Influence of Occlusal Thickness and Radicular Extension on the Fracture Resistance of Premolar Endocrowns from Different All-Ceramic Materials
Abstract
1. Introduction
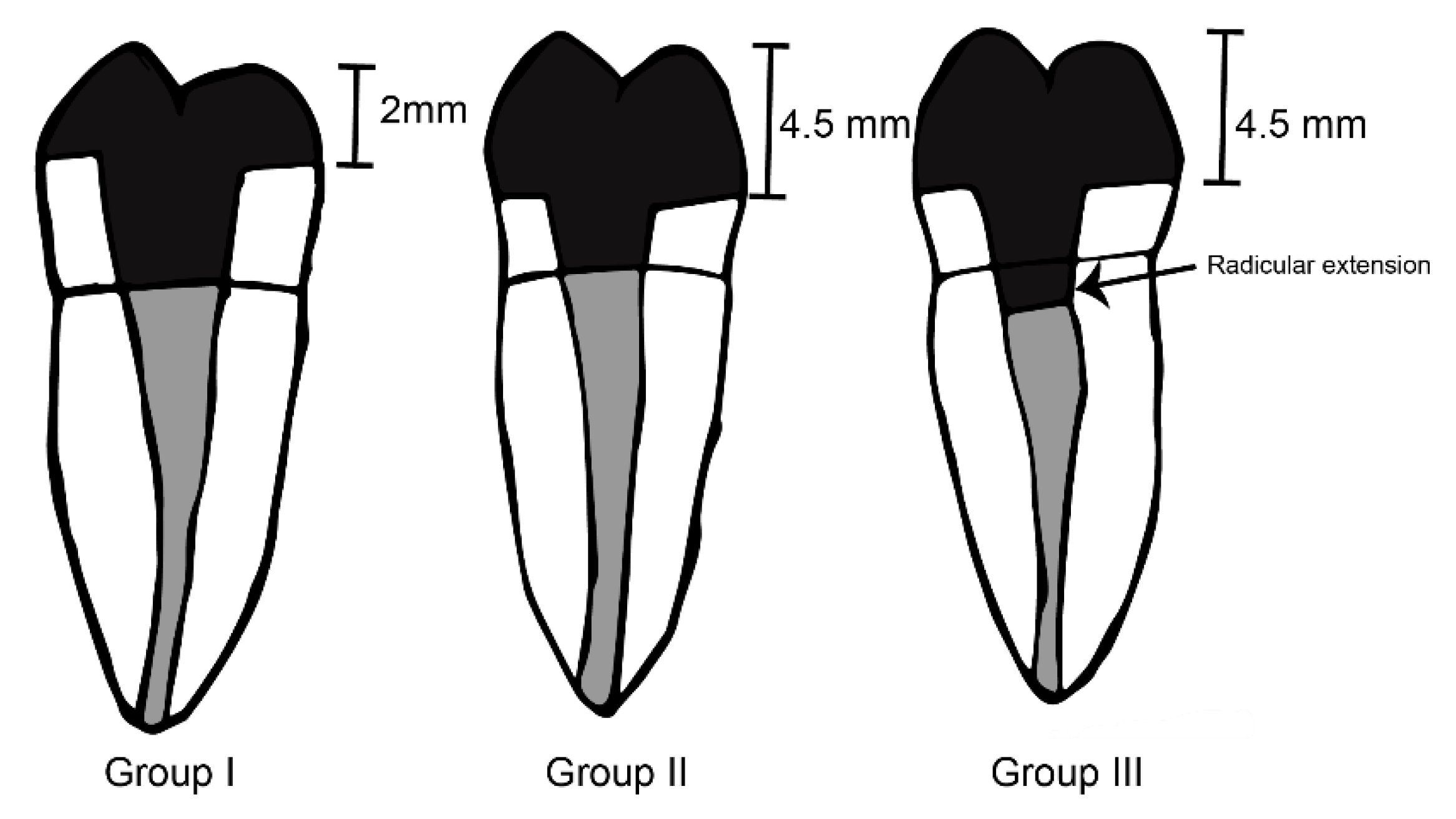
2. Materials and Methods
2.1. Endodontic Treatment
2.2. Endocrowns Preparation
2.3. Fabrication of Endocrowns and Cementation
3. Results
4. Discussion
5. Conclusions
- (1)
- LD ceramic performed better as material for endocrowns fabrication at a standard thickness of 2.5 mm as well as at increased occlusal thickness at 4.5 mm with 62.55 MPa and 45.80 respectively.
