Efficacy of Allicin against Plant Pathogenic Fungi and Unveiling the Underlying Mode of Action Employing Yeast Based Chemogenetic Profiling Approach
Abstract
1. Introduction
2. Materials and Methods
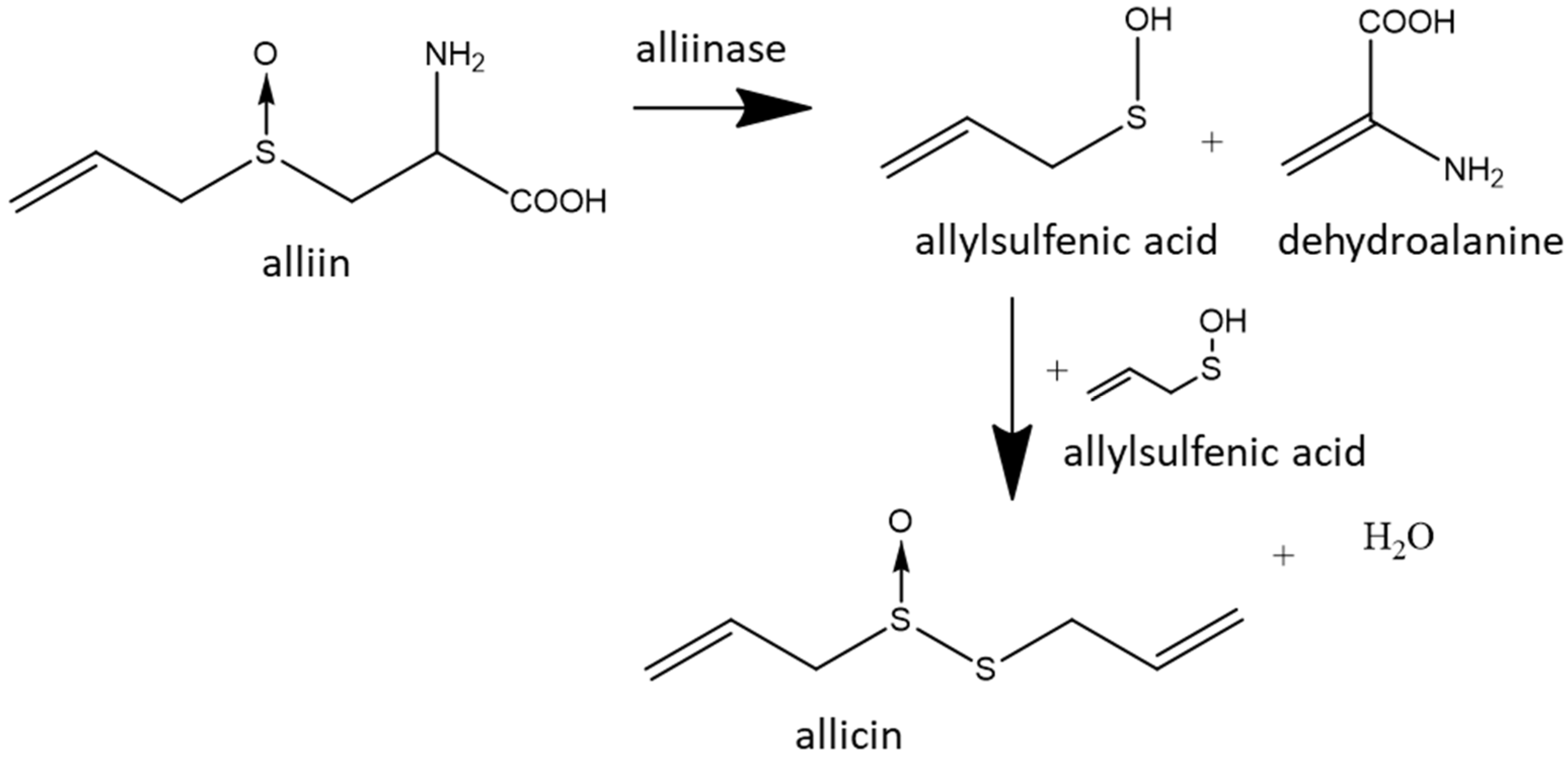
2.1. Synthesis of Allicin
2.2. Growth Media and Strains
2.2.1. PDB (Potato Dextrose Broth) and PDA (Potato Dextrose Agar) Media
2.2.2. YPD (Yeast Peptone Dextrose) Growth Medium
2.2.3. CSM (Complete Supplement Medium) Growth Medium
2.2.4. Fungal Strains
2.2.5. Yeast Strains
2.3. Assessment of Antifungal Activity
2.3.1. Zone of Growth Assay
2.3.2. Inhibition Zone Assay
2.3.3. Gas Phase Assay
2.4. Yeast Chemogenetic Screening
Measuring Yeast Growth Kinetics
3. Results and Discussion
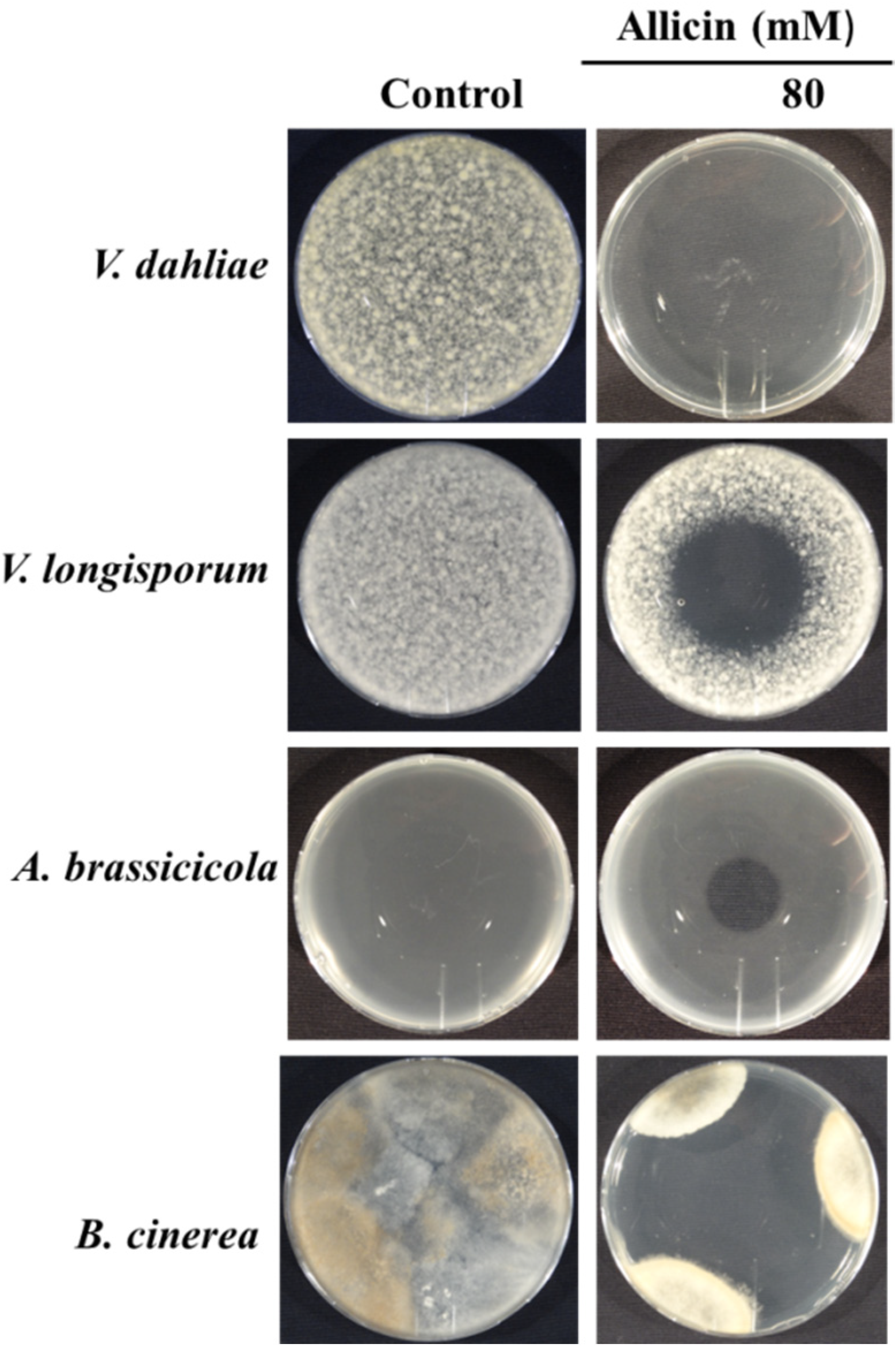
3.1. Evaluation of Antifungal Activity of Allicin
3.1.1. Determination of Zone of Growth
3.1.2. Inhibition Zone Assay
3.1.3. Gas Phase Assay
3.2. Yeast Genome Haploinsufficiency Screening
Quantitative Identification of Hypersensitive Strains
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adler, B.B.; Beuchat, L.R. Death of Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes in garlic butter as affected by storage temperature. J. Food Prot. 2002, 65, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, E.A.; Hill, D.J.; Maslin, D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef] [PubMed]
- Lemar, K.M.; Turner, M.P.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.D.; Andersen, D.O.; North, J.A.; Murray, B.K.; Lawson, L.D.; Hughes, B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992, 58, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Loi, V.V.; Huyen, N.T.T.; Busche, T.; Tung, Q.N.; Gruhlke, M.C.H.; Kalinowski, J.; Bernhardt, J.; Slusarenko, A.J.; Antelmann, H. Staphylococcus aureus responds to allicin by global S-thioallylation—Role of the Brx/BSH/YpdA pathway and the disulfide reductase MerA to overcome allicin stress. Free Radic. Biol. Med. 2019, 139, 55–69. [Google Scholar] [CrossRef]
