Some Applications of a Field Programmable Gate Array Based Time-Domain Spectrometer for NMR Relaxation and NMR Cryoporometry
Abstract
:Featured Application
Abstract
1. Introduction
2. Apparatus
2.1. Temperature Measurement and Control
- Referenced to a cell of distilled water maintained half frozen (Omega IceCell Model TRCIII), with a third thermocouple attached to a thermal mass in a Dewar provided a short-term temperature reference to help reduce the effect of the temperature cycling in the IceCell.
- Electronic diode junction 0C references have in recent years improved greatly, and these are under evaluation. They have the advantage of no temperature cycling.
3. Key Time-Domain NMR Methods
3.1. Nuclear Magnetic Resonance Relaxation (NMRR) for Material Science
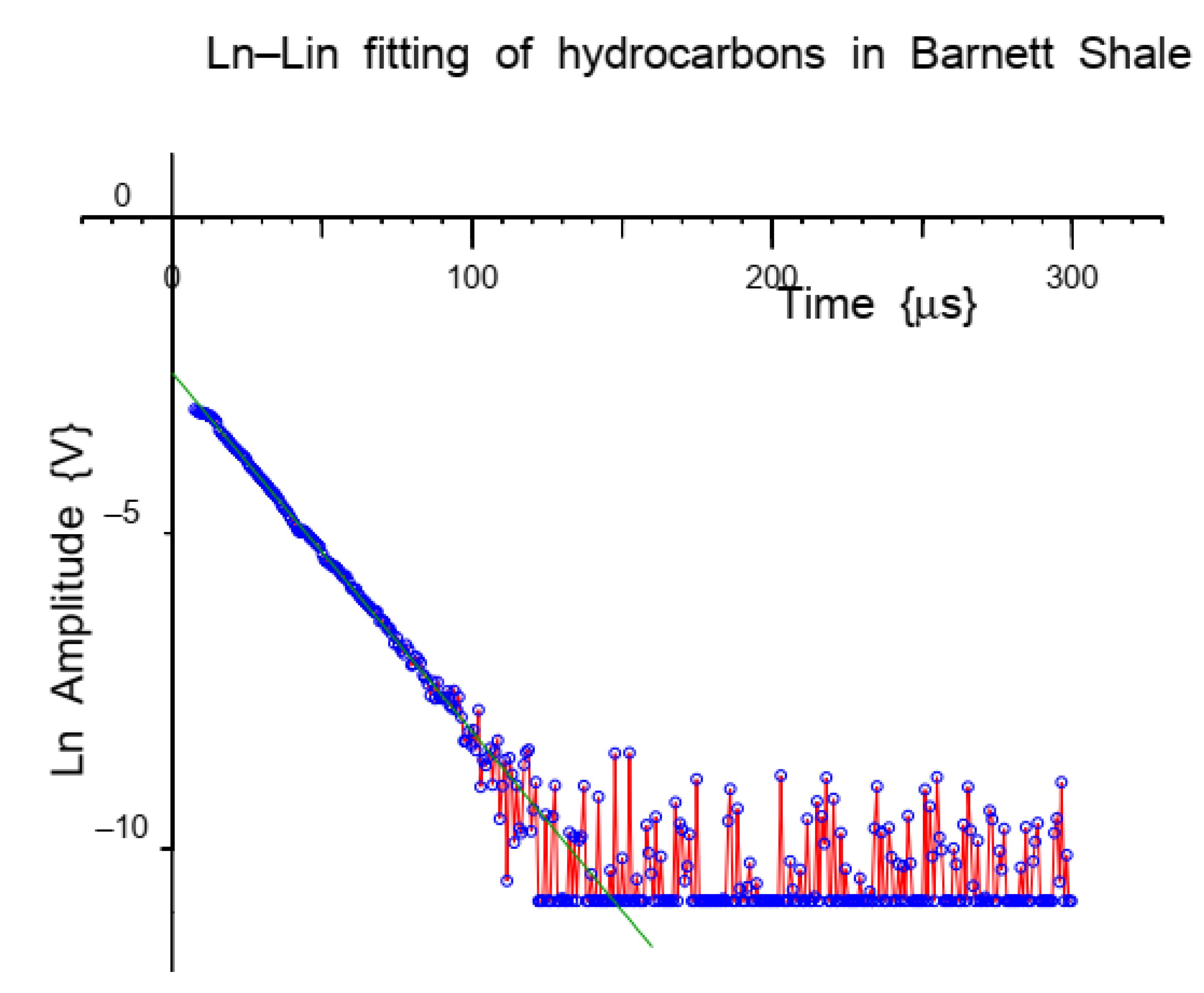
NMRR for Determining Mass and Mobility of Hydrocarbons in Porous Materials
3.2. NMR Cryoporometry
3.2.1. NMRC History
3.2.2. NMRC Theory
3.2.3. NMRC Protocol
4. NMR Relaxation Results Obtained Using the Spectrometer and Peltier Cooled Probe