- (2)
- Fracture resistance of endocrowns fabricated from PIC and HTZ was at a similar range and lower than LD ceramic.
- (3)
- The greater extension of endocrowns inside the pulp chamber and radicular extension provided better mechanical performance in LD ceramic and HTZ ceramics.
- (4)
- LD ceramic Endocrowns showed substantially higher fracture resistance than the average masticatory force. Hence, LD ceramic endocrowns can be clinically utilized for the fabrication of endocrowns in the premolar region.
Author Contributions
Funding
Conflicts of Interest
References
- Loftus, J.J.; Keating, A.P.; McCartan, B.E. Periapical status and quality of endodontic treatment in an adult Irish population. Int. Endod. J. 2005, 38, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sagsen, B.; Aslan, B. Effect of bonded restorations on the fracture resistance of root filled teeth. Int. Endod. J. 2006, 39, 900–904. [Google Scholar] [CrossRef]
- Genovese, K.; Lamberti, L.; Pappalettere, C. Finite element analysis of a new customized composite post system for endodontically treated teeth. J. Biomech. 2005, 38, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Pissis, P. Fabrication of a metal-free ceramic restoration utilizing the monobloc technique. Pract. Periodontics Aesthetic Dent. 1995, 7, 83–94. [Google Scholar]
- Bindl, A.; Mormann, W.H. Clinical evaluation of adhesively placed Cerec endo-crowns after 2 years--preliminary results. J. Adhes. Dent. 1999, 1, 255–265. [Google Scholar] [PubMed]
- Lander, E.; Dietschi, D. Endocrowns: A clinical report. Quintessence Int. 2008, 39, 99–106. [Google Scholar]
- Mormann, W.H.; Bindl, A.; Luthy, H.; Rathke, A. Effects of preparation and luting system on all-ceramic computer-generated crowns. Int. J. Prosthodont. 1998, 11, 333–339. [Google Scholar]
- Pippin, D.J.; Mixson, J.M.; Soldan-Els, A.P. Clinical evaluation of restored maxillary incisors: Veneers vs. PFM crowns. J. Am. Dent. Assoc. 1995, 126, 1523–1529. [Google Scholar] [CrossRef]
- Bindl, A.; Richter, B.; Mormann, W.H. Survival of ceramic computer-aided design/manufacturing crowns bonded to preparations with reduced macroretention geometry. Int J. Prosthodont. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Fages, M.; Bennasar, B. The endocrown: A different type of all-ceramic reconstruction for molars. J. Can. Dent. Assoc. 2013, 79, d140. [Google Scholar]
- Belleflamme, M.M.; Geerts, S.O.; Louwette, M.M.; Grenade, C.F.; Vanheusden, A.J.; Mainjot, A.K. No post-no core approach to restore severely damaged posterior teeth: An up to 10-year retrospective study of documented endocrown cases. J. Dent. 2017, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, M.; DuVall, N.; Wajdowicz, M.; Brewster, J.; Roberts, H. Preparation Ferrule Design Effect on Endocrown Failure Resistance. J. Prosthodont. 2019, 28, e237–e242. [Google Scholar] [CrossRef] [PubMed]
- Sedrez-Porto, J.A.; Rosa, W.L.; da Silva, A.F.; Munchow, E.A.; Pereira-Cenci, T. Endocrown restorations: A systematic review and meta-analysis. J. Dent. 2016, 52, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Mormann, W.H. Clinical performance of chairside CAD/CAM feldspathic ceramic posterior shoulder crowns and endocrowns up to 12 years. Int. J. Comput. Dent. 2015, 18, 147–161. [Google Scholar]
