Filtration Efficiency of Electret Air Filters Reinforced by Titanium Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
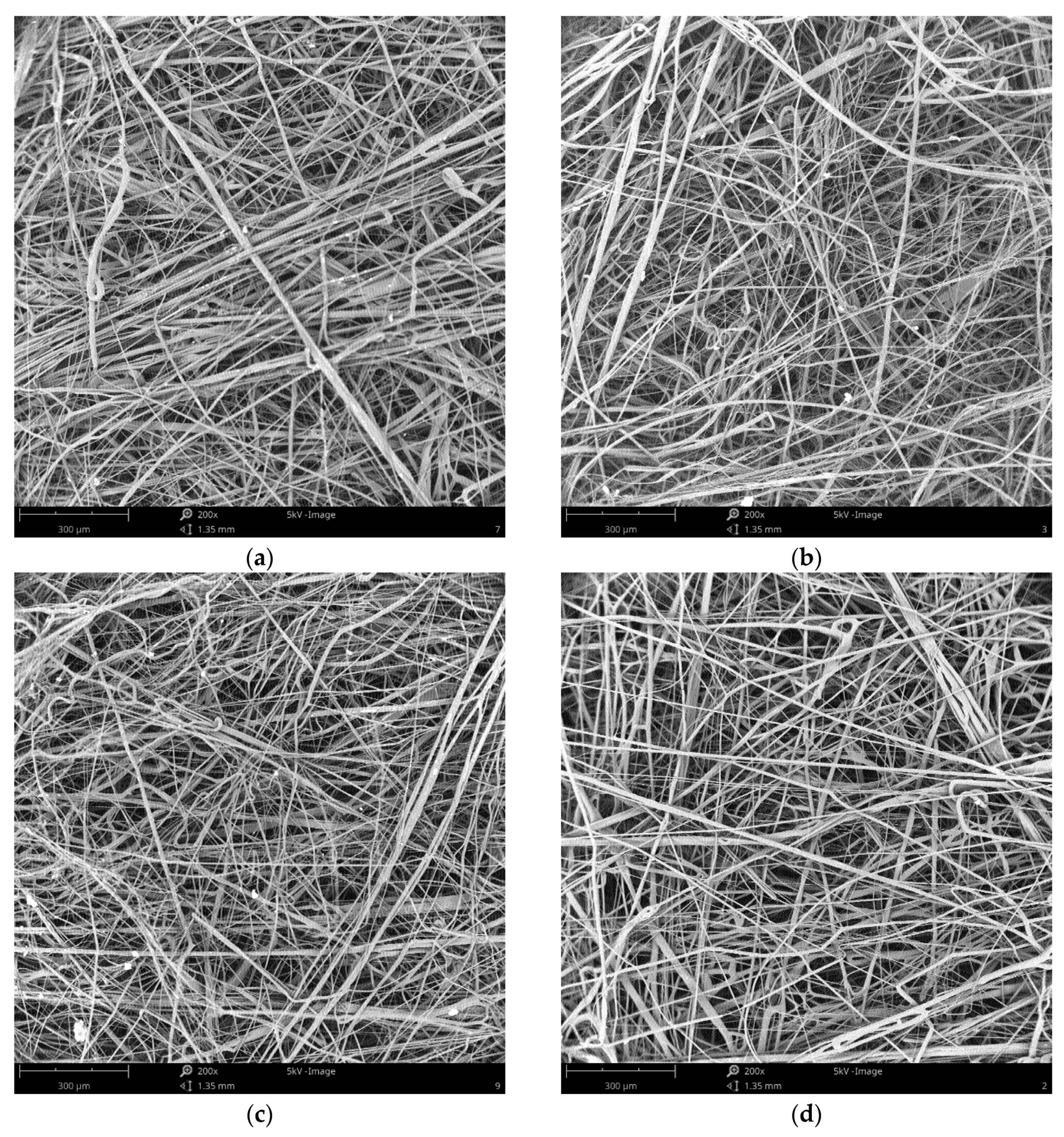
2.2. Preparation of Filtration
2.3. Testing
2.3.1. Surface Voltages
2.3.2. Surface Resistivity
2.3.3. Filtration Efficiency and Pressure Drop
2.3.4. Differential Scanning Calorimeters
3. Results and Discussion
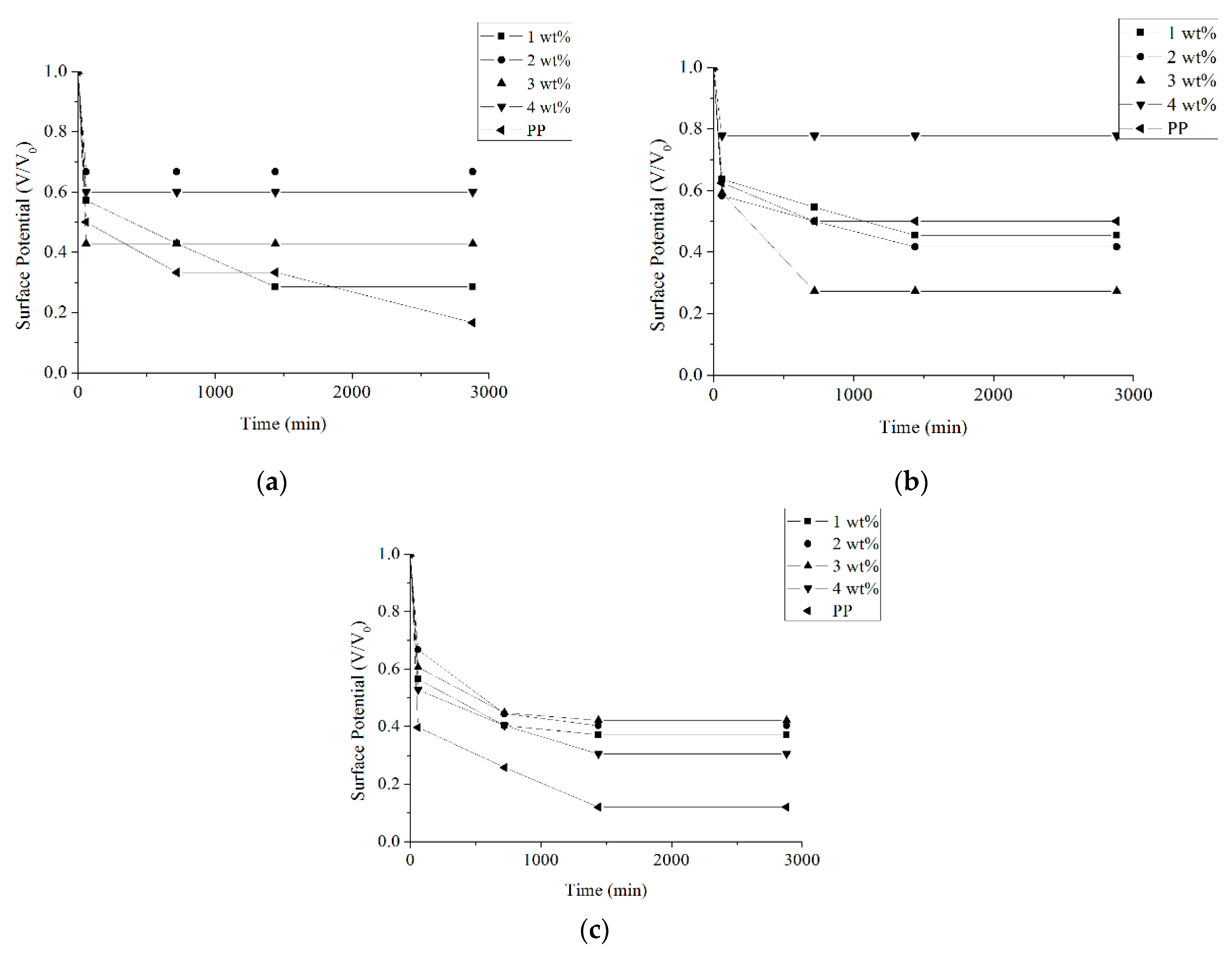
3.1. Surface Voltage
3.2. Surface Resistivity
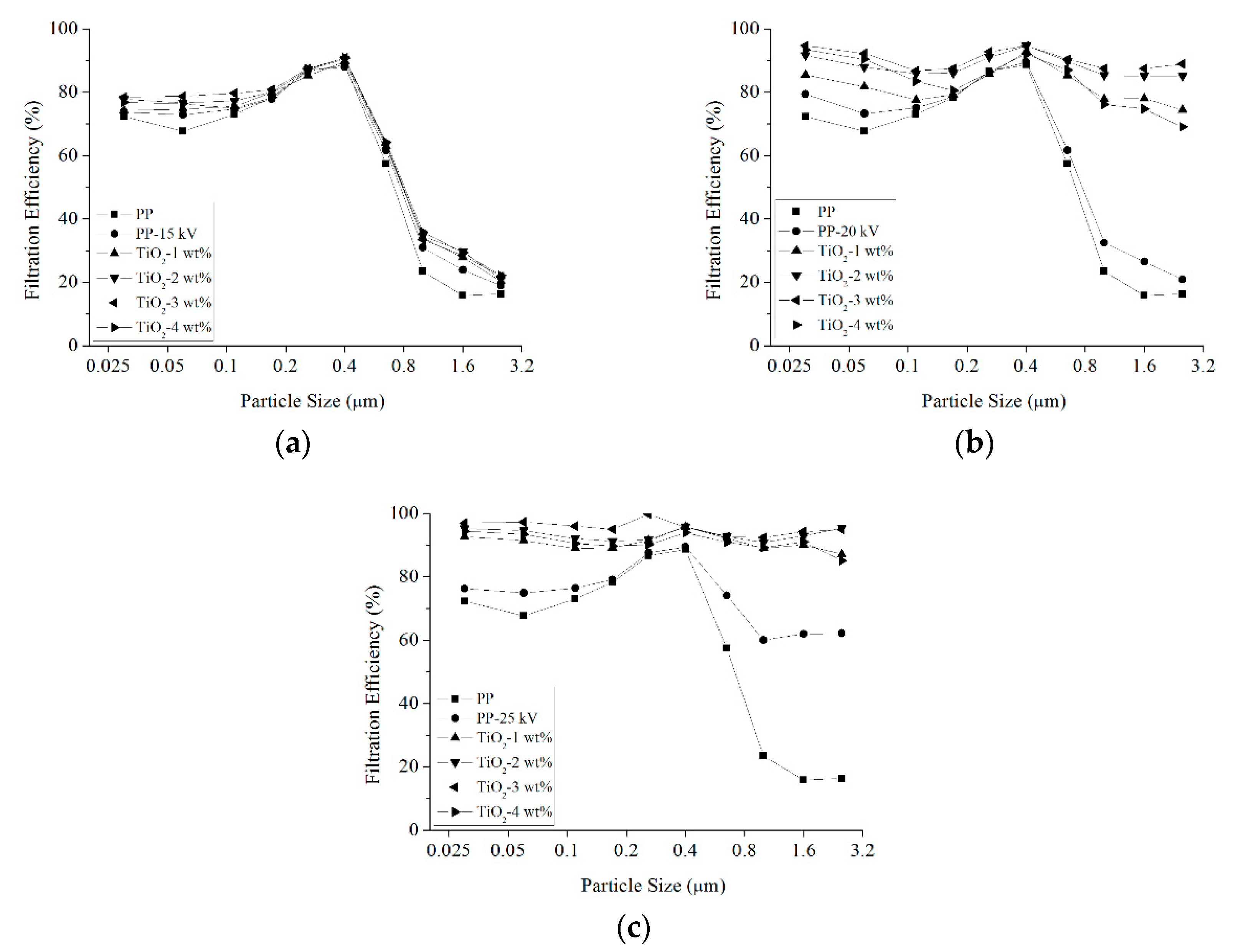
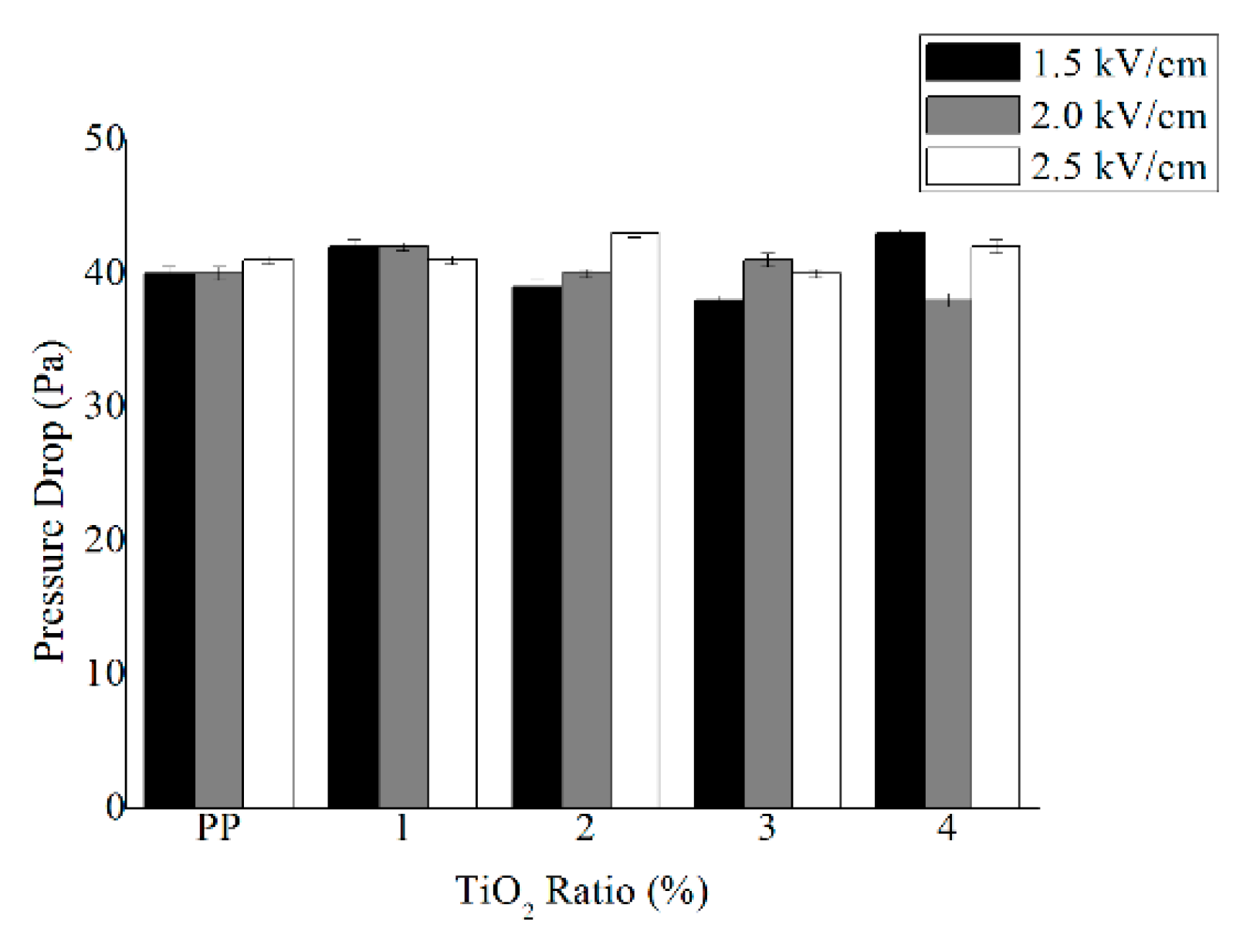
3.3. Filtration Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weber, S.A.; Insaf, T.Z.; Hall, E.S.; Talbot, T.O.; Huff, A.K. Assessing the impact of fine particulate matter (PM2.5) on respiratory cardiovascular chronic diseases in the New York City Metropolitan area using Hierarchical Bayesian Model estimates. Environ. Res. 2016, 151, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.F.; Chen, G.J.; Bhat, G.S.; Azari, H.; Pen, H.L. Electret characteristics of melt-blown polylactic acid fabrics for air filtration application. J. Appl. Polym. Sci. 2019, 137, 6. [Google Scholar] [CrossRef]
- Han, K.S.; Lee, S.; Kim, M.; Park, P.; Lee, M.H.; Nah, J. Electrically Activated Ultrathin PVDF-TrFE Air Filter for High-Efficiency PM1.0 Filtration. Adv. Funct. Mater. 2019, 29, 7. [Google Scholar] [CrossRef]