4.1. Nuclear Magnetic Resonance Relaxation for Determining Mass and Mobility of Hydrocarbons in Porous Materials
4.2. Bulk Ice T Measurement
5. NMR Cryoporometric Results Obtained Using the Spectrometer and Peltier Cooled Probe
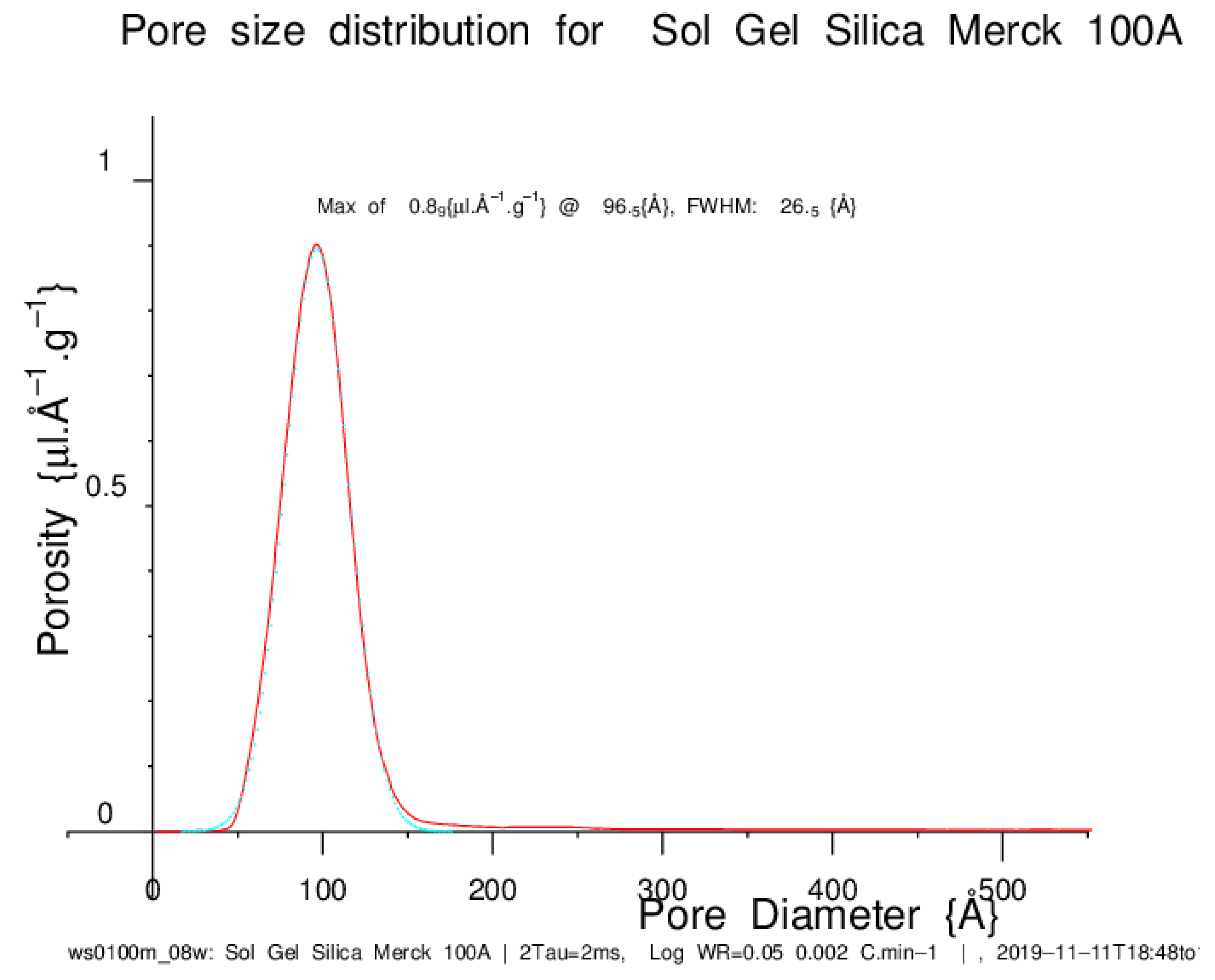
5.1. NMR Cryoporometric Measurement on a Sol-Gel Silica
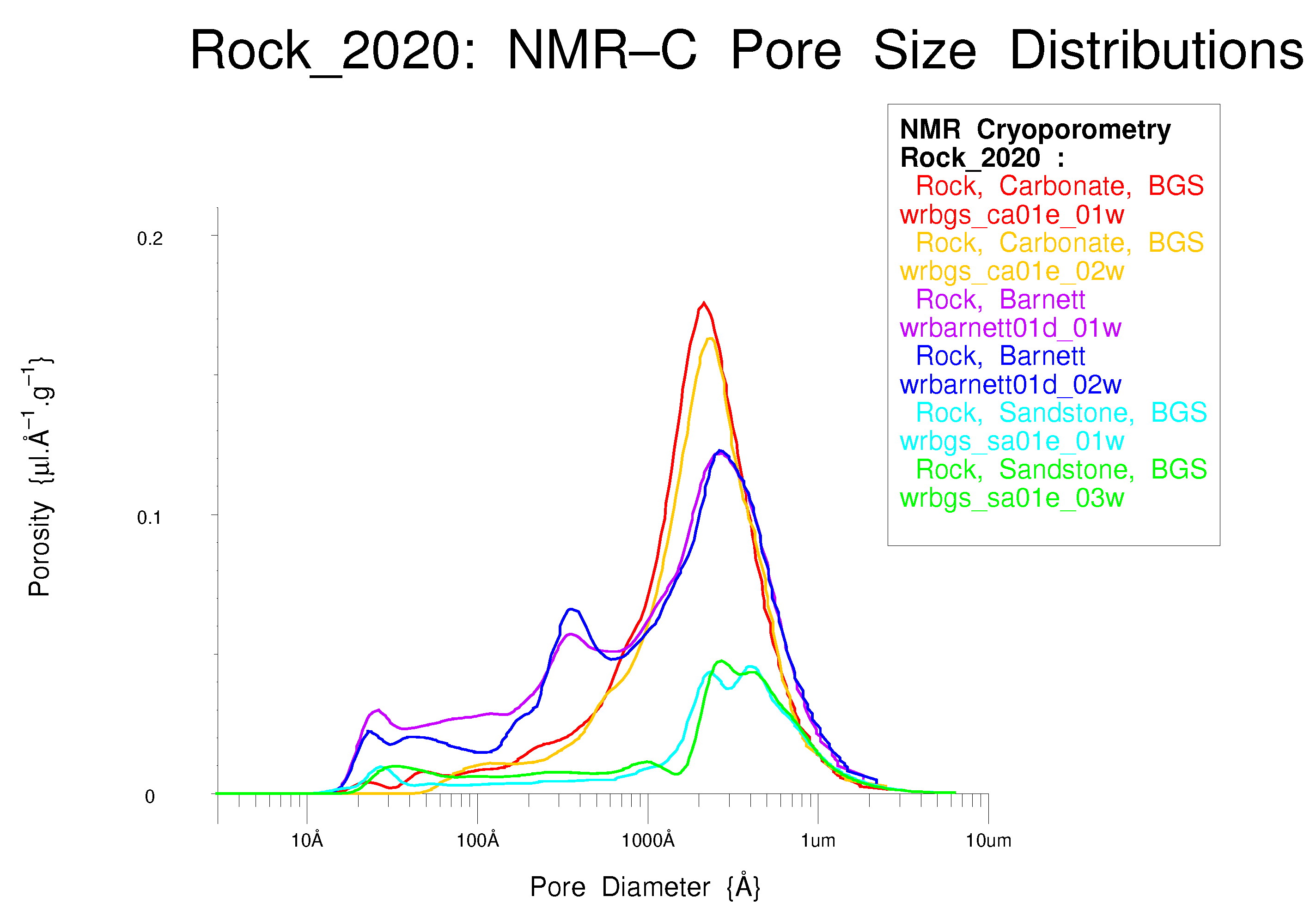
5.2. NMR Cryoporometric Measurements on Three Porous Rocks
5.3. NMR Cryoporometric Measurements on Four Biochar Samples with Different Processing
6. Measured Instrument Parameters and Capabilities
- System signal-to-noise: The controlling Graphical User Interface (GUI) configures the NMR data capture to start before the first NMR pulse, and this signal provides both a data base-line and uncontaminated signal that the instrument uses to measure the base noise-level, for each trace. A typical signal at the demodulated receiver output is shown in Figure 4. Measured noise levels appear to be around 1 mV RMS (for a typical 0.5 V signal) so a Signal-to-Noise (S/N) of about 500 for a typical 25 mg water sample in the 3 mm probe), for a signal filter setting of 250 