- Qin, F.; Zheng, S.; Luo, Z.; Li, Y.; Guo, L.; Zhao, Y.; Fu, Q. Evaluation of machinability and flexural strength of a novel dental machinable glass-ceramic. J. Dent. 2009, 37, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Gohring, T.N.; Peters, O.A. Restoration of endodontically treated teeth without posts. Am. J. Dent. 2003, 16, 313–317. [Google Scholar]
- Swain, M.; Coldea, A.; Bilkhair, A.; Guess, P. Interpenetrating network ceramic-resin composite dental restorative materials. Dent. Mater. 2016, 32, 34–42. [Google Scholar] [CrossRef]
- El Zhawi, H.; Kaizer, M.R.; Chughtai, A.; Moraes, R.R.; Zhang, Y. Polymer infiltrated ceramic network structures for resistance to fatigue fracture and wear. Dent. Mater. 2016, 32, 1352–1361. [Google Scholar] [CrossRef]
- Bernhart, J.; Bräuning, A.; Altenburger, M.; Wrbas, K. Cerec3D endocrowns--two-year clinical examination of CAD/CAM crowns for restoring endodontically treated molars. Int. J. Comput. Dent. 2010, 13, 141–154. [Google Scholar]
- Zarone, F.; Sorrentino, R.; Apicella, D.; Valentino, B.; Ferrari, M.; Aversa, R.; Apicella, A. Evaluation of the biomechanical behavior of maxillary central incisors restored by means of endocrowns compared to a natural tooth: A 3D static linear finite elements analysis. Dent. Mater. 2006, 22, 1035–1044. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Petsche, P.E.; Anusavice, K.J.; Yang, M.C. Influence of glass-ceramic thickness on Hertzian and bulk fracture mechanisms. Int. J. Prosthodont. 1998, 11, 27–32. [Google Scholar] [PubMed]
- Sorensen, J.A.; Martinoff, J.T. Clinically significant factors in dowel design. J. Prosthet. Dent. 1984, 52, 28–35. [Google Scholar] [CrossRef]
- Biacchi, G.R.; Basting, R.T. Comparison of fracture strength of endocrowns and glass fiber post-retained conventional crowns. Oper. Dent. 2012, 37, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Li, X.; Sun, C.; Gao, E.; Li, H. A comparison of the fracture resistances of endodontically treated mandibular premolars restored with endocrowns and glass fiber post-core retained conventional crowns. J. Adv. Prosthodont. 2016, 8, 489–493. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Pedrollo Lise, D.; Van Ende, A.; De Munck, J.; Umeda Suzuki, T.Y.; Cardoso Vieira, L.C.; Van Meerbeek, B. Biomechanical behavior of endodontically treated premolars using different preparation designs and CAD/CAM materials. J. Dent. 2017, 59, 54–61. [Google Scholar] [CrossRef]
- Dietschi, D.; Duc, O.; Krejci, I.; Sadan, A. Biomechanical considerations for the restoration of endodontically treated teeth: A systematic review of the literature, Part II (Evaluation of fatigue behavior, interfaces, and in vivo studies). Quintessence Int. 2008, 39, 117–129. [Google Scholar]
- Chang, C.Y.; Kuo, J.S.; Lin, Y.S.; Chang, Y.H. Fracture resistance and failure modes of CEREC endo-crowns and conventional post and core-supported CEREC crowns. J. Dent. Sci. 2009, 4, 110–117. [Google Scholar] [CrossRef]
- Forberger, N.; Gohring, T.N. Influence of the type of post and core on in vitro marginal continuity, fracture resistance, and fracture mode of lithia disilicate-based all-ceramic crowns. J. Prosthet. Dent. 2008, 100, 264–273. [Google Scholar] [CrossRef]