- Kim, M.W.; An, S.; Seok, H.; Yoon, S.S.; Yarin, A.L. Electrostatic Transparent Air Filter Membranes Composed of Metallized Microfibers for Particulate Removal. ACS Appl. Mater. Interfaces 2019, 11, 26323–26332. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Q.; Leung, W.W.F. Charged PVDF multi-layer filters with enhanced filtration performance for filtering nano-aerosols. Sep. Purif. Technol. 2019, 212, 854–876. [Google Scholar] [CrossRef]
- Chang, D.Q.; Liu, J.X.; Chen, S.C. Factors Affecting Particle Depositions on Electret Filters Used in Residential HVAC Systems and Indoor Air Cleaners. Aerosol Air Qual. Res. 2018, 18, 3211–3219. [Google Scholar] [CrossRef]
- Kilic, A.; Shim, E.; Yeom, B.Y.; Pourdeyhimi, B. Improving electret properties of PP filaments with barium titanate. J. Electrost. 2013, 71, 41–47. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Zhao, J.; Dong, C.L.; Shao, Y.Q.; Liu, Y.Y.; Li, J.Z.; Zhong, C.; Ye, L.; Song, R.; Zhang, H.; et al. Improved Electret Properties of Poly(Vinylidene Fluoride)/Lithium Niobate Nanocomposites for Applications in Air Filters. Macromol. Mater. Eng. 2019, 304, 10. [Google Scholar] [CrossRef]
- Gobi, N.; Vijayalakshmi, E.; Robert, B.; Srinivasan, N.R. Development of PAN nano fibrous filter hybridized by SiO2 nanoparticles electret for high efficiency air filtration. J. Polym. Mater. 2018, 35, 317–328. [Google Scholar] [CrossRef]
- Huang, Z.X.; Liu, X.X.; Zhang, X.; Wong, S.C.; Chase, G.G.; Qu, J.P.; Baji, A. Electrospun polyvinylidene fluoride containing nanoscale graphite platelets as electret membrane and its application in air filtration under extreme environment. Polymer 2017, 131, 143–150. [Google Scholar] [CrossRef]
