on Substances that Deplete the Ozone Layer Adopted in Kigali; European Commission: Brussels, Belgium, 2017.
- Mota-Babiloni, A.; Makhnatch, P.; Khodabandeh, R. Recent investigations in HFCs substitution with lower GWP synthetic alternatives: Focus on energetic performance and environmental impact. Int. J. Refrig. 2017, 82, 288–301. [Google Scholar] [CrossRef]
- McLinden, M.O.; Kazakov, A.F.; Steven Brown, J.; Domanski, P.A. A thermodynamic analysis of refrigerants: Possibilities and tradeoffs for Low-GWP refrigerants. Int. J. Refrig. 2014, 38, 80–92. [Google Scholar] [CrossRef]
- McLinden, M.O.; Brown, J.S.; Brignoli, R.; Kazakov, A.F.; Domanski, P.A. Limited options for low-global-warming-potential refrigerants. Nat. Commun. 2017, 8, 14476. [Google Scholar] [CrossRef]
- IIR News Current Long-Term Alternative Refrigerants and Their Possible Applications. Available online: http://www.iifiir.org/userfiles/file/publications/notes/NoteTech_31_EN.pdf (accessed on 3 February 2020).
- Sánchez, D.; Cabello, R.; Llopis, R.; Arauzo, I.; Catalán-Gil, J.; Torrella, E. Energy performance evaluation of R1234yf, R1234ze(E), R600a, R290 and R152a as low-GWP R134a alternatives. Int. J. Refrig. 2017, 74, 267–280. [Google Scholar] [CrossRef]
- Bobbo, S.; Nicola, G.D.; Zilio, C.; Brown, J.S.; Fedele, L. Low GWP halocarbon refrigerants: A review of thermophysical properties. Int. J. Refrig. 2018, 90, 181–201. [Google Scholar] [CrossRef]
- Bolaji, B.O. Experimental study of R152a and R32 to replace R134a in a domestic refrigerator. Energy 2010, 35, 3793–3798. [Google Scholar] [CrossRef]
- Cabello, R.; Sánchez, D.; Llopis, R.; Arauzo, I.; Torrella, E. Experimental comparison between R152a and R134a working in a refrigeration facility equipped with a hermetic compressor. Int. J. Refrig. 2015, 60, 92–105. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The drop-in of HFC134a with HFO1234ze in a household refrigerator. Int. J. Therm. Sci. 2018, 127, 117–125. [Google Scholar] [CrossRef]
- Sieres, J.; Santos, J.M. Experimental analysis of R1234yf as a drop-in replacement for R134a in a small power refrigerating system. Int. J. Refrig. 2018, 91, 230–238. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, H.; Meng, Z.; Qin, Y. Experimental study on R1234yf/R134a mixture (R513A) as R134a replacement in a domestic refrigerator. Appl. Therm. Eng. 2019, 146, 540–547. [Google Scholar] [CrossRef]
- Giuliani, G.; Kumar, S.; Polonara, F. A constant volume apparatus for vapour pressure and gas phase P-ν-T measurements: Validation with data for R22 and R134a. Fluid Phase Equilib. 1995, 109, 265–279. [Google Scholar] [CrossRef]
- Di Nicola, G.; Polonara, F.; Ricci, R.; Stryjek, R. PVTx measurements for the R116+ CO2 and R41+ CO2 systems. New isochoric apparatus. J. Chem. Eng. Data 2005, 50, 312–318. [Google Scholar] [CrossRef]
- Rahman, M.S. Food Properties Handbook; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Weissberger, A. Technique of Organic Chemistry; Interscience Publishers: Geneva, Switzerland; New York, NY, USA, 1949; Volume 1. [Google Scholar]
- Di Nicola, G.; Brandoni, C.; Di Nicola, C.; Giuliani, G. Triple point measurements for alternative refrigerants. J. Therm. Anal. Calorim. 2011, 108, 627–631. [Google Scholar] [CrossRef]
- Di Nicola, G.; Moglie, M.; Santori, G.; Stryjek, R. Solid-Liquid Equilibria for the CO 2+ R143a and N 2 O+ R143a Systems. Int. J. Thermophys. 2009, 30, 1155–1164. [Google Scholar] [CrossRef][Green Version]
- Tomassetti, S.; Coccia, G.; Pierantozzi, M.; Passerini, G.; Di Nicola, G. Solid--liquid equilibria for the R32+ R1234ze (E) binary system. Int. J. Refrig. 2019, 107, 128–134. [Google Scholar] [CrossRef]
- World Meteorological Organization Scientific Assessment of Ozone Depletion: 2018. Available online: https://www.esrl.noaa.gov/csd/assessments/ozone/2018/downloads/2018OzoneAssessment.pdf. (accessed on 3 February 2020).
