Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil)
Abstract
1. Introduction
2. Materials and Methods
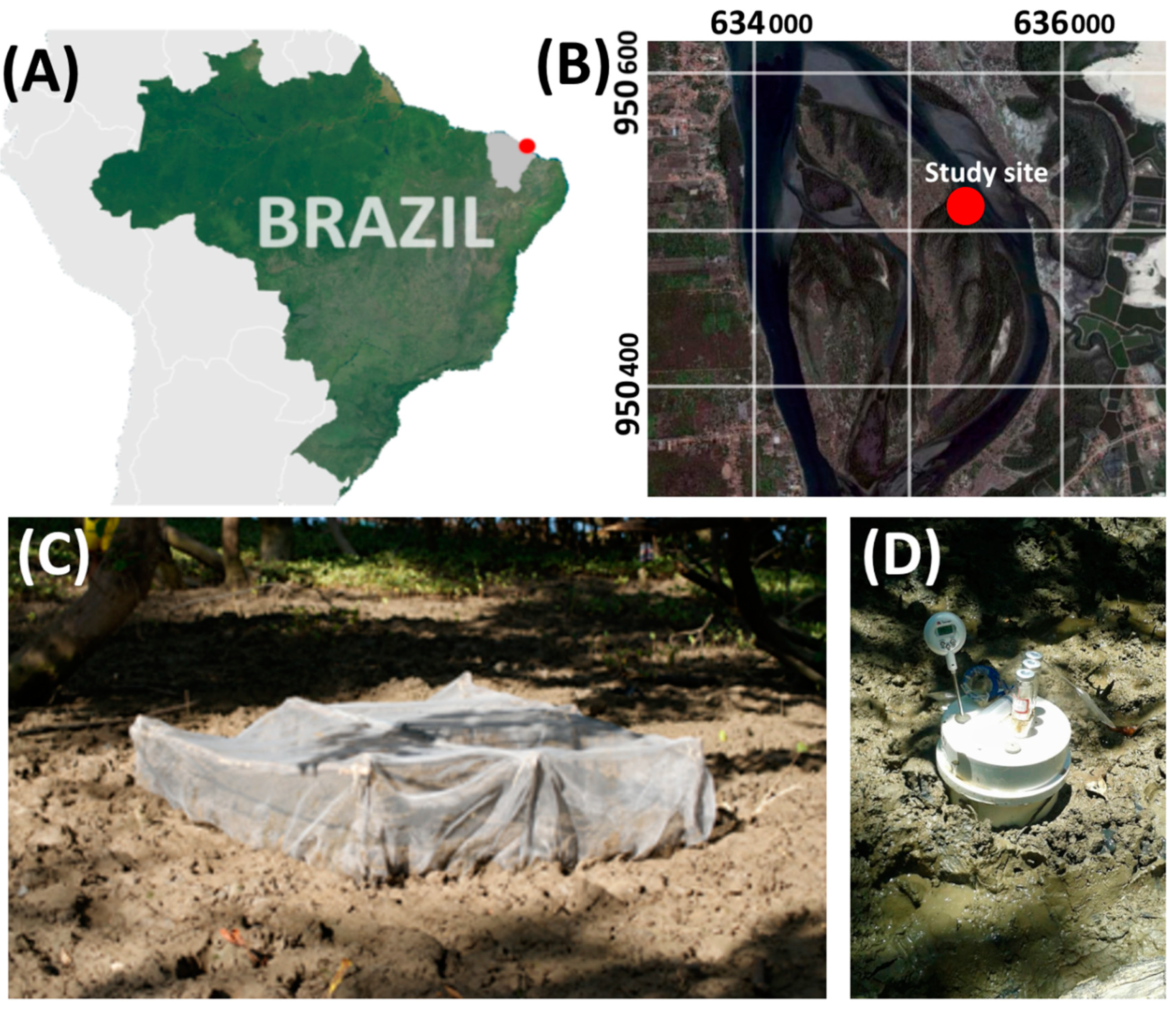
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sampling and Analyses
2.4. N2O Sampling and Analyses
2.5. Statistical Analyses
3. Results
3.1. Crab Burrow Densities and Burrow Characteristics
3.2. Soil Characterization
3.3. N2O Emissions
3.4. Effect of Seasonality and Bioturbation on Soil Composition and N2O Emissions
4. Discussion
4.1. N2O Emissions from Mangrove Soils are Controlled by Crab Activity and Seasonal Variation
4.2. Comparison of N2O Emissions from Mangroves and Estuarine Forests around the World
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alongi, D. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Chadwick, R.; Good, P.; Martin, G.; Rowell, D.P. Large rainfall changes consistently projected over substantial areas of tropical land. Nat. Clim. Chang. 2016, 6, 177–181. [Google Scholar] [CrossRef]
- Khalyani, A.H.; Gould, W.A.; Harmsen, E.; Terando, A.; Quinones, M.; Collazo, J.A. Climate Change Implications for Tropical Islands: Interpolating and Interpreting Statistically Downscaled GCM Projections for Management and Planning. J. Appl. Meteorol. Climatol. 2016, 55, 265–282. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Romero, D.J.; Nóbrega, G.N.; Otero, X.L.; Ferreira, T.O. Diffuse Reflectance Spectroscopy (Vis-Nir-Swir) as a Promising Tool for Blue Carbon Quantification in Mangrove Soils: A Case of Study in Tropical Semiarid Climatic Conditions. Soil Sci. Soc. Am. J. 2017, 81, 1661. [Google Scholar] [CrossRef]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Schaeffer-Novelli, Y.; Cintrón-Molero, G.; Adaime, R.R.; de Camargo, T.M. Variability of Mangrove Ecosystems along the Brazilian Coast. Estuaries 1990, 13, 204. [Google Scholar] [CrossRef]
- Ferreira, T.O.; Otero, X.L.; Vidal-Torrado, P.; Macias, F. Redox processes in mangrove soils under Rhizophora mangle in relation to different environmental conditions. Soil Sci. Soc. Am. J. 2007, 71, 484–491. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, GB2013. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17, 1111. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bernardino, A.F.; Ferreira, T.O.; Giovannoni, L.R.; de O. Gomes, L.E.; Romero, D.J.; Jimenez, L.C.Z.; Ruiz, F. Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biol. Lett. 2018, 14, 20180208. [Google Scholar] [CrossRef] [PubMed]
- Murdiyarso, D.; Purbopuspito, J.; Kauffman, J.B.; Warren, M.W.; Sasmito, S.D.; Donato, D.C.; Manuri, S.; Krisnawati, H.; Taberima, S.; Kurnianto, S. The potential of Indonesian mangrove forests for global climate change mitigation. Nat. Clim. Chang. 2015, 5, 1089–1092. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Kitaya, Y.; Yabuki, K.; Kiyota, M.; Tani, A.; Hirano, T.; Aiga, I. Gas exchange and oxygen concentration in pneumatophores and prop roots of four mangrove species. Trees 2002, 16, 155–158. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Buchholz, J.; Rennenberg, H. Emission of Methane and Nitrous Oxide by Australian Mangrove Ecosystems. Plant Biol. 2003, 5, 423–431. [Google Scholar] [CrossRef]