- Kilic, A.; Shim, E.; Pourdeyhimi, B. Electrostatic Capture Efficiency Enhancement of Polypropylene Electret Filters with Barium Titanate. Aerosol Sci. Technol. 2015, 49, 666–673. [Google Scholar] [CrossRef]
- Wang, N.; Cai, M.; Yang, X.; Yang, Y.Y. Electret nanofibrous membrane with enhanced filtration performance and wearing comfortability for face mask. J. Colloid Interface Sci. 2018, 530, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Liu, J.X.; Zhang, X.; Huang, C.; Jin, X.Y. Design of electret polypropylene melt blown air filtration material containing nucleating agent for effective PM2.5 capture. RSC Adv. 2018, 8, 7932–7941. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.M.; Gui, J.Y.; Chen, G.J.; Xiao, C.P. Study on correlation of filtration performance and charge behavior and crystalline structure for melt-blown polypropylene electret fabrics. J. Appl. Polym. Sci. 2015, 132, 6. [Google Scholar] [CrossRef]
- Li, T.T.; Cen, X.X.; Ren, H.T.; Sun, F.; Lin, Q.; Lou, C.W.; Lin, J.H. One-Step Bark-Like Imitated Polypropylene (PP)/Polycarbonate (PC) Nanofibrous Meltblown Membrane for Efficient Particulate Matter Removal. Polymers 2019, 11, 18. [Google Scholar] [CrossRef]
- Yu, B.; Han, J.; Sun, H.; Zhu, F.C.; Zhang, Q.; Kong, J.J. The Preparation and Property of Poly(lacticacid)/Tourmaline Blends and Melt-Blown Nonwoven. Polym. Compos. 2015, 36, 264–271. [Google Scholar] [CrossRef]
