Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
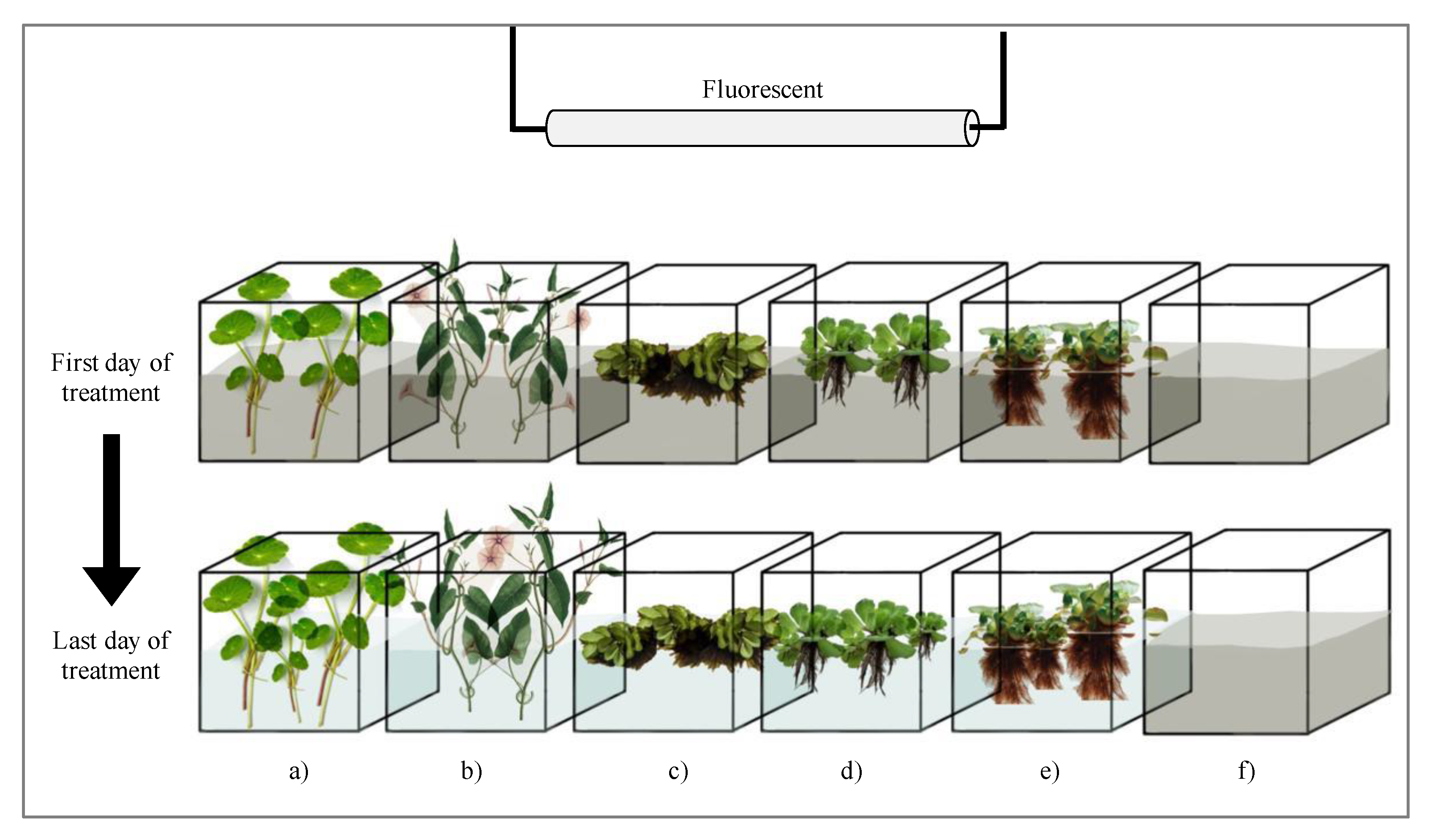
2.1. Aquatic Plants
2.2. Analysis of Wastewater Quality
- A = Weight of filter paper after filtration (weight of filter paper + dried residue), mg
- B = Weight of filter paper before filtration (weight of filter paper), mg
- V = Volume of filtered water sample, mL
2.3. Set-up of the Phytoremediation Laboratory Experiment
2.4. Statistical Analysis
3. Results and Discussion
3.1. Initial Parameters of Aquaculture Wastewater before Phytoremediation
3.2. Phytoremediation of Aquaculture Wastewater
3.3. Reduction Rate of TSS
3.4. Reduction Rate of NH3-N
3.5. Reduction Rate of Phosphate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naylor, R.L.; Williams, S.L.; Strong, D.R. Aquaculture—A gateway for exotic species. J. Sci. 2001, 294, 1655–1656. [Google Scholar]
- Nadarajah, S.; Flaaten, O. Global aquaculture growth and institutional quality. Mar. Policy 2017, 84, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Turcios, A.E.; Papenbrock, J. Sustainable treatment of aquaculture effluents—What can we learn from the past for the future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef] [Green Version]
- Ariffin, F.D.; Halim, A.A.; Hanafiah, M.M.; Awang, N.; Othman, M.S.; Azman, S.A.A.; Bakri, N.S.M. The effect of African catfish, Clarias gariepinus pond farms effluent on water quality of Kesang River in Malacca, Malaysia. Appl. Ecol. Envrion. Res. 2019, 17, 1531–1545. [Google Scholar] [CrossRef]
- Boyd, C.E. Guidelines for aquaculture effluent management at the farm-level. Aquaculture 2003, 226, 101–112. [Google Scholar] [CrossRef]