kHz and 5 point integral filter (suitable for a typical NMR Free Induction Decay (FID) and Echo experiment with a liquid)—thus, an RMS noise level, single shot, equivalent to about 50 g for water. When conducting NMR Cryoporometric experiments, a slow warming ramp is needed so that all the sample is as isothermal as possible, and thus averaging times of 100 to 300 s per captured point were commonly employed. The NMR echo is fitted with a polynomial, and the polynomial solved to obtain a peak amplitude. This gave RMS noise levels for the averaged NMR Cryoporometric signal of around 50 to 100 V, so an S/N of about 5000 to 10,000 for typical 3 mm samples containing about 25 mg of water. This gives a very useful dynamic range to the resultant pore-size distributions, see Figure 9, the data for which appears to indicate the RMS sensitivity in a typical NMRC measurement to be around 15 nl of porosity. This is particularly important for low porosity samples.
- NMR RF Pulse Power: For this spectrometer, the linear RF transmitter amplifier delivers a /2 pulse into the supplied room-temperature NMR probe (5 mm OD standard NMR tube) of about 6 s, while, for the NMR Cryoporometric probe with a 3 mm OD NMR sample, it is about 9 s.
- Recovery time after the NMR Pulse: The time from the middle of the RF pulse to the first usable signal is a key feature for spectrometers when measuring more rigid materials such as solid polymers, waxes, tars, etc. With the receiver set to full bandwidth and 2 point integration (to suppress filter ringing), the system appears to recover pleasingly quickly, even for the Cryoporometric probe, with an overall time from the centre of the NMR pulse being no greater than 7 or 8 s. This enabled the measurement of FIDs from materials as rigid as bulk ice, see Section 4.2.
- Portability: A significant feature of this Field Programmable Gate Array (FPGA) based equipment is its compactness, which might make it suitable for use in the field or even for mobile use. As such applications are still to be evaluated, these measurements having been conducted on the laboratory bench-top. For mobile use, more compact non-mains power supplies will be needed.
