Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure for Obtaining Foliar Fertilizer
2.3. Procedure for Obtaining Soil Fertilizer
2.4. Pot Experimental Design
2.5. Gas Exchange
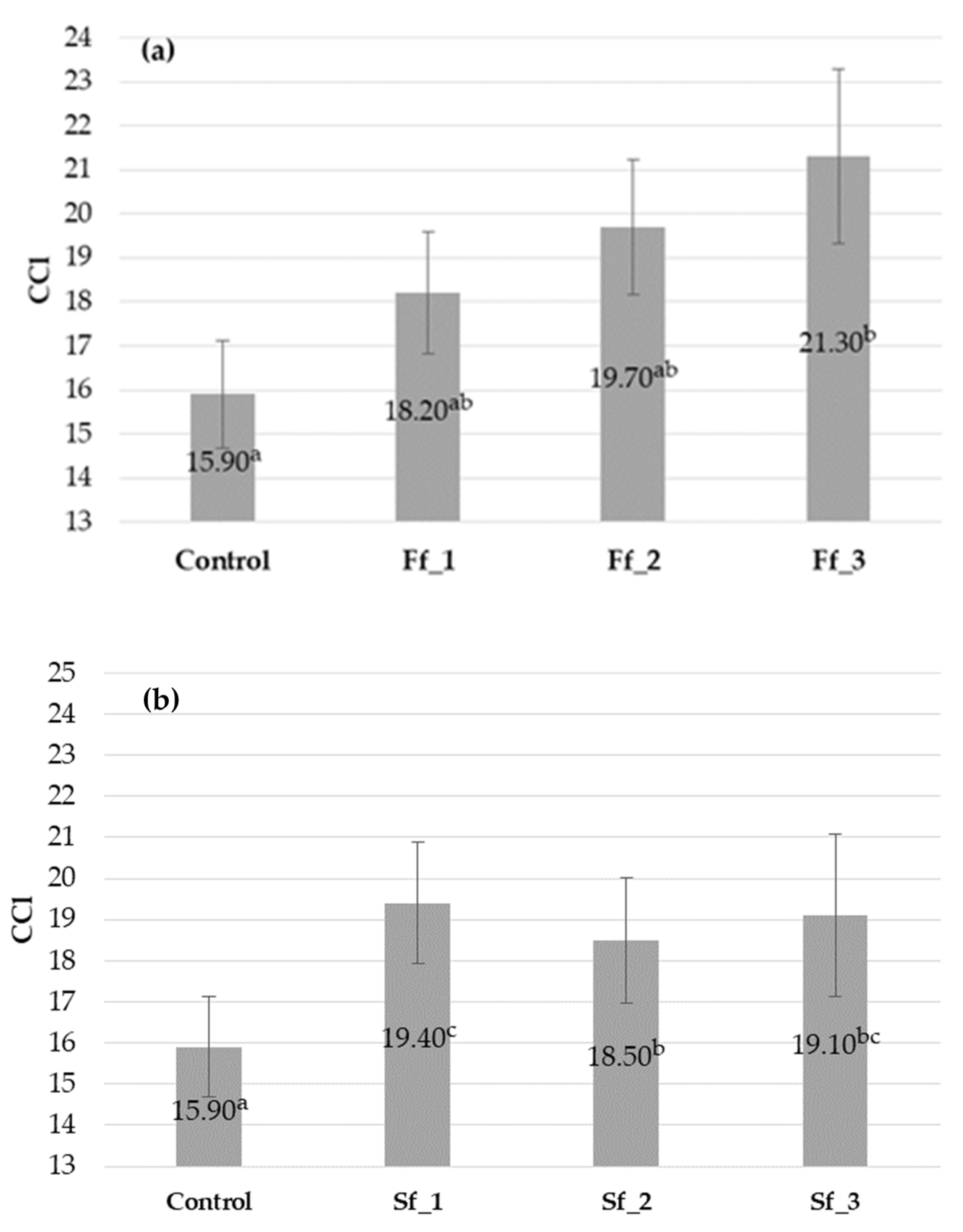
2.6. Relative Chlorophyll Content (CCl)
2.7. Chlorophyll Fluorescence
2.8. Statistical Analysis
3. Results and Discussion
3.1. Description of the Fertilizer’s Functionality
3.2. Pot Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ludwig, B.; Geisseler, D.; Michel, K.; Joergensen, R.G.; Schulz, E.; Merbach, I.; Raupp, R.; Rauber, R.; Hu, L.; Liu, X.; et al. Effects of fertilization and soil management on crop yields and carbon stabilization in soils. A review. Agron. Sust. Dev. 2011, 31, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V.; et al. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Yanai, J.; Linehan, D.J.; Robinson, D.; Young, I.M.; Hackett, C.A.; Kyuma, K.; Kosaki, T. Effects of inorganic nitrogen application on the dynamics of the soil solution composition in the root zone of maize. Plant Soil 1996, 180, 1–9. [Google Scholar] [CrossRef]
- Mollier, A.; Pellerin, S. Maize root system growth and development as influenced by phosphorus deficiency. J. Exp. Bot. 1999, 50, 487–497. [Google Scholar] [CrossRef]
- Grzebisz, W.; Szczepaniak, W. Systems of fertilization (in Polish). J. Elem. 2003, 8, 95–107. [Google Scholar]
- Wolkowski, R.P. Row-placed fertilizer for maize grown with an in-row crop residue management system in southern Wisconsin. Soil Tillage Res. 2000, 54, 55–62. [Google Scholar] [CrossRef]
- Kruczek, A. Phosphorus utilization from fertilizer and accumulation of mineral components in the initial stage of maize development. Pol. J. Environ. Stud. 2005, 14, 467–475. [Google Scholar]
- Kruczek, A.; Szulc, P. Effect of fertilization method on the uptake and accumulation of mineral components in the initial period of maize development. Int. Agrophysics 2006, 20, 11–22. [Google Scholar]
- Ling, F.; Silberbush, M. Response of Maize to foliar vs. soil application of nitrogen-phosphorus-potassium fertilizers. J. Plant Nutr. 2002, 25, 2333–2342. [Google Scholar] [CrossRef]
- Thavaprakaash, N.; Velayudham, K.; Panneerselvam, S. Foliar nutrition of baby corn (Zea mays L.). Arch. Agron. Soil Sci. 2006, 52, 419–425. [Google Scholar] [CrossRef]
- Kaur, G.; Nelson, K.A. Effect of foliar boron fertilization of fine textured soils on corn yields. Agronomy 2015, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Boote, K.J.; Gallaher, R.N.; Robertson, W.K.; Hinson, K.; Hammond, L.C. Effect of foliar fertilization on photosynthesis, leaf nutrition and yield of soybeans. Agron. J. 1978, 70, 787–791. [Google Scholar] [CrossRef]