- Nielsen, O.I.; Kristensen, E.; Macintosh, D.J. Impact of fiddler crabs (Uca spp.) on rates and pathways of benthic mineralization in deposited mangrove shrimp pond waste. J. Exp. Mar. Bio. Ecol. 2003, 289, 59–81. [Google Scholar] [CrossRef]
- Booth, J.M.; Fusi, M.; Marasco, R.; Mbobo, T.; Daffonchio, D. Fiddler crab bioturbation determines consistent changes in bacterial communities across contrasting environmental conditions. Sci. Rep. 2019, 9, 3749. [Google Scholar] [CrossRef]
- Júnior, J.M.D.C.A.; Ferreira, T.O.; Suarez-Abelenda, M.; Nóbrega, G.N.; Albuquerque, A.G.B.M.; de Carvalho Bezerra, A.; Otero, X.L. The role of bioturbation by Ucides cordatus crab in the fractionation and bioavailability of trace metals in tropical semiarid mangroves. Mar. Pollut. Bull. 2016, 111, 194–202. [Google Scholar] [CrossRef]
- Kristensen, E.; Alongi, D.M. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron, and sulfur biogeochemistry in mangrove sediment. Limnol. Oceanogr. 2006, 51, 1557–1571. [Google Scholar] [CrossRef]
- Kristensen, E.; Flindt, M.; Ulomi, S.; Borges, A.; Abril, G.; Bouillon, S. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Mar. Ecol. Prog. Ser. 2008, 370, 53–67. [Google Scholar] [CrossRef]
- Neue, H.U.; Gaunt, J.L.; Wang, Z.P.; Becker-Heidmann, P.; Quijano, C. Carbon in tropical wetlands. Geoderma 1997, 79, 163–185. [Google Scholar] [CrossRef]
- Van Cleemput, O.; Samater, A.H. Nitrite in soils: Accumulation and role in the formation of gaseous N compounds. Fertil. Res. 1995, 45, 81–89. [Google Scholar] [CrossRef]
- Corredor, J.E.; Morell, J.M.; Bauza, J. Atmospheric nitrous oxide fluxes from mangrove sediments. Mar. Pollut. Bull. 1999, 38, 473–478. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Russow, R.; Sich, I.; Neue, H.U. The formation of the trace gases NO and N2O in soils by the coupled processes of nitrification and denitrification: Results of kinetic 15N tracer investigations. Chemosph. Glob. Chang. Sci. 2000, 2, 359–366. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 2010, 408, 2761–2767. [Google Scholar] [CrossRef]
- Meyer, R.L.; Allen, D.E.; Schmidt, S. Nitrification and denitrification as sources of sediment nitrous oxide production: A microsensor approach. Mar. Chem. 2008, 110, 68–76. [Google Scholar] [CrossRef]
- Firestone, M.K.; Davidson, E.A. Microbial basis of NO and N2O production and consumptino in soil. In Exchange of Trace Gases between Terrestrial Ecosystems and Atmosphere; Andreae, M.O., Schimel, D.S., Eds.; John Wiley & Sons: Berkeley, CA, USA, 1989; pp. 7–21. [Google Scholar]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Hollocher, T.C.; Tate, M.E.; Nicholas, D.J. Oxidation of ammonia by Nitrosomonas europaea. Definite 18O-tracer evidence that hydroxylamine formation involves a monooxygenase. J. Biol. Chem. 1981, 256, 10834–10836. [Google Scholar]