- Horn, T.; Bettray, W.; Slusarenko, J.A.; Gruhlke, C.M. S-allylmercaptoglutathione is a Substrate for Glutathione Reductase (E.C. 1.8.1.7) from Yeast (Saccharomyces cerevisiae). Antioxidants 2018, 7, 86. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Portz, D.; Stitz, M.; Anwar, A.; Schneider, T.; Jacob, C.; Schlaich, N.L.; Slusarenko, A.J. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Rad. Biol. Med. 2010, 49, 1916–1924. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Bolger, A.; Schier, C.; Vogel, A.; Usadel, B.; Gruhlke, M.C.H.; Slusarenko, A.J. Genetic and molecular characterization of multicomponent resistance of Pseudomonas against allicin. Life Sci. 2020, 3. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Antelmann, H.; Bernhardt, J.; Kloubert, V.; Rink, L.; Slusarenko, A.J. The human allicin-proteome: S-thioallylation of proteins by the garlic defence substance allicin and its biological effects. Free Radic. Biol. Med. 2019, 131, 144–153. [Google Scholar] [CrossRef]
- Reiter, J.; Hubbers, A.M.; Albrecht, F.; Leichert, L.I.O.; Slusarenko, A.J. Allicin, a natural antimicrobial defence substance from garlic, inhibits DNA gyrase activity in bacteria. Int. J. Med. Microbiol. 2020, 310, 151359. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Seebeck, E. Chemical investigations on alliin, the specific principle of garlic. Adv. Enzymol. Relat. Subj. Biochem. 1951, 11, 377–400. [Google Scholar] [PubMed]
- Kuettner, E.B.; Hilgenfeld, R.; Weiss, M.S. The active principle of garlic at atomic resolution. J. Biol. Chem. 2002, 277, 46402–46407. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Fujisawa, H.; Watanabe, K.; Suma, K.; Origuchi, K.; Matsufuji, H.; Seki, T.; Ariga, T. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci. Biotechnol. Biochem. 2009, 73, 1948–1955. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef]