- Gontarczyk, M. Photosynthesis and transpiration of C4 plants under drought condition. Plant Physiol. Biochein. 1996, 328–329. [Google Scholar]
- Górny, A.G. Photosynthetic activity of flag leaves in diallel crosses of spring har ley under varied nutrition and soil moisture. Cercal Res. Commun. 2001, 29, 159–166. [Google Scholar] [CrossRef]
- Michałek, S. Response of some soyhean cultivars to foliar fertilization with Insol W under herbicide slress condition. In Biostimulators in Modern Agriculture—Field Crops; Dąbrowski, Z., Ed.; Editorial House Wieś Jutra: Warszawa, Poland, 2008; pp. 92–99. [Google Scholar]
- Borkowski, E.; Michałek, S. The effect of placement and light conditions during foliar application of Insol U fertilizer on gas exchange, yield and quality of spinach (Spinacia oleracea L.). Folia Hortic. 2009, 21, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Balawejder, M.; Matłok, N.; Gorzelany, J.; Pieniążek, M.; Antos, P.; Witek, G.; Szostek, M. Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production. Sustainability 2019, 11, 5287. [Google Scholar] [CrossRef] [Green Version]
- Hycnar, E.; Ratajczak, T.; Jończyk, M. Kreda jeziorna z Bełchatowa jako sorbent SO2 w paleniskach fluidalnych. In Sorbenty Mineralne. Surowce, Energetyka, Ochrona Środowiska, Nowoczesne Technologie; AGH: Kraków, Poland, 2013; pp. 153–168. [Google Scholar]
- Nielsson, F.T.; Yates, L.D.; Roy, L.F.; Heil, F.G. Nitric Phosphates from Phosphate Rock, Nitric Acid, Ammonia, and Carbon Dioxide. J. Agric. Food Chem. 1953, 1, 1050–1054. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Gorzelany, J.; Kania, K.; Witek, G. Sposób Wytwarzania Rozpuszczalnego Nawozu. PL Patent PL424243A1, 10 January 2018. [Google Scholar]
- Balawejder, M.; Matłok, N.; Gorzelany, J.; Antos, D.; Piątkowski, W.; Bochenek, R.; Przywara, M.; Olbrycht, M.; Kołodziej, M.; Antos, P.; et al. Sposób Wytwarzania Nawozu Wieloskładnikowego o Kontrolowanym Uwalnianiu Składników. PL Patent 429318, 11 May 2019. [Google Scholar]
- Mulinns, G.L. Phosphorus, Agriculture & the Environment. Virginia Cooperative Extension; Virginia State University: Petersburg, Russia, 2009. [Google Scholar]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms. In Phosphorus in Action; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–198. [Google Scholar]
- Möhr, P.J.; Dickinson, E.B. Mineral nutrition in maize. Tech. Monogr. 1978, 26–32. [Google Scholar]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [Green Version]