- Thomson, A.J.; Giannopoulos, G.; Pretty, J.; Baggs, E.M.; Richardson, D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Chang. Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef] [PubMed]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Bhomia, R.K.; MacKenzie, R.A.; Murdiyarso, D.; Sasmito, S.D.; Purbopuspito, J. Impacts of land use on Indian mangrove forest carbon stocks: Implications for conservation and management. Ecol. Appl. 2016, 26, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, G.N.; Ferreira, T.O.; Neto, M.S.; Queiroz, H.M.; Artur, A.G.; Mendonça, E.D.S.; Silva, E.D.O.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gopal, B.; Chauhan, M. Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquat. Sci. 2006, 68, 338–354. [Google Scholar] [CrossRef]
- Duke, N.C.; Meynecke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A World Without Mangroves? Science 2007, 317, 41b–42b. [Google Scholar] [CrossRef]
- Araújo Júnior, J.M.C.; Otero, X.L.; Marques, A.G.B.; Nóbrega, G.N.; Silva, J.R.F.; Ferreira, T.O. Selective geochemistry of iron in mangrove soils in a semiarid tropical climate: Effects of the burrowing activity of the crabs Ucides cordatus and Uca maracoani. Geo-Mar. Lett. 2012, 32, 289–300. [Google Scholar] [CrossRef]
- Barcellos, D.; Queiroz, H.M.; Nóbrega, G.N.; de Oliveira Filho, R.L.; Santaella, S.T.; Otero, X.L.; Ferreira, T.O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Artur, A.G.; Taniguchi, C.A.K.; da Silveira, M.R.S.; do Nascimento, J.C.; Nóbrega, G.N.; Otero, X.L.; Ferreira, T.O. Hidden contribution of shrimp farming effluents to greenhouse gas emissions from mangrove soils. Estuar. Coast. Shelf Sci. 2019, 221, 8–14. [Google Scholar] [CrossRef]
- Tanaka, M.O.; Maia, R.C. Shell Morphological Variation of Littoraria angulifera among and within Mangroves in NE Brazil. Hydrobiologia 2006, 559, 193–202. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Instituto de Pesquisa e Estratégia Econômica do Ceará. Perfil Básico Municipal—Aracati; IPECE: Fortaleza, Brazil, 2014. [Google Scholar]
- De Sales, J.C. Climatic Characterization and Comparison of Estimated Methods of the Reference Evapotranspiration in the Regions of Ceará State. Ph.D. Thesis, São Paulo State University, São Paulo, Brazil, 2008. [Google Scholar]
- Schmidt, A.J. Estudo da Dinâmica Populacional do Caranguejo-uçá, Ucides Cordatus Cordatus (Linnaeus, 1763) (Crustacea-Decapoda-Brachyura), e dos Efeitos de Uma Mortalidade em Massa Desta Espécie em Manguezais do Sul da Bahia. Ph.D. Thesis, São Paulo State University, São Paulo, Brazil, 2006. [Google Scholar]
- Kauffman, J.B.; Donato, D. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests; CIFOR: Bongor, Indonesia, 2012. [Google Scholar]
- Branco, J.O. Aspectos bioecológicos do caranguejo Ucides cordatus (Linnaeus, 1763) (Crustacea, Decapoda) do manguezal do Itacorubi, Santa Catarina, BR. Braz. Arch. Biol. Technol. 1993, 36, 133–148. [Google Scholar]