- Khodavandi, A.; Alizadeh, F.; Harmal, N.S.; Sidik, S.M.; Othman, F.; Sekawi, Z.; Jahromi, M.A.; Ng, K.P.; Chong, P.P. Comparison between efficacy of allicin and fluconazole against Candida albicans in vitro and in a systemic candidiasis mouse model. Fems Microbiol. Lett. 2011, 315, 87–93. [Google Scholar] [CrossRef]
- Daniel, C.K.; Lennox, C.L.; Vries, F.A. IN-Vitro Effects OF Garlic Extracts On Pathogenic Fungi Botrytis Cinerea, Penicillium Expansum And Neofabraea Alba. S. Afr. J. Sci. 2015, 111, 1–8. [Google Scholar] [CrossRef]
- Kutawa, A.; Danladi, M.; Haruna, A. Antifungal Activity of Garlic (Allium sativum) Extract on Some Selected Fungi. J. Med. Herbs Ethnomed. 2018, 4, 12–14. [Google Scholar]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Perello, A.; Gruhlke, M.C.; Slusarenko, A.J. Effect of garlic extract on seed germination, seedling health, and vigour of pathogen-infested wheat. J. Plant Prot. Res. 2013, 53, 317–323. [Google Scholar] [CrossRef]
- Perelló, A.; Noll, U.; Slusarenko, A.J. In vitro efficacy of garlic extract to control fungal pathogens of wheat. J. Med. Plant Res. 2013, 7, 1809–1817. [Google Scholar]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Hobbelen, P.H.; Paveley, N.D.; van den Bosch, F. The emergence of resistance to fungicides. PLoS ONE 2014, 9, e91910. [Google Scholar] [CrossRef]
- Barbara, D.J.; Clewes, E. Plant pathogenic Verticillium species: How many of them are there? Mol. Plant Pathol. 2003, 4, 297–305. [Google Scholar] [CrossRef]
- Daayf, F. Verticillium wilts in crop plants: Pathogen invasion and host defence responses. Can. J. Plant Pathol. 2014, 37, 8–20. [Google Scholar] [CrossRef]
- Bhat, R.G.; Subbarao, K.V. Host Range Specificity in Verticillium dahliae. Phytopathology 1999, 89, 1218–1225. [Google Scholar] [CrossRef]
- Keykhasaber, M.; Thomma, B.P.H.J.; Hiemstra, J.A. Verticillium wilt caused by Verticillium dahliae in woody plants with emphasis on olive and shade trees. Eur. J. Plant Pathol. 2018, 150, 21–37. [Google Scholar] [CrossRef]