- Michałek, S.; Chwil, S.; Pranagal, J.; Ligęza, S. Maize early growth under conditions of foliar nutrition with VIFLO fertilizers (in Polish). Zesz. Probl. Postępów Nauk Rol. 2009, 542, 333–339. [Google Scholar]
- Correia, C.M.; Moutinho Pereira, J.M.; Coutinho, J.F.; Björn, L.O.; Torres-Pereira, J.M.G. Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: A Mediterranean field study. Eur. J. Agron. 2005, 22, 337–347. [Google Scholar] [CrossRef]
- Huang, Z.A.; Jiang, D.A.; Yang, Y.; Sun, J.W.; Jin, S.H. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Foyer, C.; Spencer, C. The relationship between phosphate status and photosynthesis in leaves. Planta 1986, 167, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Tuffers, A.; Naidoo, G.; Von Willert, D.J. Low salinites adversely affect photosynthetic performance of the mangrove, Avicennia marina. Wetl. Ecol. Manag. 2001, 9, 225–232. [Google Scholar] [CrossRef]
- Kalaji, H.M. Oddziaływanie abiotycznych czynników stresowych na fluorescencję chlorofilu w roślinach wybranych odmian jęczmienia Hordeum vulgare L. Rozpr. Nauk. Monogr. 2011. [Google Scholar]
- Kalaji, H.M.; Guo, P. Chlorophyll fluorescence: A usefull tool in barley plant breeding programs. In Photochemistry Research Progress; Sánchez, A., Gutierrez, S.J., Eds.; Nova Science Publishers: New York, NY, USA, 2008; pp. 439–463. [Google Scholar]
- Kalaji, H.M.; Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W. Chlorophyll fluorescence measurements in environmental studies. Kosmos 2016, 65, 197–205. [Google Scholar]
- Furmańczuk, A. Response of the photosynthetic apparatus of string-bean to cobalt excess in the substrate (in Polish). Proc. Ecopole 2013, 7, 207–213. [Google Scholar]
- Angelini, G.; Ragni, P.; Esposito, D.; Giardi, M.T. A device to study the effect of space radiation on photosynthesis organizms. Phys. Med. 2001, 17 (Suppl. 1), 267–268. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. Screening the vitality and photo-synthetic activity of plants by fluorescent transient. Crop Improv. Food Secur. 1999, 79, 72–115. [Google Scholar]
- Smethurst, C.F.; Garnett, T.; Shabala, S. Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant Soil 2005, 270, 31–45. [Google Scholar] [CrossRef]
| Object | Intensity of Photosynthesis Net (PN) | Transpiration Rate (E) | Stomatal Conductance (gs) | Intercellular CO2 Concentration (Ci) |
|---|---|---|---|---|
| μmol(CO2)m−2s−1 | mmol(H2O)m−2s−1 | mmol m−2s−1 | mmol L−1 | |