- Kilic, A.; Shim, E.; Pourdeyhimi, B.; Yeom, B.Y. Aerosol Filtration Properties of Nucleating Agent Containing Electret Filters. Polym. Eng. Sci. 2014, 54, 1533–1539. [Google Scholar] [CrossRef]
- Dreno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.; Singh, N.; Sharma, Y.; Gupta, R.K. Improved supercapacitive performance in electrospun TiO2 nanofibers through Ta-doping for electrochemical capacitor applications. Catal. Today 2019, 325, 33–40. [Google Scholar] [CrossRef]
- Prateek; Bhunia, R.; Siddiqui, S.; Garg, A.; Gupta, R.K. Significantly Enhanced Energy Density by Tailoring the Interface in Hierarchically Structured TiO2-BaTiO3-TiO2 Nanofillers in PVDF-Based Thin-Film Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 14329–14339. [Google Scholar] [CrossRef]
- Viraneva, A.; Yovcheva, T.; Bodurov, I.; Galikhanov, M. Effect of TiO2 particle incorporation on the electret properties of corona charged polypropylene composite films. Bulg. Chem. Commun. 2013, 45, 73–76. [Google Scholar]
- Zha, J.W.; Dang, Z.M.; Song, H.T.; Yin, Y.; Chen, G. Dielectric properties and effect of electrical aging on space charge accumulation in polyimide/TiO2 nanocomposite films. J. Appl. Phys. 2010, 108, 6. [Google Scholar] [CrossRef]
