Investigation of the Role of the Environment on the Photoluminescence and the Exciton Relaxation of CsPbBr3 Nanocrystals Thin Films
Abstract
:1. Introduction
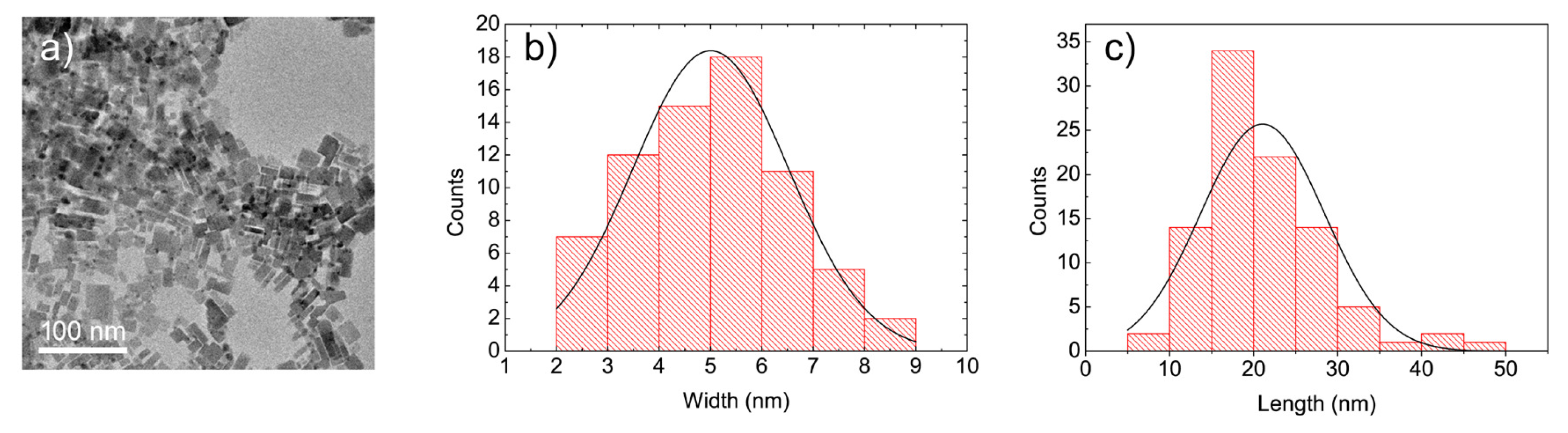
2. Results
- The peak intensity slightly increases (about 13%) (see inset of Figure 4a).
- The two faster decay times remain essentially unchanged ( 1.9 ns and 11.5 ns), while a clear increase of the longest decay time from about 70 ns to about 100 ns is observed.
- The pre-exponential amplitude of the fastest process A1 slightly decreases (about 4%), while the amplitudes A2 and A3 increase of about 40% and 36%, respectively.
- The peak intensity increases of about 2 times (see inset of Figure 4b).
- A clear increase of the two faster decay times is observed ( from 1.2 to about 2.0 ns and from about 7.5 to about 9.8 ns), while the slowest time remains basically constant at about 65 ns.
- All the pre-exponential amplitudes clearly increase of about 1.9, 4.3, and 3.9 times for A1, A2, and A3, respectively.
3. Discussion
4. Materials and Methods
4.1. Synthesis of NCs
4.2. Samples Preparation and Characterization
4.3. PL and Time Resolved PL Measurements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yu, J.; Zhang, M.; Chen, D.; Li, C.; Chen, R.; Jia, G.; Rogach, A.L.; Yang, X. Stable, Strongly Emitting Cesium Lead Bromide Perovskite Nanorods with High Optical Gain Enabled by an Intermediate Monomer Reservoir Synthetic Strategy. Nano Lett. 2019, 19, 6315–6322. [Google Scholar] [CrossRef] [PubMed]
- Rodá, C.; Abdelhady, A.L.; Shamsi, J.; Lorenzon, M.; Pinchetti, V.; Gandini, M.; Meinardi, F.; Manna, L.; Brovelli, S. O2 as a molecular probe for nonradiative surface defects in CsPbBr3 perovskite nanostructures and single crystals. Nanoscale 2019, 11, 7613–7623. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Pan, J.; Yin, J.; Bakr, O.M.; Mohammed, O.F. Room-Temperature Engineering of All-Inorganic Perovskite Nanocrsytals with Different Dimensionalities. Chem. Mat. 2017, 29, 8978–8982. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, A.y.; Yu, J.C.; Nam, Y.S.; Choi, Y.; Park, J.; Song, M.H. Surface Ligand Engineering for Efficient Perovskite Nanocrystal-Based Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 8428–8435. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-Threshold Amplified Spontaneous Emission and Lasing From Colloidal Nanocrystals of Caesium Lead Halide Perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef]