- Howard, J.; Hoyt, S.; Isensee, K.; Telszewski, M.; Pidgeon, E.; Telszewski, M. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrasses; International Union for Conservation of Nature: Arlington, VA, USA, 2014; Volume 1, ISBN 9782831717623. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.G.; Dutter, R. Statistical Data Analysis Explained; John Wiley & Sons, Ltd.: Chichester, UK, 2008; ISBN 9780470987605. [Google Scholar]
- McBratney, A.B.; Minasny, B.; Viscarra Rossel, R. Spectral soil analysis and inference systems: A powerful combination for solving the soil data crisis. Geoderma 2006, 136, 272–278. [Google Scholar] [CrossRef]
- Johnson, R.W. An Introduction to the Bootstrap. Teach. Stat. 2001, 23, 49–54. [Google Scholar] [CrossRef]
- Ferreira, T.O. Bioturbation and its role in iron and sulfur geochemistry in mangrove soils. In Biogeochemistry and Pedogenetic Process in Saltmarsh and Mangrove Systems; Nova Science Publishers: New York, NY, USA, 2010; pp. 199–222. ISBN 9781617282690. [Google Scholar]
- Allen, D.E.; Dalal, R.C.; Rennenberg, H.; Meyer, R.L.; Reeves, S.; Schmidt, S. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol. Biochem. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Ferreira, T.O.; Otero, X.L.; Vidal-Torrado, P.; Macías, F. Effects of bioturbation by root and crab activity on iron and sulfur biogeochemistry in mangrove substrate. Geoderma 2007, 142, 36–46. [Google Scholar] [CrossRef]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Chauhan, R.; Datta, A.; Ramanathan, A.; Adhya, T.K. Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmos. Environ. 2015, 107, 95–106. [Google Scholar] [CrossRef]
- Krithika, K.; Purvaja, R.; Ramesh, R. Fluxes of methane and nitrous oxide from an Indian mangrove. Curr. Sci. 2008, 94, 218–224. [Google Scholar]
- Hashimoto, S.; Gojo, K.; Hikota, S.; Sendai, N.; Otsuki, A. Nitrous oxide emissions from coastal waters in Tokyo Bay. Mar. Environ. Res. 1999, 47, 213–223. [Google Scholar] [CrossRef]
- Allen, D.; Dalal, R.C.; Rennenberg, H.; Schmidt, S. Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia. Plant Biol. 2011, 13, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J.; Xu, J.; Zhang, F. Distributions, sources and atmospheric fluxes of nitrous oxide in Jiaozhou Bay. Estuar. Coast. Shelf Sci. 2006, 68, 557–566. [Google Scholar] [CrossRef]
- Barnes, J.; Ramesh, R.; Purvaja, R.; Rajkumar, A.N.; Kumar, B.S.; Krithika, K.; Ravichandran, K.; Uher, G.; Upstill-Goddard, R. Tidal dynamics and rainfall control N2O and CH4 emissions from a pristine mangrove creek. Geophys. Res. Lett. 2006, 33, 4–9. [Google Scholar] [CrossRef]
- Livesley, S.J.; Andrusiak, S.M. Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuar. Coast. Shelf Sci. 2012, 97, 19–27. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment 2005: Thematic Study on Mangroves: Brazil, Country Profile; FAO - Forestry Department: Rome, Italy, 2005; p. 10. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Spalding, M.; Kainuma, M.; Collins, L. Book review: World atlas of mangroves. Wetlands 2010, 31, 1003–1005. [Google Scholar]