7. Conclusions and Suggestions
7.1. Diffusion and Controlled Magnetic Field Gradient Experiments
7.2. Portability
8. Patents
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NMR | Nuclear Magnetic Resonance |
| NMRR | Nuclear Magnetic Resonance Relaxation |
| NMRC | Nuclear Magnetic Resonance Cryoporometry |
| FID | Free Induction Decay |
| CPMG | Carr–Purcell–Meiboom–Gill (NMR sequence) |
| T | Longitudinal Relaxation time—Time for NMR spins to equilibrate with the lattice |
| T | Transverse Relaxation time—Time for NMR spins to inter-equilibrate with each other |
| T | T1 in the Rotating Frame |
| k | Gibbs–Thomson coefficient |
| V-T | Variable-Temperature |
| RF | Radio Frequency |
| EMF | Electro-Motive-Force—voltage |
| CPU | Central Processing Unit |
| ADC | Analogue to Digital Convertor |
| DAC | Digital to Analogue Convertor |
| DVM | Digital Volt Meter |
| FPGA | Field Programmable Gate Array |
| OD | Outside Diameter |
| 3D | Three-Dimensional |
| Mk3 | Mark 3 |
| NIST | (USA) National Institute of Science and Technology |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
References
- Abragam, A. The Principles of Nuclear Magnetism; Clarendon Press: Oxford, UK, 1961; Available online: https://books.google.co.uk/books/about/The_Principles_of_Nuclear_Magnetism.html?id=9M8U_JK7K54C (accessed on 14 April 2020).
- Denise Besghini, M.M.; Simonutti, R. Time Domain NMR in Polymer Science: From the Laboratory to the Industry. Appl. Sci. 2019, 9, 1801. [Google Scholar] [CrossRef] [Green Version]
- Fabian Vaca Chavez, K.S. Time-Domain NMR Observation of Entangled Polymer Dynamics: Universal Behavior of Flexible Homopolymers and Applicability of the Tube Model. Macromolecules 2011, 44, 1549–1559. [Google Scholar] [CrossRef]
- Todt, H.; Guthausen, G.; Burk, W.; Schmalbein, D.; Kamlowski, A. Water/moisture and fat analysis by time-domain NMR. Food Chem. 2006, 96, 436–440. [Google Scholar] [CrossRef]
- Brownstein, K.; Tarr, C. Importance of classical diffusion in NMR studies of water in biological cells. Phys. Rev. A. 1979, 19, 2446–2453. [Google Scholar] [CrossRef]
- Gladden, L. Nuclear-magnetic-resonance studies of porous-media. Chem. Eng. Res. Des. 1993, 71, 657–674. [Google Scholar]
- Callaghan, P.; Godefroy, S.; Ryland, B. Diffusion-relaxation correlation in simple pore structures. J. Magn. Reson. 2003, 162, 320–327. [Google Scholar] [CrossRef]
- Fantazzini, P.; Brown, R.; Borgia, G. Bone tissue and porous media: Common features and differences studied by NMR relaxation. Magn. Reson. Imaging 2003, 21, 227–234. [Google Scholar] [CrossRef]
- Kimmich, R.; Fatkullin, N.; Mattea, C.; Fischer, E. Polymer chain dynamics under nanoscopic confinements. Magn. Reson. Imaging 2005, 23, 191–196. [Google Scholar] [CrossRef]
- Hansen, E.; Fonnum, G.; Weng, E. Pore morphology of porous polymer particles probed by NMR relaxometry and NMR cryoporometry. J. Phys. Chem. 2005, 109, 24295–24303. [Google Scholar] [CrossRef]
- McDonald, P.J.; Mitchell, J.; Mulheron, M.; Aptaker, P.S.; Korb, J.P.; Monteilhet, L. Two-dimensional correlation relaxometry studies of cement pastes performed using a new one-sided NMR magnet. Cem. Concr. Res. 2007, 37, 303–309. [Google Scholar] [CrossRef]