- Goicoechea, N. To what extent are soil amendments useful to control Verticillium wilt? Pest Manag. Sci. 2009, 65, 831–839. [Google Scholar] [CrossRef]
- Short, D.P.; Sandoya, G.; Vallad, G.E.; Koike, S.T.; Xiao, C.L.; Wu, B.M.; Gurung, S.; Hayes, R.J.; Subbarao, K.V. Dynamics of verticillium species microsclerotia in field soils in response to fumigation, cropping patterns, and flooding. Phytopathology 2015, 105, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Van Den Oever, R.U.; Roosels, D.; Lahaye, D. Actual hazard of methyl bromide fumigation in soil disinfection. Br. J. Ind. Med. 1982, 39, 140–144. [Google Scholar] [CrossRef][Green Version]
- Olivier, C.; Vaughn, S.F.; Mizubuti, E.S.G.; Loria, R. Variation in Allyl Isothiocyanate Production Within Brassica Species and Correlation with Fungicidal Activity. J. Chem. Ecol. 1999, 25, 2687–2701. [Google Scholar] [CrossRef]
- Porter, I.J.; Brett, R.W.; Wiseman, B.M. Alternatives to methyl bromide: Chemical fumigants or integrated pest management systems? Australas. Plant Pathol. 1999, 28, 65–71. [Google Scholar] [CrossRef]
- Neubauer, C.; Heitmann, B.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant Pathol. 2014, 140, 341–352. [Google Scholar] [CrossRef]
- Auger, J.; Arnault, I.; Diwo-Allain, S.; Ravier, M.l.; Molia, F.; Pettiti, M. Insecticidal and fungicidal potential of Allium substances as biofumigants. Agroindustria 2004, 3, 5–8. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Mamgain, A.; Roychowdhury, R.; Tah, J. Alternaria pathogenicity and its strategic controls. Res. J. Biol. 2013, 1, 1–9. [Google Scholar]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta 1998, 1379, 233–244. [Google Scholar] [CrossRef]