- Pan, J.; Sarmah, S.P.; Murali, B.; Dursun, I.; Peng, W.; Parida, M.R.; Liu, J.; Sinatra, L.; Alyami, N.; Zhao, C.; et al. Air-Stable Surface-Passivated Perovskite Quantum Dots for Ultra-Robust, Single- and Two-Photon-Induced Amplified Spontaneous Emission. J. Phys. Chem. Lett. 2015, 6, 5027–5033. [Google Scholar] [CrossRef] [Green Version]
- Balena, A.; Perulli, A.; Fernandez, M.; De Giorgi, M.L.; Nedelcu, G.; Kovalenko, M.V.; Anni, M. Temperature Dependence of the Amplified Spontaneous Emission from CsPbBr3 Nanocrystal Thin Films. J. Phys. Chem. C 2018, 122, 5813–5819. [Google Scholar] [CrossRef]
- De Giorgi, M.L.; Krieg, F.; Kovalenko, M.V.; Anni, M. Amplified Spontaneous Emission Threshold Reduction and Operational Stability Improvement in CsPbBr3 Nanocrystals Films by Hydrophobic Functionalization of the Substrate. Sci. Rep. 2019, 9, 17964. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory. Research Cell Record Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 20 March 2020).
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- De Giorgi, M.L.; Anni, M. Amplified Spontaneous Emission and Lasing in Lead Halide Perovskites: State of the Art and Perspectives. Appl. Sci. 2019, 9, 4591. [Google Scholar] [CrossRef] [Green Version]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef] [Green Version]
- Quitsch, W.A.; de Quilettes, D.W.; Pfingsten, O.; Schmitz, A.; Ognjanovic, S.; Jariwala, S.; Koch, S.; Winterer, M.; Ginger, D.S.; Bacher, G. The Role of Excitation Energy in Photobrightening and Photodegradation of Halide Perovskite Thin Films. J. Phys. Chem. Lett. 2018, 9, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Motti, S.G.; Gandini, M.; Barker, A.J.; Ball, J.M.; Srimath Kandada, A.R.; Petrozza, A. Photoinduced Emissive Trap States in Lead Halide Perovskite Semiconductors. ACS Energy Lett. 2016, 1, 726–730. [Google Scholar] [CrossRef]
- Barker, A.J.; Sadhanala, A.; Deschler, F.; Gandini, M.; Senanayak, S.P.; Pearce, P.M.; Mosconi, E.; Pearson, A.J.; Wu, Y.; Srimath Kandada, A.R.; et al. Defect-Assisted Photoinduced Halide Segregation in Mixed-Halide Perovskite Thin Films. ACS Energy Lett. 2017, 2, 1416–1424. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, C.; Ecker, B.; Yang, J.; Huang, J.; Gao, Y. Light-Induced Degradation of CH3NH3PbI3 Hybrid Perovskite Thin Film. J. Phys. Chem. C 2017, 121, 3904–3910. [Google Scholar] [CrossRef]
- Hodes, G.; Cahen, D. Perovskite cells roll forward. Nat. Photonics 2014, 8, 87–88. [Google Scholar] [CrossRef]