- D’Agostino, C.; Mitchell, J.; Mantle, M.D.; Gladden, L.F. Interpretation of NMR Relaxation as a Tool for Characterising the Adsorption Strength of Liquids inside Porous Materials. Chemistry 2014, 20, 13009–13015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, J.B.W. Biological, Medical and Nano Structured materials—NMR done Simply. Arch. Biomed. Eng. Biotechnol. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Prado, P.; Balcom, B.; Beyea, S.; Bremner, T.; Armstrong, R.; Pishe, R.; Gratten-Bellew, P. Spatially resolved relaxometry and pore size distribution by single-point MRI methods: Porous media calorimetry. J. Phys. D Appl. Phys. 1998, 31, 2040–2050. [Google Scholar] [CrossRef]
- Friedemann, K.; Schoenfelder, W.; Stallmach, F.; Kaerger, J. NMR relaxometry during internal curing of Portland cements by lightweight aggregates. Mater. Struct. 2008, 41, 1647–1655. [Google Scholar] [CrossRef]
- Schoenfelder, W.; Glaeser, H.R.; Mitreiter, I.; Stallmach, F. Two-dimensional NMR relaxometry study of pore space characteristics of carbonate rocks from a Permian aquifer. J. Appl. Geophys. 2008, 65, 21–29. [Google Scholar] [CrossRef]
- Strange, J.; Mitchell, J.; Webber, J. Pore surface exploration by NMR. Magn. Reson. Imaging 2003, 21, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.; Webber, J.; Strange, J. Nuclear magnetic resonance cryoporometry. Phys. Rep. 2008, 461, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Petrov, O.V.; Furo, I. NMR cryoporometry: Principles, applications and potential. Prog. Nucl. Magn. Res. Spectrosc. 2009, 54, 97–122. [Google Scholar] [CrossRef]
- Rottreau, T.J.; Parkes, G.E.; Schirru, M.; Harries, J.L.; Mesa, M.G.; Topham, P.D.; Evans, R. NMR Cryoporometry of Polymers: Cross-linking, Porosity and the Importance of Probe Liquid. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 256–263. [Google Scholar] [CrossRef]
- Gibbs, J. Collected Works; Longmans, Green and Co.: New York, NY, USA, 1928. [Google Scholar]
- Gibbs, J. The Scientific Papers of J. Willward Gibbs; New Dover ed.; Volume 1: Thermodynamics; Dover Publications, Inc., Constable and Co.: New York, NY, USA; London, UK, 1906. [Google Scholar]
- Gibbs, J. On the equilibrium of hetrogeneous substances. Trans. Conn. Acad. 1878, 96, 441–458. [Google Scholar]
- Belmonte, S.B.; Sarthour, R.S.; Oliveira, I.S.; Guimaes, A.P. A field-programmable gate-array-based high-resolution pulse programmer. Meas. Sci. Technol. 2003, 14, N1. [Google Scholar] [CrossRef]
- Hemnani, P.; Rajarajan, A.; Joshi, G.; Ravindranath, S. FPGA Based RF Pulse Generator for NQR/NMR Spectrometer. Procedia Comput. Sci. 2016, 93, 161–168. [Google Scholar] [CrossRef]
- Takeda, K. A highly integrated FPGA-based nuclear magnetic resonance spectrometer. Rev. Sci. Instrum. 2007, 78, 033103. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.B.W.; Corbett, P.; Semple, K.T.; Ogbonnaya, U.; Teel, W.S.; Masiello, C.A.; Fisher, Q.J.; Valenza, J.J., II; Song, Y.Q.; Hu, Q. An NMR study of porous rock and biochar containing organic material. Microporous Mesoporous Mater. 2013, 178, 94–98. [Google Scholar] [CrossRef]
- Webber, J.B.W. Thermostatted NMR Probe. EU Patent EP2818885, 26 June 2014. [Google Scholar]
- Webber, J.B.W. Nuclear Magnetic Resonance Probes. U.S. Patent 9,810,750 B2, 7 November 2017. [Google Scholar]