- Chawengkijwanich, C.; Pokhum, C.; Srisitthiratkul, C.; Subjalearndee, N.; Pongsorrarith, V.; Yaipimai, W.; Phanomkate, N.; Intasanta, V. Fabrication of Water-Based TiO2-Coated Pleated Synthetic Fiber toward Photocatalytic Oxidation of VOCs and CO for Indoor Air Quality Improvement. J. Environ. Eng. ASCE 2019, 145, 9. [Google Scholar] [CrossRef]
- Wongwatcharapaiboon, J.; Gan, G.H.; Riffat, S.B. A new air PM2.5 filtrative lamp with a combination of fabric filter and TiO2 coating mop. Int. J. Low Carbon Technol. 2019, 14, 394–399. [Google Scholar] [CrossRef]
- Xu, C.W.; Xie, W.X.; Yu, Y.; Zhang, J.; Yang, J.G. Photocatalytic and Filtration performance study of TiO2/CNTs-Filter for oil particle. Process. Saf. Environ. Protect. 2019, 123, 72–78. [Google Scholar] [CrossRef]
- Ho, C.C.; Kang, F.; Chang, G.M.; You, S.J.; Wang, Y.F. Application of recycled lanthanum-doped TiO2 immobilized on commercial air filter for visible-light photocatalytic degradation of acetone and NO. Appl. Surf. Sci. 2019, 465, 31–40. [Google Scholar] [CrossRef]
- Li, Y.H.; Cheng, S.W.; Yuan, C.S.; Lai, T.F.; Hung, C.H. Removing volatile organic compounds in cooking fume by nano-sized TiO2 photocatalytic reaction combined with ozone oxidation technique. Chemosphere 2018, 208, 808–817. [Google Scholar] [CrossRef]
- Cao, G.X.; Zhang, X.Q.; Zhao, D.; Zhang, C.; Wang, B.; Zeng, C.C. Charge Storage and Transport in Oriented and Non-oriented Polytetrafluoroethylene Films. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1108–1115. [Google Scholar] [CrossRef]