- Christians, J.; Miranda Herrera, P.; Kamat, P. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.; Young, T.; Patterson, R.J.; Kim, D.; Seidel, J.; Lim, S.; Green, M.A.; Huang, S.; Ho-Baillie, A. Humidity-Induced Degradation via Grain Boundaries of HC(NH2)2PbI3 Planar Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1705363. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Liu, Y.; Deng, Y.; Bai, Y.; Dong, Q.; Huang, J. Scaling behavior of moisture-induced grain degradation in polycrystalline hybrid perovskite thin films. Energy Environ. Sci. 2017, 10, 516–522. [Google Scholar] [CrossRef]
- Brenes, R.; Guo, D.; Osherov, A.; Noel, N.K.; Eames, C.; Hutter, E.M.; Pathak, S.K.; Niroui, F.; Friend, R.H.; Islam, M.S.; et al. Metal Halide Perovskite Polycrystalline Films Exhibiting Properties of Single Crystals. Joule 2017, 1, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Godding, J.S.; Ramadan, A.J.; Lin, Y.H.; Schutt, K.; Snaith, H.J.; Wenger, B. Oxidative Passivation of Metal Halide Perovskites. Joule 2019, 3, 2716–2731. [Google Scholar] [CrossRef]
- Péan, E.V.; Castro, C.S.D.; Davies, M.L. Shining a light on the photoluminescence behaviour of methylammonium lead iodide perovskite: Investigating the competing photobrightening and photodarkening processes. Mater. Lett. 2019, 243, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.M.; Tennyson, E.M.; Barik, S.; Szostak, R.; Waks, E.; Toney, M.F.; Nogueira, A.F.; Neves, B.R.A.; Leite, M.S. Humidity-Induced Photoluminescence Hysteresis in Variable Cs/Br Ratio Hybrid Perovskites. J. Phys. Chem. Lett. 2018, 9, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Lu, H.; Deng, W.; Yang, K.; Deng, Z.; Zhang, X.; Yuan, S.; Wang, J.; Niu, J.; et al. Reversible air-induced optical and electrical modulation of methylammonium lead bromide (MAPbBr3) single crystals. Appl. Phys. Lett. 2017, 111, 103904. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ren, Y.; Zhang, S.; Wu, J.; Song, J.; Li, X.; Xu, J.; Sow, C.H.; Zeng, H.; Sun, H. Switching excitonic recombination and carrier trapping in cesium lead halide perovskites by air. Commun. Phys. 2018, 1, 96. [Google Scholar] [CrossRef]
- Lorenzon, M.; Sortino, L.; Akkerman, Q.; Accornero, S.; Pedrini, J.; Prato, M.; Pinchetti, V.; Meinardi, F.; Manna, L.; Brovelli, S. Role of Nonradiative Defects and Environmental Oxygen on Exciton Recombination Processes in CsPbBr3 Perovskite Nanocrystals. Nano Lett. 2017, 17, 3844–3853. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Wang, B.; Zhu, N.; Zhang, C.; Kong, L.; Zhang, Q.; Shan, A.; Li, L. Morphology Evolution and Degradation of CsPbBr3 Nanocrystals under Blue Light-Emitting Diode Illumination. ACS Appl. Mat. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef]