- Webber, J.; Strange, J.; Dore, J. An evaluation of NMR cryoporometry, density measurement and neutron scattering methods of pore characterisation. Magn. Reson. Imaging 2001, 19, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.B.W. Accessible Catalyst Pore Volumes, for Water and Organic Liquids, as probed by NMR Cryoporometry. Diffusion-fundamentals.org 2014, 22, 1–8. [Google Scholar]
- Andreev, A.S.; Beau Webber, P.S. Advanced NMR for Industrial Applications: Structure, Porosity, and Acidity; Characterization of Catalytic Materials through a Facile Approach to Probe OH Groups; Total Research and Technology Feluy (TRTF), Lab-Tools Ltd.: Ramsgate, UK, 2019. [Google Scholar]
- Boguszynska, J.; Tritt-Goc, J. H-1 NMR cryoporometry study of the melting behavior of water in white cement. Z. Naturforsch. Sect. A J. Phys. Sci. 2004, 59, 550–558. [Google Scholar] [CrossRef]
- Fleury, M.; Fabre, R.; Webber, J.W. Comparison of Pore Size Distribution by NMR Relaxation and NMR Cryoporometry in Shales. In Proceedings of the International Symposium of the Society of Core Analysts, St. John’s, NL, Canada, 16–21 August 2015. [Google Scholar]
- Ankathi, S.K.; Zhou, W.; Webber, J.B.; Patil, R.; Chaudhari, U.; Shonnard, D. Synergistic Effects between Hydrolysis Time and Microporous Structure in Poplar. ACS Sustain. Chem. Eng. 2019, 7, 12920–12929. [Google Scholar] [CrossRef]
- Aditya Rawal, S.D.J.; Webber, J.B.W. Mineral-Biochar Composites: Molecular Structure and Porosity. Environ. Sci. Technol. 2016, 50, 7706–7714. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; Webber, J.; Ogbonnaya, U. Characteristics of biochar porosity by NMR and study of ammonium ion adsorption. J. Anal. Appl. Pyrolysis 2019, 143, 104687. [Google Scholar] [CrossRef]
- Strange, J.; Webber, J. Characterization of Porous Solids by NMR. In Proceedings of the 12th Specialized Colloque Ampere, Corfu, Greece, 10–15 September 1995. [Google Scholar]
- Webber, J.B.W. Lab-Tools Mk3 NMR Relaxometer, Lab-Tools (Nano-Science); Ramsgate: Kent, UK, 2019. [Google Scholar]
- Webber, J.B.W. Lab-Tools Mk3 NMR Cryoporometer, Lab-Tools (Nano-Science); Ramsgate: Kent, UK, 2019. [Google Scholar]
- Red Pitaya FPGA Module. Available online: www.redpitaya.com (accessed on 16 February 2019).
- Meyer, C.W.; Garrity, K.M. Updated uncertainty budgets for NIST thermocouple calibrations. In Proceedings of the AIP Conference Proceedings, Physical Measurement Laboratory/Sensor Science Division, Thermodynamic Metrology Group, Los Angeles, CA, USA, 19–23 March 2012; p. 1552. [Google Scholar] [CrossRef] [Green Version]
- Strange, J.H.; Rahman, M.; Smith, E.G. Characterization of Porous Solids By Nmr. Phys. Rev. Lett. 1993, 71, 3589–3591. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J. On Crystallization and Liquefaction, as influenced by Stresses tending to change form in the Crystals. Proc. R. Soc. 1862, xi, 473–481. [Google Scholar]
- Thomson, J. Theoretical Considerations on the Effect of Pressure in Lowering the Freezing Point of Water. Trans. R. Soc. 1849, xvi, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Thomson, W. On the Equilibrium of Vapour at a Curved Surface of Liquid. Philos. Mag. 1871, 42, 448–452. [Google Scholar] [CrossRef]
- Thomson, J. Application of dynamics to Physics and Chemistry; Macmillan & Co: London, UK, 1888. [Google Scholar]
- Webber, J. A generalisation of the thermoporisimetry Gibbs–Thomson equation for arbitrary pore geometry. Magn. Reson. Imaging 2003, 21, 428. [Google Scholar] [CrossRef]