- Xia, Z.F.; Gerhard-Multhaupt, R.; Kunstler, W.; Wedel, A.; Danz, R. High surface-charge stability of porous polytetrafluoroethylene electret films at room and elevated temperatures. J. Phys. D Appl. Phys. 1999, 32, L83–L85. [Google Scholar] [CrossRef]
- Wang, C.S.; Otani, Y. Removal of Nanoparticles from Gas Streams by Fibrous Filters: A Review. Ind. Eng. Chem. Res. 2013, 52, 5–17. [Google Scholar] [CrossRef]
- Zhang, H.; Zhen, Q.; Liu, Y.; Liu, R.T.; Zhang, Y.F. One-step melt blowing process for PP/PEG micro-nanofiber filters with branch networks. Results Phys. 2019, 12, 1421–1428. [Google Scholar] [CrossRef]




| TiO2 Ratio (wt%) | Electric Field Intensity (kV/cm) | Basis Weight (g/m2) | Thickness (mm) | Fiber Diameter (μm) | Air Permeability (cm3/s/cm2) |
|---|---|---|---|---|---|
| 1 | 1.5 | 68.3 ± 4.8 | 0.63 ± 0.04 | 5.73 ± 2.97 | 64.43 ± 3.52 |
| 2 | 76.5 ± 6.4 | 0.65 ± 0.09 | 6.18 ± 3.13 | 60.81 ± 4.59 | |
| 2.5 | 69.4 ± 3.4 | 0.64 ± 0.09 | 5.91 ± 3.67 | 63.64 ± 8.07 | |
| 2 | 1.5 | 75.1 ± 4.5 | 0.65 ± 0.05 | 6.47 ± 3.25 | 61.67 ± 4.09 |
| 2 | 73.3 ± 6.8 | 0.64 ± 0.04 | 6.33 ± 1.98 | 67.16 ± 5.24 | |
| 2.5 | 67.0 ± 8.9 | 0.62 ± 0.04 | 6.76 ± 3.91 | 66.87 ± 5.12 | |
| 3 | 1.5 | 63.5 ± 11.3 | 0.68 ± 0.07 | 6.02 ± 3.28 | 61.06 ± 4.23 |
| 2 | 70.0 ± 8.6 | 0.61 ± 0.06 | 5.97 ± 3.77 | 64.50 ± 4.84 | |
| 2.5 | 66.5 ± 8.2 | 0.65 ± 0.05 | 6.73 ± 4.12 | 67.31 ± 3.9 | |
| 4 | 1.5 | 73.5 ± 5.8 | 0.64 ± 0.02 | 5.39 ± 2.49 | 62.00 ± 4.17 |
| 2 | 71.6 ± 4.4 | 0.62 ± 0.04 | 6.74 ± 3.74 | 59.3 ± 5.72 | |
| 2.5 | 78.8 ± 7.1 | 0.68 ± 0.06 | 6.47 ± 3.81 | 68.65 ± 8.36 |