- Wills, E.D. Enzyme Inhibition by Allicin, the Active Principle of Garlic. Biochem. J. 1956, 63, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability throughphospholipid membranes may contribute to its biological activity. Biochim. Et Biophys. Acta 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Prescott, T.A.K.; Panaretou, B. A Mini HIP HOP Assay Uncovers a Central Role for Copper and Zinc in the Antifungal Mode of Action of Allicin. J. Agric. Food Chem. 2017, 65, 3659–3664. [Google Scholar] [CrossRef]
- Yu, L.; Guo, N.; Meng, R.; Liu, B.; Tang, X.; Jin, J.; Cui, Y.; Deng, X. Allicin-induced global gene expression profile of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 88, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C.; Thompson, N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef]
- Giaever, G.; Shoemaker, D.D.; Jones, T.W.; Liang, H.; Winzeler, E.A.; Astromoff, A.; Davis, R.W. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat. Genet. 1999, 21, 278–283. [Google Scholar] [CrossRef]
- Giaever, G.; Flaherty, P.; Kumm, J.; Proctor, M.; Nislow, C.; Jaramillo, D.F.; Chu, A.M.; Jordan, M.I.; Arkin, A.P.; Davis, R.W. Chemogenomic profiling: Identifying the functional interactions of small molecules in yeast. Proc. Natl. Acad. Sci. USA 2004, 101, 793–798. [Google Scholar] [CrossRef]
- Albrecht, F.; Leontiev, R.; Jacob, C.; Slusarenko, A.J. An Optimized Facile Procedure to Synthesize and Purify Allicin. Molecules 2017, 22, 770. [Google Scholar] [CrossRef]
- Leontiev, R.; Slusarenko, A.J. Finding the Starting Point for Mode-of-Action Studies of Novel Selenium Compounds: Yeast as a Genetic Toolkit. Curr. Org. Synth. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Portz, D.; Koch, E.; Slusarenko, A.J. Effects of garlic (Allium sativum) juice containing allicin on Phytophthora infestans and downy mildew of cucumber caused by Pseudoperonospora cubensis. Eur. J. Plant Pathol. 2008, 122, 197–206. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, J.; Wang, Z.; Liu, F.; Wan, Z. Fumigation toxicity of allicin against three stored product pests. J. Stored Prod. Res. 2013, 55, 48–54. [Google Scholar] [CrossRef]