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0, National Institute of Standards and Technology. 2018. Available online: http//www. nist. gov/srd/nist23. cfm (accessed on 3 February 2020).
- Makhnatch, P.; Khodabandeh, R. The role of environmental metrics (GWP, TEWI, LCCP) in the selection of low GWP refrigerant. Energy Procedia 2014, 61, 2460–2463. [Google Scholar] [CrossRef]
- Technology and Economic Assessment Panel 2010 Progress Report: Assessment of HCFCs and Environmentally Sound Alternatives and Scoping Study on Alternatives to HCFC Refrigerants under High Ambient Temperature Conditions. Available online: https://unep.ch/ozone/Assessment_Panels/TEAP/Reports/TEAP_Reports/teap-2010-progress-report-volume1-May2010.pdf (accessed on 3 February 2020).
- Di Nicola, G.; Polonara, F.; Santori, G. Saturated pressure measurements of 2, 3, 3, 3-tetrafluoroprop-1-ene (HFO-1234yf). J. Chem. Eng. Data 2010, 55, 201–204. [Google Scholar] [CrossRef][Green Version]
- Di Nicola, C.; Di Nicola, G.; Pacetti, M.; Polonara, F.; Santori, G. P-V-T Behavior of 2, 3, 3, 3-Tetrafluoroprop-1-ene (HFO-1234yf) in the Vapor Phase from (243 to 373) K. J. Chem. Eng. Data 2010, 55, 3302–3306. [Google Scholar] [CrossRef]
- Di Nicola, G.; Brown, J.S.; Fedele, L.; Bobbo, S.; Zilio, C. Saturated pressure measurements of trans-1, 3, 3, 3-tetrafluoroprop-1-ene (R1234ze (E)) for reduced temperatures ranging from 0.58 to 0.92. J. Chem. Eng. Data 2012, 57, 2197–2202. [Google Scholar] [CrossRef]
- Brown, J.S.; Nicola, G.D.; Zilio, C.; Fedele, L.; Bobbo, S.; Polonara, F. Subcooled liquid density measurements and PvT measurements in the vapor phase for trans-1, 3, 3, 3-Tetrafluoroprop-1-ene (R1234ze (E)). J. Chem. Eng. Data 2012, 57, 3710–3720. [Google Scholar] [CrossRef]
- Di Nicola, G.; Di Nicola, C.; Arteconi, A.; Stryjek, R. PVTx measurements of the carbon dioxide+ 2, 3, 3, 3-Tetrafluoroprop-1-ene binary system. J. Chem. Eng. Data 2012, 57, 450–455. [Google Scholar] [CrossRef]
- Di Nicola, G.; Passerini, G.; Polonara, F.; Stryjek, R. PVTx measurements of the carbon dioxide+trans-1,3,3,3-tetrafluoroprop-1-ene binary system. Fluid Phase Equilib. 2013, 360, 124–128. [Google Scholar] [CrossRef]
- Di Nicola, G.; Arteconi, A.; Nardini, G.; Stryjek, R. Solid-liquid equilibria measurements of the carbon dioxide+2,3,3,3-tetrafluoroprop-1-ene and carbon dioxide+trans-1,3,3,3-tetrafluoropropene mixtures. Fluid Phase Equilib. 2013, 354, 54–58. [Google Scholar] [CrossRef]
- Di Nicola, G.; Giuliani, G.; Passerini, G.; Polonara, F.; Stryjek, R. Vapor-Liquid-Equilibrium (VLE) properties of R-32 + R-134a system derived from isochoric measurements. Fluid Phase Equilib. 1998, 153, 143–165. [Google Scholar] [CrossRef]