- Heintze, S.D. Clinical relevance of tests on bond strength, microleakage and marginal adaptation. Dent. Mater. 2013, 29, 59–84. [Google Scholar] [CrossRef]
- Gresnigt, M.M.; Ozcan, M.; van den Houten, M.L.; Schipper, L.; Cune, M.S. Fracture strength, failure type and Weibull characteristics of lithium disilicate and multiphase resin composite endocrowns under axial and lateral forces. Dent. Mater. 2016, 32, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Widmalm, S.E.; Ericsson, S.G. Maximal bite force with centric and eccentric load. J. Oral Rehabil. 1982, 9, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Bantleon, H.P.; Hnat, W.P.; Freudenthaler, J.W.; Marcotte, M.R.; Johnson, B.E. A study of bite force, part 1: Relationship to various physical characteristics. Angle Orthod. 1995, 65, 367–372. [Google Scholar] [PubMed]
- Lin, C.L.; Chang, Y.H.; Chang, C.Y.; Pai, C.A.; Huang, S.F. Finite element and Weibull analyses to estimate failure risks in the ceramic endocrown and classical crown for endodontically treated maxillary premolar. Eur. J. Oral Sci. 2010, 118, 87–93. [Google Scholar] [CrossRef]
- Sakaguchi, L.R.; Powers, J.M. Craig’s Restorative Dental Materials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 256–264. [Google Scholar]
- Denry, I.; Holloway, J.A. Ceramics for Dental Applications: A Review. Materials 2010, 3, 351–368. [Google Scholar] [CrossRef]
- Hallmann, L.; Ulmer, P.; Kern, M. Effect of microstructure on the mechanical properties of lithium disilicate glass-ceramics. J. Mech. Behav. Biomed. Mater. 2018, 82, 355–370. [Google Scholar] [CrossRef]
- Kang, S.H.; Chang, J.; Son, H.H. Flexural strength and microstructure of two lithium disilicate glass ceramics for CAD/CAM restoration in the dental clinic. Restor Dent. Endod. 2013, 38, 134–140. [Google Scholar] [CrossRef]
- Alex, G. CE 1-Preparing Porcelain Surfaces for Optimal Bonding. Compendium 2008, 29, 324. [Google Scholar]
- El-Damanhoury, H.M.; Haj-Ali, R.N.; Platt, J.A. Fracture resistance and microleakage of endocrowns utilizing three CAD-CAM blocks. Oper. Dent. 2015, 40, 201–210. [Google Scholar] [CrossRef]
- Li, R.; Ma, S.Q.; Zang, C.C.; Zhang, W.Y.; Liu, Z.H.; Sun, Y.C.; Feng, Y.Y. Enhanced bonding strength between lithium disilicate ceramics and resin cement by multiple surface treatments after thermal cycling. PLoS ONE 2019, 14, e0220466. [Google Scholar] [CrossRef]
- GÜNGÖR, M.B.; Bal, B.T.; Yilmaz, H.; Aydin, C.; Nemli, S.K. Fracture strength of CAD/CAM fabricated lithium disilicate and resin nano ceramic restorations used for endodontically treated teeth. Dent. Mater. J. 2017. [Google Scholar] [CrossRef]