- Pillay, T.V.R. Aquaculture and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Mora-Ravelo, S.G.; Alarcon, A.; Rocandio-Rodriguez, M.; Vanoye-Eligio, V. Bioremediation of wastewater for reutilization in agricultural systems: A review. Appl. Ecol. Environ. Res. 2017, 15, 33–50. [Google Scholar] [CrossRef]
- Pillay, T.V.R. Waste production in aquaculture. Aquac. Fish. 2019, 4, 81–88. [Google Scholar]
- Hanafiah, M.M.; Mohamad, N.H.S.M.; Aziz, N.I.H.A. Salvinia Molesta Dan Pistia Stratiotes Sebagai Agen Fitoremediasi Dalam Rawatan Air Sisa Kumbahan. Sains Malays. 2018, 47, 1625–1634. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Hanafiah, M.M. Sustaining life on earth system through clean air, pure water, and fertile soil. Environ. Sci. Pollut. R. 2019, 26, 13679–13680. [Google Scholar] [CrossRef] [Green Version]
- Esmaili, Z.; Cheshmberah, F.; Solaimany Nazar, A.R.; Farhadian, M. Treatment of florfenicol of synthetic trout fish farm wastewater through nanofiltration and photocatalyst oxidation. Environ. Technol. 2017, 38, 2040–2047. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valipour, A.; Hamnabard, N.; Woo, K.S.; Ahn, Y.H. Performance of high-rate constructed phytoremediation process with attached growth for domestic wastewater treatment: Effect of high TDS and Cu. J. Environ. Manag. 2014, 145, 108. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Clemens, S.; Palmgren, M.G.; Krämer, U. A long way ahead: Understanding and engineering plant metal accumulation. Trends Plant Sci. 2002, 7, 309–315. [Google Scholar] [CrossRef]
- Hazmi, N.I.A.; Hanafiah, M.M. Phytoremediation of Livestock Wastewater Using Azolla filiculoides and Lemna minor. Environ. Ecosyst. Sci. 2018, 2, 13–16. [Google Scholar] [CrossRef]
- Standard Methods. 5210B: BOD: 5-Day Test—Standard Methods for the Examination of Water and Wastewater. Available online: http://standardmethods.org/ (accessed on 20 April 2018).