- Prowse, G.M.; Galloway, T.S.; Foggo, A. Insecticidal activity of garlic juice in two dipteran pests. Agric. For. Entomol. 2006, 8, 1–6. [Google Scholar] [CrossRef]
- Park, I.K.; Shin, S.C. Fumigant activity of plant essential oils and components from garlic (Allium sativum) and clove bud (Eugenia caryophyllata) oils against the Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2005, 53, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Choi, K.S.; Kim, D.H.; Choi, I.H.; Kim, L.S.; Bak, W.C.; Choi, J.W.; Shin, S.C. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae). Pest Manag. Sci. 2006, 62, 723–728. [Google Scholar] [CrossRef]
- Prescott, T.A.; Rigby, L.P.; Veitch, N.C.; Simmonds, M.S. The haploinsufficiency profile of alpha-hederin suggests a caspofungin-like antifungal mode of action. Phytochemistry 2014, 101, 116–120. [Google Scholar] [CrossRef]
- Prescott, T.A.K.; Panaretou, B.; Veitch, N.C.; Simmonds, M.S.J. A yeast chemical genetics approach identifies the compound 3,4,5-trimethoxybenzyl isothiocyanate as a calcineurin inhibitor. Febs Lett. 2014, 588, 455–458. [Google Scholar] [CrossRef]
- Manikova, D.; Letavayova, L.M.; Vlasakova, D.; Kosik, P.; Estevam, E.C.; Nasim, M.J.; Gruhlke, M.; Slusarenko, A.; Burkholz, T.; Jacob, C.; et al. Intracellular diagnostics: Hunting for the mode of action of redox-modulating selenium compounds in selected model systems. Molecules 2014, 19, 12258–12279. [Google Scholar] [CrossRef]
- Snyder, M.; He, W.; Zhang, J.J. The DNA replication factor MCM5 is essential for Stat1-mediated transcriptional activation. Proc. Natl. Acad. Sci. USA 2005, 102, 14539–14544. [Google Scholar] [CrossRef]
- Alonso, B.; Chaussinand, G.; Armengaud, J.; Godon, C. A role for GPN-loop GTPase yGPN1 in sister chromatid cohesion. Cell Cycle 2011, 10, 1828–1837. [Google Scholar] [CrossRef]
- Chiu, M.I.; Mason, T.L.; Fink, G.R. Hts1 Encodes Both the Cytoplasmic and Mitochondrial Histidyl-Trna Synthetase of Saccharomyces Cerevisiae: Mutations Alter the Specificity of Compartmentation. Genetics 1992, 132, 987–1001. [Google Scholar]