- Rocca, G.T.; Daher, R.; Saratti, C.M.; Sedlacek, R.; Suchy, T.; Feilzer, A.; Krejci, I. Restoration of severely damaged endodontically treated premolars: The influence of the endo-core length on marginal integrity and fatigue resistance of lithium disilicate CAD-CAM ceramic endocrowns. J. Dent. 2018, 68, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Dartora, N.R.; de Conto Ferreira, M.B.; Moris, I.C.M.; Brazão, E.H.; Spazin, A.O.; Sousa-Neto, M.D.; Silva-Sousa, Y.T.; Gomes, E.A. Effect of intracoronal depth of teeth restored with endocrowns on fracture resistance: In vitro and 3-dimensional finite element analysis. J. Endod. 2018, 44, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Frentzen, M.; Utz, K.H.; Hoyer, D.; Langenbach, A.; Bourauel, C. Finite element analysis of adhesive endo-crowns of molars at different height levels of buccally applied load. J. Dent. Biomech. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Adel, S.; Abo-Madina, M.M.; Abo-El Farag, S.A. Fracture Strength of Hybrid Ceramic Endocrown Restoration with Different Preparation Depths and Designs. IOSR J. Dent. Med. Sci. 2019, 18, 17–23. [Google Scholar]
- Zhu, J.; Rong, Q.; Wang, X.; Gao, X. Influence of remaining tooth structure and restorative material type on stress distribution in endodontically treated maxillary premolars: A finite element analysis. J. Prosthet. Dent. 2017, 117, 646–655. [Google Scholar] [CrossRef]
- Argyrou, R.; Thompson, G.A.; Cho, S.H.; Berzins, D.W. Edge chipping resistance and flexural strength of polymer infiltrated ceramic network and resin nanoceramic restorative materials. J. Prosthet. Dent. 2016, 116, 397–403. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Giasimakopoulos, P.; Rigos, A.E. Strength and aging resistance of monolithic zirconia: An update to current knowledge. Jpn. Dent. Sci. Rev. 2020, 56, 1–23. [Google Scholar] [CrossRef]
- Elsayed, A.; Meyer, G.; Wille, S.; Kern, M. Influence of the yttrium content on the fracture strength of monolithic zirconia crowns after artificial aging. Quintessence Int. 2019, 50, 344–348. [Google Scholar]
- Tong, H.; Tanaka, C.B.; Kaizer, M.R.; Zhang, Y. Characterization of three commercial Y-TZP ceramics produced for their high-translucency, high-strength and high-surface area. Ceram. Int. 2016, 42, 1077–1085. [Google Scholar] [CrossRef]
- Heuer, A.; Claussen, N.; Kriven, W.M.; Ruhle, M. Stability of tetragonal ZrO2 particles in ceramic matrices. J. Am. Ceram. Soc. 1982, 65, 642–650. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, S.; Lai, R.; Liu, R.; Ma, S.; Zhou, Z.; Longquan, S. Load-bearing capacity and the recommended thickness of dental monolithic zirconia single crowns. J. Mech. Behav. Biomed. Mater. 2014, 35, 93–101. [Google Scholar] [CrossRef]
- Ozer, F.; Naden, A.; Turp, V.; Mante, F.; Sen, D.; Blatz, M.B. Effect of thickness and surface modifications on flexural strength of monolithic zirconia. J. Prosthet. Dent. 2018, 119, 987–993. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, J.J.W.; Srikanth, R.; Lawn, B.R. Edge chipping and flexural resistance of monolithic ceramics. Dent. Mater. 2013, 29, 1201–1208. [Google Scholar] [CrossRef]
- Yamanel, K.; Çaglar, A.; Gülsahi, K.; Özden, U.A. Effects of different ceramic and composite materials on stress distribution in inlay and onlay cavities: 3-D finite element analysis. Dent. Mater. J. 2009, 28, 661–670. [Google Scholar] [CrossRef]
- Aversa, R.; Apicella, D.; Perillo, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Apicella, A. Non-linear elastic three-dimensional finite element analysis on the effect of endocrown material rigidity on alveolar bone remodeling process. Dent. Mater. 2009, 25, 678–690. [Google Scholar] [CrossRef]