| Control | 14.4 a ± 0.1 | 2.11 a ± 0.05 | 0.07 a ± 0.002 | 45 a ± 0.5 |
| Ff_1 | 17.5 b ± 0.1 | 2.36 a ± 0.02 | 0.10 b ± 0.001 | 65 b ± 0.5 |
| Ff_2 | 18.3 bc ± 0.1 | 2.51 a ± 0.23 | 0.11b c ± 0.002 | 67 b ± 0.5 |
| Ff_3 | 19.4 c ± 0.1 | 2.64 a ± 0.02 | 0.13 c ± 0.002 | 81 c ± 0.7 |
| Object | Intensity of Photosynthesis Net (PN) | Transpiration Rate (E) | Stomatal Conductance (gs) | Intercellular CO2 Concentration (Ci) |
|---|---|---|---|---|
| μmol(CO2)m−2s−1 | mmol(H2O)m−2s−1 | mmol m−2s−1 | mmol L−1 | |
| Control | 14.4 a ± 0.1 | 2.11 a ± 0.05 | 0.07 a ± 0.002 | 45 a ± 0.5 |
| Sf_1 | 17.9 c ± 0.2 | 2.61 c ± 0.01 | 0.12 c ± 0.003 | 71 c ± 0.5 |
| Sf_2 | 17.2 bc ± 0.1 | 2.49 bc ± 0.05 | 0.09 b ± 0.002 | 62 bc ± 0.5 |
| Sf_3 | 16.1 b ± 0.1 | 2.34 ab ± 0.03 | 0.08 b ± 0.001 | 58 b ± 0.7 |
| Object | Maximal Photochemical Efficiency of PSII (Fv/Fm) | Maximum Quantum Yield of Primary Photochemistry (Fv/F0) | Performance Index (PI) |
|---|---|---|---|
| Control | 0.753 a ± 0.001 | 3.06 a ± 0.1 | 2.72 a ± 0.1 |
| Ff_1 | 0.769 a ± 0.001 | 3.44 b ± 0.1 | 2.84 a ± 0.1 |
| Ff_2 | 0.777 a ± 0.001 | 3.59 b ± 0.1 | 3.15 b ± 0.2 |
| Ff_3 | 0.798 a ± 0.002 | 3.72 b ± 0.1 | 3.33 b ± 0.1 |
| Object | Maximal Photochemical Efficiency of PSII (Fv/Fm) | Maximum Quantum Yield of Primary Photochemistry (Fv/F0) | Performance Index (PI) |
|---|---|---|---|
| Control | 0.753 a ± 0.001 | 3.06 a ± 0.1 | 2.72 a ± 0.1 |
| Sf_1 | 0.787 b ± 0.002 | 3.56 b ± 0.3 | 3.39 a ± 0.1 |
| Sf_2 | 0.771 ab ± 0.004 | 3.41 b ± 0.1 | 3.14 b ± 0.2 |
| Sf_3 | 0.762 ab ± 0.001 | 3.19 a ± 0.1 | 2.99 b ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matlok, N.; Szostek, M.; Antos, P.; Gajdek, G.; Gorzelany, J.; Bobrecka-Jamro, D.; Balawejder, M. Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants. Appl. Sci. 2020, 10, 2579. https://doi.org/10.3390/app10072579
Matlok N, Szostek M, Antos P, Gajdek G, Gorzelany J, Bobrecka-Jamro D, Balawejder M. Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants. Applied Sciences. 2020; 10(7):2579. https://doi.org/10.3390/app10072579
Chicago/Turabian StyleMatlok, Natalia, Małgorzata Szostek, Piotr Antos, Grażyna Gajdek, Józef Gorzelany, Dorota Bobrecka-Jamro, and Maciej Balawejder. 2020. "Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants" Applied Sciences 10, no. 7: 2579. https://doi.org/10.3390/app10072579
APA StyleMatlok, N., Szostek, M., Antos, P., Gajdek, G., Gorzelany, J., Bobrecka-Jamro, D., & Balawejder, M. (2020). Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants. Applied Sciences, 10(7), 2579. https://doi.org/10.3390/app10072579