- Webber, J. The dimensional term in the Gibbs–Thomson equation, describing the behaviour of liquids in porous media, is being investigated by NMR relaxation, novel protocol NMR cryoporometry, neutron scattering and ab initio QM-MD simulation. Magn. Reson. Imaging 2006, 25, 589–590. [Google Scholar] [CrossRef]
- Webber, J.B.W. Studies of nano-structured liquids in confined geometry and at surfaces. Prog. Nucl. Magn. Res. Spectrosc. 2010, 56, 78–93. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.B.W. The Characterisation of Porous Media. Ph.D. Thesis, Physics, University of Kent, Canterbury, UK, 2000. [Google Scholar]
- Webber, J.B.W. A bi-symmetric log transformation for wide-range data. Meas. Sci. Technol. 2013, 24, 027001. [Google Scholar] [CrossRef] [Green Version]
- Carlton, K.J.; Halse, M.R.; Strange, J.H. Diffusion-weighted imaging of bacteria colonies in the STRAFI plane. J. Magn. Reson. 2000, 143, 24–29. [Google Scholar] [CrossRef]
- Zielinski, L.; Sen, P. Restricted diffusion in grossly inhomogeneous fields. J. Magn. Reson. 2003, 164, 145–153. [Google Scholar] [CrossRef]
- Mitzithras, A.; Strange, J.H. Diffusion of Fluids In Confined Geometry. Magn. Reson. Imaging 1994, 12, 261–263. [Google Scholar] [CrossRef]
- Kimmich, R.; Zavada, T.; Stapf, S. NMR experiments on molecular dynamics in nanoporous media: Evidence for Levy walk statistics. In Dynamics in Small Confining Systems III; Drake, J., Klafter, J., Kopelman, R., Eds.; Materials Research Society: Warrendale, PA, USA, 1997; Volume 464, pp. 313–324. [Google Scholar]
- Kimmich, R.; Fatkullin, N.; Fischer, E.; Mattea, C.; Beginn, U. Reptation in artificial tubes and the corset effect of confined polymer dynamics. In Dynamics in Small Confining Systems-2003; Fourkas, J.T., Levitz, P., Urbakh, M., Wahl, K.J., Eds.; Materials Research Society: Warrendale, PA, USA, 2004; Volume 790, pp. 107–118. [Google Scholar]
- Mallett, M.J.D.; Strange, J.H. Diffusion measurements using oscillating gradients. Appl. Magn. Reson. 1997, 12, 193–198. [Google Scholar] [CrossRef]
- Eidmann, G.; Savelsberg, R.; Blümler, P.; Blümich, B. The NMR MOUSE, a Mobile Universal Surface Explorer. J. Magn. Reson. 1996, 122, 104–109. [Google Scholar] [CrossRef]
- Webber, J.B.W.; Demin, P. Credit-card sized field and benchtop NMR relaxometers using field programmable gate arrays. Magn. Reson. Imaging 2019, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]









© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webber, J.B.W. Some Applications of a Field Programmable Gate Array Based Time-Domain Spectrometer for NMR Relaxation and NMR Cryoporometry. Appl. Sci. 2020, 10, 2714. https://doi.org/10.3390/app10082714
Webber JBW. Some Applications of a Field Programmable Gate Array Based Time-Domain Spectrometer for NMR Relaxation and NMR Cryoporometry. Applied Sciences. 2020; 10(8):2714. https://doi.org/10.3390/app10082714
Chicago/Turabian StyleWebber, J. Beau W. 2020. "Some Applications of a Field Programmable Gate Array Based Time-Domain Spectrometer for NMR Relaxation and NMR Cryoporometry" Applied Sciences 10, no. 8: 2714. https://doi.org/10.3390/app10082714
APA StyleWebber, J. B. W. (2020). Some Applications of a Field Programmable Gate Array Based Time-Domain Spectrometer for NMR Relaxation and NMR Cryoporometry. Applied Sciences, 10(8), 2714. https://doi.org/10.3390/app10082714