| Group | Occlusal Thickness (mm) | N | Mean | SD |
|---|---|---|---|---|
| Lithium disilicate | 2 mm | 10 | 62.55 | 5.14 |
| 4.5 mm | 10 | 45.80 | 5.91 | |
| Radicular extension | 10 | 74.27 | 3.67 | |
| Polymer infiltrated ceramic | 2 mm | 10 | 26.30 | 1.57 |
| 4.5 mm | 10 | 21.65 | 1.41 | |
| Radicular extension | 10 | 25.66 | 2.51 | |
| High Translucency Zirconia | 2 mm | 10 | 23.47 | 1.45 |
| 4.5 mm | 10 | 27.30 | 1.40 | |
| Radicular extension | 10 | 37.29 | 4.00 |
| Group | Source | df | SS | MS | F | p |
|---|---|---|---|---|---|---|
| LD | Between the Groups | 2 | 4094.571 | 2047.286 | 81.806 | 0.000 * |
| Within Groups | 27 | 675.705 | 25.026 | |||
| Total | 29 | 4770.276 | ||||
| PIC | Between the Groups | 2 | 127.083 | 63.541 | 30.289 | 0.000 * |
| Within Groups | 27 | 56.642 | 2.098 | |||
| Total | 29 | 183.724 | ||||
| HTZ | Between the Groups | 2 | 1017.262 | 508.631 | 75.824 | 0.000 * |
| Within Groups | 27 | 181.117 | 6.708 | |||
| Total | 29 | 1198.379 |
| Dependent Variable | (I) Group | (J) Group | ||
|---|---|---|---|---|
| 2 mm | 4.5 mm | Radicular Extension | ||
| LD | 2 mm | - | 0.000 * | 0.000 * |
| 4.5 mm | 0.000 | - | 0.000 | |
| Radicular extension | 0.000 * | 0.000 * | ||
| PIC | 2 mm | - | 0.000 * | 0.597 |
| 4.5 mm | 0.000 * | - | 0.000 * | |
| Radicular extension | 0.597 | 0.000 * | - | |
| HTZ | 2 mm | - | 0.007 * | 0.000 * |
| 4.5 mm | 0.007 * | - | 0.000 * | |
| Radicular extension | 0.000 * | 0.000 * | ||
| Group | Occlusal Thickness (mm) | Favorable | Unfavorable |
|---|---|---|---|
| Lithium disilicate | 2 mm | 7 | 3 |
| 4.5 mm | 2 | 8 | |
| Radicular extension | 0 | 10 | |
| Polymer infiltrated ceramic | 2 mm | 8 | 2 |
| 4.5 mm | 7 | 3 | |
| Radicular extension | 7 | 3 | |
| High Translucency Zirconia | 2 mm | 8 | 2 |
| 4.5 mm | 5 | 5 | |
| Radicular extension | 6 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haralur, S.B.; Alamri, A.A.; Alshehri, S.A.; Alzahrani, D.S.; Alfarsi, M. Influence of Occlusal Thickness and Radicular Extension on the Fracture Resistance of Premolar Endocrowns from Different All-Ceramic Materials. Appl. Sci. 2020, 10, 2696. https://doi.org/10.3390/app10082696
Haralur SB, Alamri AA, Alshehri SA, Alzahrani DS, Alfarsi M. Influence of Occlusal Thickness and Radicular Extension on the Fracture Resistance of Premolar Endocrowns from Different All-Ceramic Materials. Applied Sciences. 2020; 10(8):2696. https://doi.org/10.3390/app10082696
Chicago/Turabian StyleHaralur, Satheesh B., Alaa Ali Alamri, Shatha Abdulrahman Alshehri, Danyah Saeed Alzahrani, and Mohammed Alfarsi. 2020. "Influence of Occlusal Thickness and Radicular Extension on the Fracture Resistance of Premolar Endocrowns from Different All-Ceramic Materials" Applied Sciences 10, no. 8: 2696. https://doi.org/10.3390/app10082696
APA StyleHaralur, S. B., Alamri, A. A., Alshehri, S. A., Alzahrani, D. S., & Alfarsi, M. (2020). Influence of Occlusal Thickness and Radicular Extension on the Fracture Resistance of Premolar Endocrowns from Different All-Ceramic Materials. Applied Sciences, 10(8), 2696. https://doi.org/10.3390/app10082696