- Lee, S.M.; Moon, C.J.; Lim, H.; Lee, Y.; Choi, M.Y.; Bang, J. Temperature-Dependent Photoluminescence of Cesium Lead Halide Perovskite Quantum Dots: Splitting of the Photoluminescence Peaks of CsPbBr3 and CsPb(Br/I)3 Quantum Dots at Low Temperature. J. Phys. Chem. C 2017, 121, 26054–26062. [Google Scholar] [CrossRef]
- Dey, A.; Rathod, P.; Kabra, D. Role of Localized States in Photoluminescence Dynamics of High Optical Gain CsPbBr3 Nanocrystals. Adv. Opt. Mat. 2018, 6, 1800109. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Jing, P.; Li, J.; Wei, M.; Hua, J.; Zhao, J.; Tian, L. Temperature-Dependent Photoluminescence of Inorganic Perovskite Nanocrystal Films. RSC Adv. 2016, 6, 78311–78316. [Google Scholar] [CrossRef]
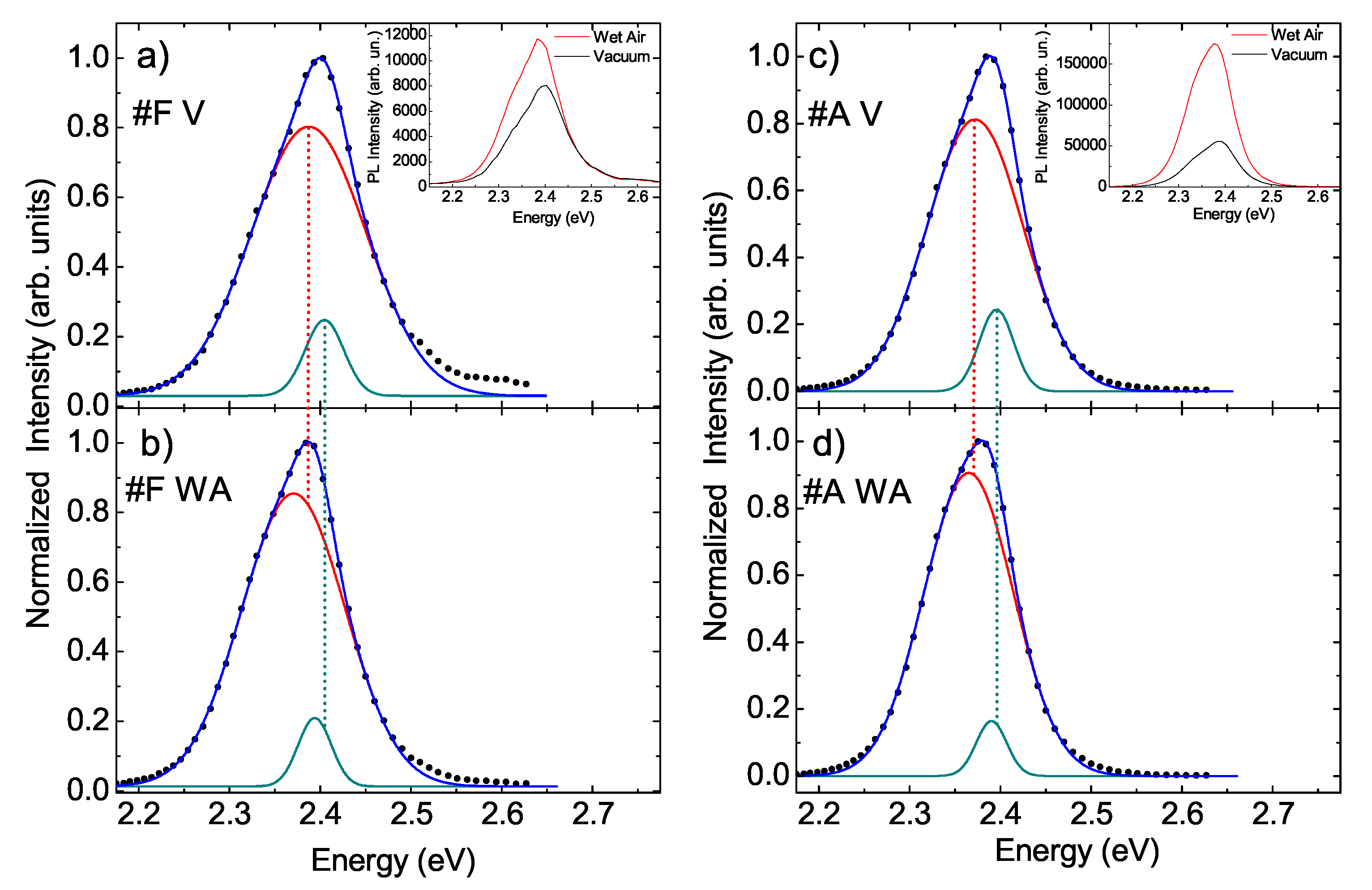



| Sample | E1 (eV) | FWHM1 (meV) | A1 | E2 (eV) | FWHM2 (meV) | A2 |
|---|---|---|---|---|---|---|
| #F V | 2.3873 ± 0.0009 | 149 ± 2 | 0.913 ± 0.018 | 2.405 ± 0.002 | 50 ± 6 | 0.087 ± 0.017 |
| #F WA | 2.3705 ± 0.0005 | 134.4 ± 0.9 | 1.209 ± 0.010 | 2.3939 ± 0.0009 | 43 ± 3 | 0.91 ± 0.09 |
| #A V | 2.3720 ± 0.0003 | 125.1 ± 0.5 | 0.903 ± 0.005 | 2.3955 ± 0.0004 | 44.5 ± 1.3 | 0.097 ± 0.004 |
| #A WA | 2.3650 ± 0.0004 | 116.1 ± 0.6 | 2.64 ± 0.02 | 2.3901 ± 0.0008 | 40 ± 3 | 0.163 ± 0.016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anni, M.; Cretì, A.; Zhang, Y.; De Giorgi, M.L.; Lomascolo, M. Investigation of the Role of the Environment on the Photoluminescence and the Exciton Relaxation of CsPbBr3 Nanocrystals Thin Films. Appl. Sci. 2020, 10, 2148. https://doi.org/10.3390/app10062148
Anni M, Cretì A, Zhang Y, De Giorgi ML, Lomascolo M. Investigation of the Role of the Environment on the Photoluminescence and the Exciton Relaxation of CsPbBr3 Nanocrystals Thin Films. Applied Sciences. 2020; 10(6):2148. https://doi.org/10.3390/app10062148
Chicago/Turabian StyleAnni, Marco, Arianna Cretì, Yuhai Zhang, Maria Luisa De Giorgi, and Mauro Lomascolo. 2020. "Investigation of the Role of the Environment on the Photoluminescence and the Exciton Relaxation of CsPbBr3 Nanocrystals Thin Films" Applied Sciences 10, no. 6: 2148. https://doi.org/10.3390/app10062148
APA StyleAnni, M., Cretì, A., Zhang, Y., De Giorgi, M. L., & Lomascolo, M. (2020). Investigation of the Role of the Environment on the Photoluminescence and the Exciton Relaxation of CsPbBr3 Nanocrystals Thin Films. Applied Sciences, 10(6), 2148. https://doi.org/10.3390/app10062148