- Corral, M.J.; Benito-Pena, E.; Jimenez-Anton, M.D.; Cuevas, L.; Moreno-Bondi, M.C.; Alunda, J.M. Allicin Induces Calcium and Mitochondrial Dysregulation Causing Necrotic Death in Leishmania. PLoS Negl. Trop. Dis. 2016, 10, e0004525. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, H.; Zhang, S.; Wang, X.; Yu, L.; Lu, J.; Li, J. Initial study on naturally occurring products from traditional Chinese herbs and vegetables for chemoprevention. J. Cell. Biochem. Suppl. 1997, 27, 106–112. [Google Scholar] [CrossRef]
- Hassan, H.T. Ajoene (natural garlic compound): A new anti-leukaemia agent for AML therapy. Leuk. Res. 2004, 28, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, S.J.; Kwon, H.C.; Lee, K.R.; Rhee, D.K.; Pyo, S. Caspase-independent cell death by allicin in human epithelial carcinoma cells: Involvement of PKA. Cancer Lett. 2005, 224, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sendl, A.; Schliack, M.; Loser, R.; Stanislaus, F.; Wagner, H. Inhibition of cholesterol synthesis in vitro by extracts and isolated compounds prepared from garlic and wild garlic. Atherosclerosis 1992, 94, 79–85. [Google Scholar] [CrossRef]
- Gebhardt, R.; Beck, H.; Wagner, K.G. Inhibition of cholesterol biosynthesis by allicin and ajoene in rat hepatocytes and HepG2 cells. Biochim. Biophys. Acta 1994, 1213, 57–62. [Google Scholar] [CrossRef]
- Stevinson, C.; Pittler, M.H.; Ernst, E. Garlic for treating hypercholesterolemia. A meta-analysis of randomized clinical trials. Ann. Intern. Med. 2000, 133, 420–429. [Google Scholar] [CrossRef]
- Yeh, Y.Y.; Liu, L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: Human and animal studies. J. Nutr. 2001, 131, 989S–993S. [Google Scholar] [CrossRef]
- Lu, Y.; He, Z.; Shen, X.; Xu, X.; Fan, J.; Wu, S.; Zhang, D. Cholesterol-lowering effect of allicin on hypercholesterolemic ICR mice. Oxidative Med. Cell. Longev. 2012, 2012, 489690. [Google Scholar] [CrossRef]