- Otero, X.L.; Macias, F. Spatial variation in pyritization of trace metals in salt-marsh soils. Biogeochemistry 2003, 62, 59–86. [Google Scholar] [CrossRef]
- Ferreira, T.O.; Otero, X.L.; de Souza Junior, V.S.; Vidal-Torrado, P.; Macías, F.; Firme, L.P. Spatial patterns of soil attributes and components in a mangrove system in Southeast Brazil (São Paulo). J. Soils Sediments 2010, 10, 995–1006. [Google Scholar] [CrossRef]
- Otero, X.L.; Méndez, A.; Nóbrega, G.N.; Ferreira, T.O.; Meléndez, W.; Macías, F. High heterogeneity in soil composition and quality in different mangrove forests of Venezuela. Environ. Monit. Assess. 2017, 189, 511. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.H.; Erler, D.V.; Eyre, B.D. Nitrous Oxide Fluxes in Estuarine Environments: Response to Global Change. Glob. Change Biol. 2015, 21, 3219–3245. [Google Scholar] [CrossRef]
- Sanchez-Rubio, G.; Perry, H.M.; Biesiot, P.M.; Johnson, D.R.; Lipcius, R.N. Climate-related hydrological regimes and their effects on abundance of juvenile blue crabs (Callinectes sapidus) in the northcentral Gulf of Mexico. Fish. Bull. 2011, 109, 139–146. [Google Scholar]
- Vinueza, L.R.; Branch, G.M.; Branch, M.L.; Bustamante, R.H. Top-down herbivory and bottom-up El Niño effects on Galápagos rocky-shore communities. Ecol. Monogr. 2006, 76, 111–131. [Google Scholar] [CrossRef]
- Alongi, D. The Energetics of Mangrove Forests; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-4270-6. [Google Scholar]
- Wolanski, E. Hydrodynamics of mangrove swamps and their coastal waters. Hydrobiologia 1992, 247, 141–161. [Google Scholar] [CrossRef]
- Woodroffe, C. Mangrove sediments and geomorphology. In Tropical Mangrove Ecosystems; Alongi, D.M., Robertson, A.I., Eds.; American Geophysical Union (AGU): Washington, DC, USA, 1992; Volume 41, pp. 7–41. [Google Scholar]
- Chen, Y.; Chen, G.; Ye, Y. Coastal vegetation invasion increases greenhouse gas emission from wetland soils but also increases soil carbon accumulation. Sci. Total Environ. 2015, 526, 19–28. [Google Scholar] [CrossRef]
- Chen, G.C.; Ulumuddin, Y.I.; Pramudji, S.; Chen, S.Y.; Chen, B.; Ye, Y.; Ou, D.Y.; Ma, Z.Y.; Huang, H.; Wang, J.K. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 2014, 487, 91–96. [Google Scholar] [CrossRef]





| Site | Environment Characterization | Season | N2O flux (µg m−2 h−1) | Reference |
|---|---|---|---|---|
| Brazil. Ceará State | Crab-exclusion/Control area (without crabs Ucides cordatus) | Rainy | 47.0 ± 18.0 | This study |
| Dry | 6.0 ± 5.0 | |||
| Naturally bioturbated area (with crabs Ucides cordatus) | Rainy | 36.5 ± 12.0 | ||
| Dry | 4.50 ± 2.0 | |||
| Australia. Queensland | Fringe river with high human influence. High nitrate and phosphate concentration | Autumn (April) | 28.5 ± 9.10 | [56] |
| Summer (February). Rainy season | 15.2 ± 8.80 | |||
| Winter (July) | 24.5 ± 5.20 | |||
| Avicennia marina forest | Autumn (April) | 29.17 ± 11.6 | ||
| Summer (February). Rainy season | 6.0 ± 3.0 | |||