| TiO2 Ratio (wt%) | Tc (°C) | Tm (°C) | Crystallinity (%) |
|---|---|---|---|
| Pure PP | 121.0 | 146.6 | 6.9 |
| 1 | 120.0 | 159.6 | 15.3 |
| 2 | 122.2 | 149.9 | 15.1 |
| 3 | 118.3 | 147.7 | 22.3 |
| 4 | 118.2 | 144.5 | 16.2 |
| TiO2 Ratio (wt%) | Surface Resistivity (Ω/SQ) |
|---|---|
| Pure PP | 2.71 × 1011 ± 4.96 × 1010 |
| 1 | 1.79 × 1011 ± 7.12 × 109 |
| 2 | 1.63 × 1011 ± 5.62 × 109 |
| 3 | 1.43 × 1011 ± 1.50 × 1010 |
| 4 | 9.15 × 1010 ± 1.24 × 1010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, C.-W.; Shih, Y.-H.; Huang, C.-H.; Lee, S.-A.; Chen, Y.-S.; Lin, J.-H. Filtration Efficiency of Electret Air Filters Reinforced by Titanium Dioxide. Appl. Sci. 2020, 10, 2686. https://doi.org/10.3390/app10082686
Lou C-W, Shih Y-H, Huang C-H, Lee S-A, Chen Y-S, Lin J-H. Filtration Efficiency of Electret Air Filters Reinforced by Titanium Dioxide. Applied Sciences. 2020; 10(8):2686. https://doi.org/10.3390/app10082686
Chicago/Turabian StyleLou, Ching-Wen, Ying-Huei Shih, Chen-Hung Huang, Shu-An Lee, Yueh-Sheng Chen, and Jia-Horng Lin. 2020. "Filtration Efficiency of Electret Air Filters Reinforced by Titanium Dioxide" Applied Sciences 10, no. 8: 2686. https://doi.org/10.3390/app10082686
APA StyleLou, C.-W., Shih, Y.-H., Huang, C.-H., Lee, S.-A., Chen, Y.-S., & Lin, J.-H. (2020). Filtration Efficiency of Electret Air Filters Reinforced by Titanium Dioxide. Applied Sciences, 10(8), 2686. https://doi.org/10.3390/app10082686