- Ha, M.W.; Yuan, Y. Allicin induced cell cycle arrest in human gastric cancer cell lines. Zhonghua Zhong Liu Za Zhi Chin. J. Oncol. 2004, 26, 585–589. [Google Scholar]
- Iciek, M.; Kwiecien, I.; Wlodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagenesis 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.; Nicco, C.; Batteux, F.; Slusarenko, A.J. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]


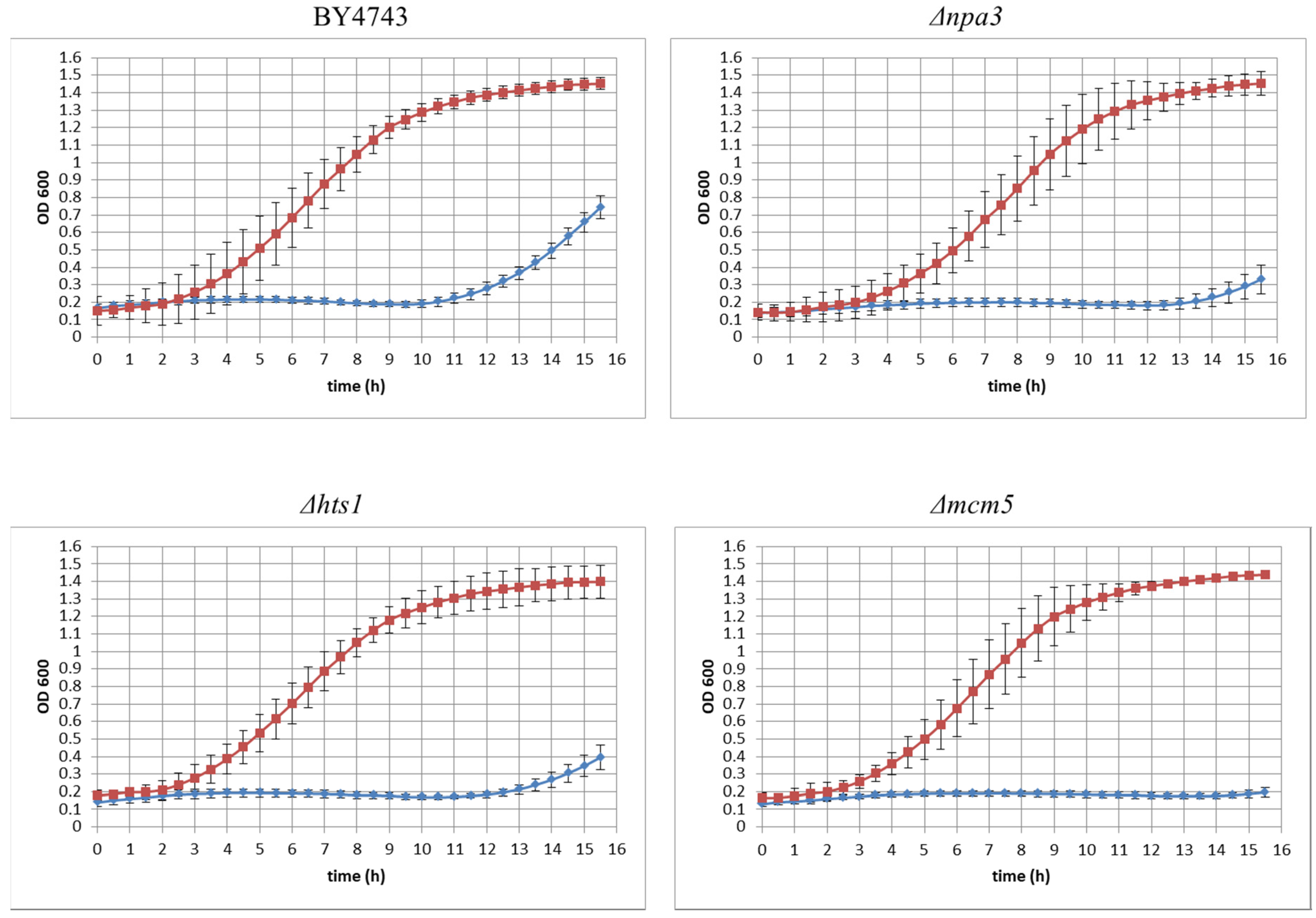
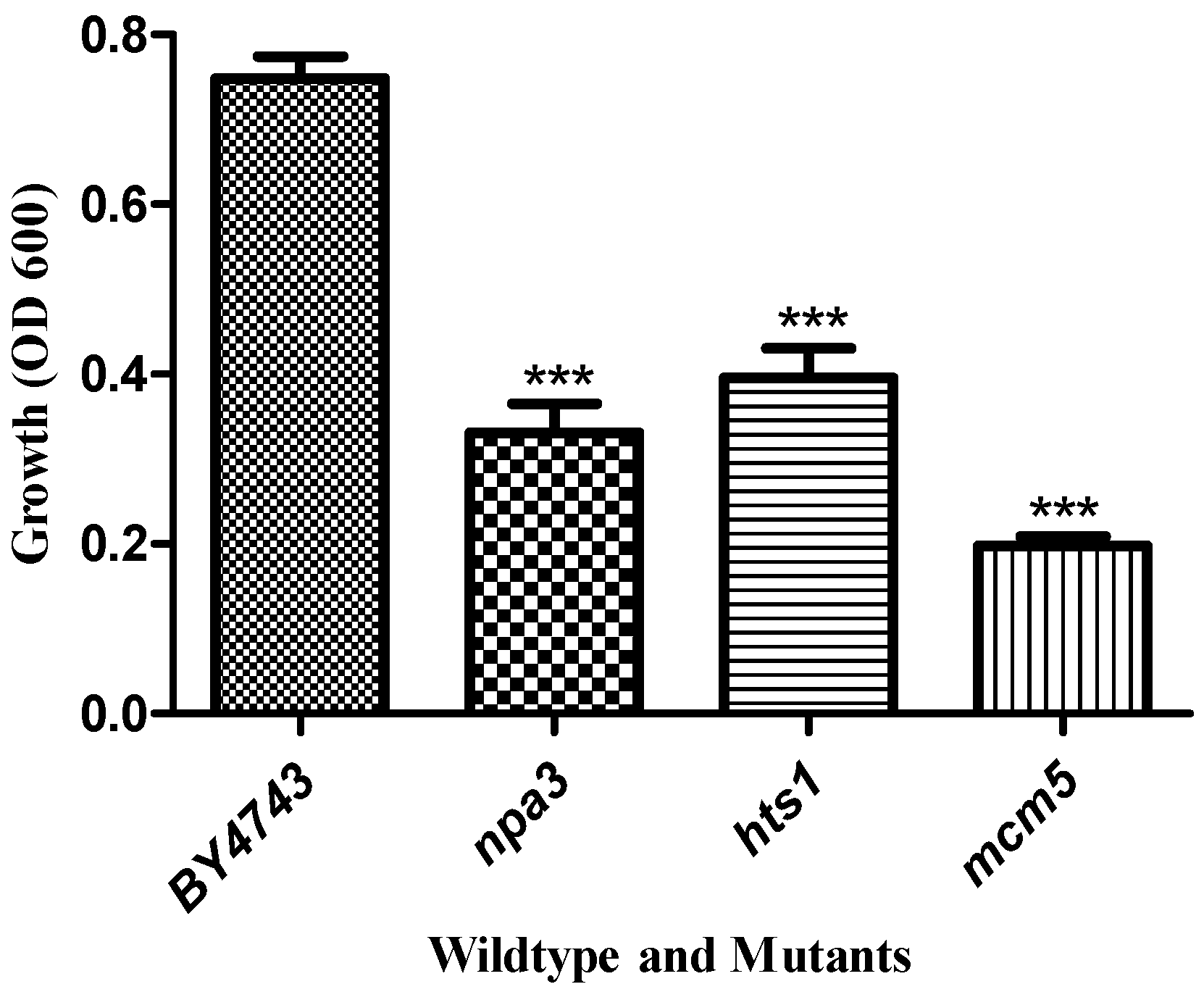
| ORF | Gene | Biological/Molecular Function |
|---|---|---|
| YLR274W | MCM5 | double-strand break repair via break-induced replication |
| YJR072C | NPA3 | mitotic sister chromatid cohesion |
| YPR033C | HTS1 | mitochondrial translation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, M.; Nasim, M.J.; Jacob, C.; Gruhlke, M.C.H. Efficacy of Allicin against Plant Pathogenic Fungi and Unveiling the Underlying Mode of Action Employing Yeast Based Chemogenetic Profiling Approach. Appl. Sci. 2020, 10, 2563. https://doi.org/10.3390/app10072563
Sarfraz M, Nasim MJ, Jacob C, Gruhlke MCH. Efficacy of Allicin against Plant Pathogenic Fungi and Unveiling the Underlying Mode of Action Employing Yeast Based Chemogenetic Profiling Approach. Applied Sciences. 2020; 10(7):2563. https://doi.org/10.3390/app10072563
Chicago/Turabian StyleSarfraz, Muhammad, Muhammad Jawad Nasim, Claus Jacob, and Martin C. H. Gruhlke. 2020. "Efficacy of Allicin against Plant Pathogenic Fungi and Unveiling the Underlying Mode of Action Employing Yeast Based Chemogenetic Profiling Approach" Applied Sciences 10, no. 7: 2563. https://doi.org/10.3390/app10072563
APA StyleSarfraz, M., Nasim, M. J., Jacob, C., & Gruhlke, M. C. H. (2020). Efficacy of Allicin against Plant Pathogenic Fungi and Unveiling the Underlying Mode of Action Employing Yeast Based Chemogenetic Profiling Approach. Applied Sciences, 10(7), 2563. https://doi.org/10.3390/app10072563