| Winter (July) | 11.2 ± 6.34 | |||
| India. South Andaman island | Pristine area. Mangrove creek | Rainy | 9.24 (water creek) | [64] |
| Dry | 11.04 (water creek) | |||
| Mangrove forest of Rhizophora sp. | Rainy | 9.66 | ||
| Dry | 5.52 | |||
| India. Odisha state. | Avicennia sp. | Monsoon | 16.55 | |
| Winter | 26.54 | [59] | ||
| Rhizophora mucronate | Monsoon | 18.79 | ||
| Winter (high nitrate concentration) | 112.8 | |||
| Mangrove eutrophicated with nitrate | Monsoon | 14.13 | ||
| Winter | 39.00 | |||
| China. Jiulong estuary | Vegetation: Kandelia obovata | Winter-dry season | 16.0 ± 6.0 | [78] |
| Spring | 52.0 ± 0.010 | |||
| Summer-Rainy season | 37.0 ± 7.0 | |||
| Autumn | 31.0 ± 3.0 | |||
| Vegetation Sonneratia apetala | Winter-dry season | 25.0 ± 3.0 | ||
| Spring | 51.0 ± 11.0 | |||
| Summer-Rainy season | 50.0 ± 7.0 | |||
| Autumn | 80.0 ± 7.0 | |||
| Vegetation: Cyperus malaccensis | Winter-dry season | 13.0 ± 7.0 | ||
| Spring | 46.0 ± 9.0 | |||
| Summer-Rainy season | 90.0 ± 7.0 | |||
| Autumn | 50.0 ± 10.0 | |||
| Vegetation: Spartina alterniflora | Winter-dry season | 6.0 ± 1.0 | ||
| Spring | 0.081 ± 0.008 | |||
| Summer-Rainy season | 60.0 ± 5.0 | |||
| Autumn | 56.0 ± 3.0 | |||
| Indonesia. North Sulawesi. | Rhizophora apiculata, Bruguiera gymnorrhiza, | −16.8–29.3 | [79] | |
| Puerto Rico | Magueyes Island. Vegetation: Rhizophora mangue | January/December. Rainy season | ~28.8 | [23] |
| Enrique reef. Vegetation: R. mangue | November/February. Rainy season | 12 | ||
| Bird Island. Vegetation: R. mangue affected by guano | November/February. Rainy season | 374 | ||
| Seawage treatment plant. Vegetation R. mangue/ Laguncularia racemosa. High nitrate concentration | January-June. Rainy-dry season | ~33.6 | ||
| Seawage treatment plant.Vegetation: Avicennia germinans. High nitrate concentration | January/June. Rainy/dry season | 5.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, X.L.; Araújo, J.M.C., Jr.; Barcellos, D.; Queiroz, H.M.; Romero, D.J.; Nóbrega, G.N.; Siqueira Neto, M.; Ferreira, T.O. Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil). Appl. Sci. 2020, 10, 2215. https://doi.org/10.3390/app10072215
Otero XL, Araújo JMC Jr., Barcellos D, Queiroz HM, Romero DJ, Nóbrega GN, Siqueira Neto M, Ferreira TO. Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil). Applied Sciences. 2020; 10(7):2215. https://doi.org/10.3390/app10072215
Chicago/Turabian StyleOtero, Xosé L., José M. C. Araújo, Jr., Diego Barcellos, Hermano M. Queiroz, Danilo J. Romero, Gabriel N. Nóbrega, Marcos Siqueira Neto, and Tiago O. Ferreira. 2020. "Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil)" Applied Sciences 10, no. 7: 2215. https://doi.org/10.3390/app10072215
APA StyleOtero, X. L., Araújo, J. M. C., Jr., Barcellos, D., Queiroz, H. M., Romero, D. J., Nóbrega, G. N., Siqueira Neto, M., & Ferreira, T. O. (2020). Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil). Applied Sciences, 10(7), 2215. https://doi.org/10.3390/app10072215