- Raabe, G. Molecular Simulation Studies on the Vapor–Liquid Phase Equilibria of Binary Mixtures of R-1234yf and R-1234ze(E) with R-32 and CO2. J. Chem. Eng. Data 2013, 58, 1867–1873. [Google Scholar] [CrossRef]

| Refrigerant | GWP | M (kg kmol−1) | Tb (K) | Tc (K) | Pc (kPa) |
|---|---|---|---|---|---|
| R744 | 1 | 44.010 | 194.69 | 304.13 | 7377.3 |
| R32 | 705 | 52.024 | 221.50 | 351.26 | 5782.0 |
| R600a | <1 | 58.122 | 261.40 | 407.81 | 3629.0 |
| R134a | 1360 | 102.030 | 247.08 | 374.21 | 4059.3 |
| R1234yf | <1 | 114.040 | 243.67 | 367.85 | 3382.2 |
| R1234ze(E) | <1 | 114.040 | 254.18 | 382.51 | 3634.9 |
| Series | N. Points | z1 | v (m3 kg−1) | ΔT (K) | ΔP (kPa) |
|---|---|---|---|---|---|
| 1 | 17 | 0.051 | 0.026 | 225–373 | 62–976 |
| 2 | 17 | 0.160 | 0.029 | 223–373 | 96–973 |
| 3 | 16 | 0.266 | 0.027 | 224–373 | 148–1119 |
| 4 | 15 | 0.365 | 0.026 | 223–373 | 198–1273 |
| 5 | 16 | 0.480 | 0.026 | 225–373 | 271–1381 |
| 6 | 16 | 0.574 | 0.029 | 224–373 | 312–1354 |
| 7 | 16 | 0.662 | 0.018 | 223–373 | 420–2292 |
| 8 | 15 | 0.754 | 0.029 | 224–373 | 460–1667 |
| Series | N. Points | z1 | v (m3 kg−1) | ΔT (K) | ΔP (kPa) |
|---|---|---|---|---|---|
| 1 | 14 | 0.155 | 0.028 | 233–363 | 104–943 |
| 2 | 14 | 0.254 | 0.029 | 233–363 | 149–1001 |
| 3 | 14 | 0.331 | 0.029 | 233–363 | 171–1063 |
| 4 | 14 | 0.459 | 0.029 | 233–363 | 255–1178 |
| 5 | 14 | 0.563 | 0.028 | 233–363 | 340–1366 |
| 6 | 14 | 0.642 | 0.030 | 233–363 | 397–1380 |
| 7 | 14 | 0.733 | 0.028 | 233–363 | 504–1601 |
| 8 | 14 | 0.859 | 0.028 | 233–363 | 634–1909 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierantozzi, M.; Tomassetti, S.; Di Nicola, G. Climate Change and Refrigerants: Thermodynamic Properties of Low-GWP Fluids for Domestic Applications and Binary Systems for Low-Temperature Options. Appl. Sci. 2020, 10, 2014. https://doi.org/10.3390/app10062014
Pierantozzi M, Tomassetti S, Di Nicola G. Climate Change and Refrigerants: Thermodynamic Properties of Low-GWP Fluids for Domestic Applications and Binary Systems for Low-Temperature Options. Applied Sciences. 2020; 10(6):2014. https://doi.org/10.3390/app10062014
Chicago/Turabian StylePierantozzi, Mariano, Sebastiano Tomassetti, and Giovanni Di Nicola. 2020. "Climate Change and Refrigerants: Thermodynamic Properties of Low-GWP Fluids for Domestic Applications and Binary Systems for Low-Temperature Options" Applied Sciences 10, no. 6: 2014. https://doi.org/10.3390/app10062014
APA StylePierantozzi, M., Tomassetti, S., & Di Nicola, G. (2020). Climate Change and Refrigerants: Thermodynamic Properties of Low-GWP Fluids for Domestic Applications and Binary Systems for Low-Temperature Options. Applied Sciences, 10(6), 2014. https://doi.org/10.3390/app10062014