- Akinbile, C.O.; Yusoff, M.S. Assessing water hyacinth (Eichhornia crassopes) and lettuce (Pistia stratiotes) effectiveness in aquaculture wastewater treatment. Int. J. Phytoremediat. 2012, 14, 201–211. [Google Scholar] [CrossRef]
- Manan, F.A.; Chai, T.T.; Samad, A.A.; Mamat, D.D. Evaluation of the phytoremediation potential of two medicinal plants. Sains Malays. 2015, 44, 503–509. [Google Scholar] [CrossRef]
- Sundaralingam, T.; Gnanavelrajah, N. Phytoremediation potential of selected plants for Nitrate and Phosphorus from ground water. Int. J. Phytoremediat. 2014, 16, 275–284. [Google Scholar] [CrossRef]
- Ebel, M.; Evangelou, M.W.; Schaeffer, A. Cyanide phytoremediation by water hyacinths Eichhornia crassipes). Chemosphere 2007, 66, 816–823. [Google Scholar] [CrossRef]
- Sa’at, S.K.M.; Zaman, N.Q. Suitability of Ipomoea aquatica for the treatment of effluent from palm oil mill. J. Built Environ. Technol. Eng. 2017, 2, 39–44. [Google Scholar]
- Banch, T.J.; Hanafiah, M.M.; Alkarkhi, A.F.M.; Amr, S.S.A.; Nizam, N.U.M. Evaluation of different treatment processes for landfill leachate using low-cost agro-industrial materials. Processes 2020, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, D. Phytoremediation of heavy metals from industrial effluent using constructed wetland technology. Appl. Ecol. Environ. Res. 2013, 1, 92–97. [Google Scholar] [CrossRef]
- Akhtar, A.B.T.; Yasar, A.; Ali, R.; Irfan, R. Phytoremediation using aquatic macrophytes. In Phytoremediation; Ansari, A., Gill, S., Gill, R.R., Lanza, G., Newman, L., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–276. [Google Scholar]
- Hanafiah, M.M.; Yussof, M.K.M.; Hasan, M.; AbdulHasan, M.J.; Toriman, M.E. Water quality assessment of Tekala River, Selangor, Malaysia. Appl. Ecol. Environ. Res. 2018, 16, 5157–5174. [Google Scholar] [CrossRef]
- Umar, K.J.; Muhammad, M.J.; Sani, N.A.; Muhammad, S.; Umar, M.T. Comparative Study of Antioxidant Activities of the Leaves and Stem of Ipomoea aquatica Forsk (Water Spinach). Niger. J. Basic Appl. Sci. 2015, 23, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Fonkou, T.; Agendia, P.; Kengne, I.; Akoa, A.; Nya, J. Potentials of water lettuce (Pistia stratiotes) in domestic sewage treatment with macrophytic lagoon systems in Cameroon. Proc. Int. Symp. Environ. Pollut. Control Waste Manag. 2002, 709–714. [Google Scholar]
- Lu, Q.; He, Z.L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Uptake and distribution of metals by water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. R. 2011, 18, 978–986. [Google Scholar] [CrossRef]
- Le, P.T.T.; Boyd, C.E. Comparison of phenate and salicylate methods for determination of total ammonia nitrogen in freshwater and saline water. J. World Aquacult. Soc. 2012, 43, 885–889. [Google Scholar] [CrossRef]
- Bağdat, R.B.; Eid, E.M. Phytoremedation behaviour of some medicinal and aromatic plants to various pollutants. J. Field Crops Cent. Res. 2007, 16, 1–10. [Google Scholar]
- Henry-Silva, G.G.; Camargo, A.F.M. Efficiency of aquatic macrophytes to treat Nile tilapia pond effluents. Sci. Agric. 2006, 63, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Austin, D.F. Water spinach (Ipomoea aquatica, Convolvulaceae): A food gone wild. Ethnobot. Res. Appl. 2007, 5, 123–146. [Google Scholar] [CrossRef] [Green Version]
- Aziz, H.A.; Adlan, M.N.; Zahari, M.S.M.; Alias, S. Removal of ammoniacal nitrogen (NH3-N) from municipal solid waste leachate by using activated carbon and limestone. Waste Manag. Res. 2004, 22, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, F.D.; Halim, A.A.; Hanafiah, M.M.; Ramlee, N.A. Phytoremediation capability by Azolla pinnata in aquaculture wastewater treatment. Sains Malays. 2019, 48, 281–289. [Google Scholar] [CrossRef]
- Carlozzi, P.; Padovani, G. The aquatic fern Azolla as a natural plant-factory for ammonia removal from fish-breeding fresh wastewater. Environ. Sci. Pollut. R. 2016, 23, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Mylavarapu, R. Impact of Phosphorus on Water Quality. Available online: https://edis.ifas.ufl.edu/pdffiles/SS/SS49000.pdf (accessed on 20 December 2019).
- Yang, X.E.; Wu, X.; Hao, H.L.; He, Z.L. Mechanisms and assessment of water eutrophication. Zhejiang Da Xue Xue Bao Li Xue Ban 2008, 9, 197–209. [Google Scholar] [CrossRef]
- Victor, K.K.; Séka, Y.; Norbert, K.K.; Sanogo, T.A.; Celestin, A.B. Phytoremediation of wastewater toxicity using water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes). Int. J. Phytoremediat. 2016, 18, 949–955. [Google Scholar] [CrossRef]
- Ng, Y.S.; Chan, D.J.C. Phytoremediation capabilities of Spirodela polyrhiza, Salvinia molesta and Lemna sp. in synthetic wastewater: A comparative study. Int. J. Phytoremediat. 2018, 20, 1179–1186. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Basri, N.K. Removal of COD, ammoniacal nitrogen and colour from stabilized landfill leachate by anaerobic organism. Appl. Water Sci. 2013, 3, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.O. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Azarpira, H.; Behdarvand, P.; Dhumal, K.; Pondhe, G. Phytoremediation of municipal wastewater by using aquatic plants. Adv. Environ. Biol. 2013, 7, 4649–4654. [Google Scholar]
- Bhaduri, A.M.; Fulekar, M. Assessment of Arbuscular Mycorrhizal Fungi on the Phytoremediation Potential of Ipomoea aquatica on Cadmium Uptake. 3 Biotech 2012, 2, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Chanu, L.B.; Gupta, A. Phytoremediation of Lead Using Ipomoea aquatica Forsk. In Hydroponic Solution. Chemosphere 2016, 156, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, H.; Morad, N.; Fizri, F.F.A. Phytoaccumulation of copper from aqueous solutions using Eichhornia crassipes and Centella asiatica. Int. J. Environ. Sci. Dev. 2011, 2, 205. [Google Scholar] [CrossRef]
- Rane, N.R.; Patil, S.M.; Chandanshive, V.V.; Kadam, S.K.; Khandare, R.V.; Jadhav, J.P.; Govindwar, S.P. Ipomoea hederifolia rooted soil bed and Ipomoea aquatica rhizofiltration coupled phytoreactors for efficient rreatment of textile wastewater. Water Res. 2016, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Standard Method (APHA 2007) | Equipment |
|---|---|---|
| BOD5 | BOD5 (APHA 2007) | YSI multiparameter 5000 |
| TSS | Gravimetric (APHA 2007) | Vacuum pump |
| NH3-N | Nessler Method (Method 8038) | HACH DR 2800 Spectrophotometer |
| Phosphate | Ascorbic Acid Method (Method 8048) | HACH DR 2800 Spectrophotometer |
| Parameter | Unit | Average Value | Class/Standard |
|---|---|---|---|
| Temperature | °C | 27.77 ± 0.06 | Standard A |
| pH | - | 8.29 ± 0.02 | Standard A |
| DO | mg/L | 4.63 ± 0.04 | Standard A |
| Conductivity | μs/cm | 95.73 ± 0.15 | - |
| Turbidity | NTU | 205.00 ± 1.00 | - |
| TSS | mg/L | 45.67 ± 0.60 | Standard A |
| TDS | mg/L | 59.49 ± 0.45 | - |
| BOD5 | mg/L | 1.06 ± 0.03 | - |
| NH3-N | mg/L | 4.20 ± 0.10 | Standard A |
| Phosphate | mg/L | 0.35 ± 0.10 | - |
| Temperature | °C | 27.77 ± 0.06 | Standard A |
| pH | - | 8.29 ± 0.02 | Standard A |
| DO | mg/L | 4.63 ± 0.04 | Standard A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Nizam, N.U.; Mohd Hanafiah, M.; Mohd Noor, I.; Abd Karim, H.I. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Appl. Sci. 2020, 10, 2712. https://doi.org/10.3390/app10082712
Mohd Nizam NU, Mohd Hanafiah M, Mohd Noor I, Abd Karim HI. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Applied Sciences. 2020; 10(8):2712. https://doi.org/10.3390/app10082712
Chicago/Turabian StyleMohd Nizam, Nurul Umairah, Marlia Mohd Hanafiah, Izzati Mohd Noor, and Hazwani Izzati Abd Karim. 2020. "Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater" Applied Sciences 10, no. 8: 2712. https://doi.org/10.3390/app10082712
APA StyleMohd Nizam, N. U., Mohd Hanafiah, M., Mohd Noor, I., & Abd Karim, H. I. (2020). Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Applied Sciences, 10(8), 2712. https://doi.org/10.3390/app10082712





