Clinical Significance of Cough Peak Flow and Its Non-Contact Measurement via Cough Sounds: A Narrative Review
Abstract
1. Introduction
2. Cough Mechanism and Related Factors Influencing CPF
3. Clinical Significance of CPF Evaluation
3.1. Conventional CPF Measurement Methods
3.2. Neuromuscular Disease
3.3. Risk Management in Aspiration Pneumonitis
4. Non-Contact Measurement of CPF via Cough Sounds
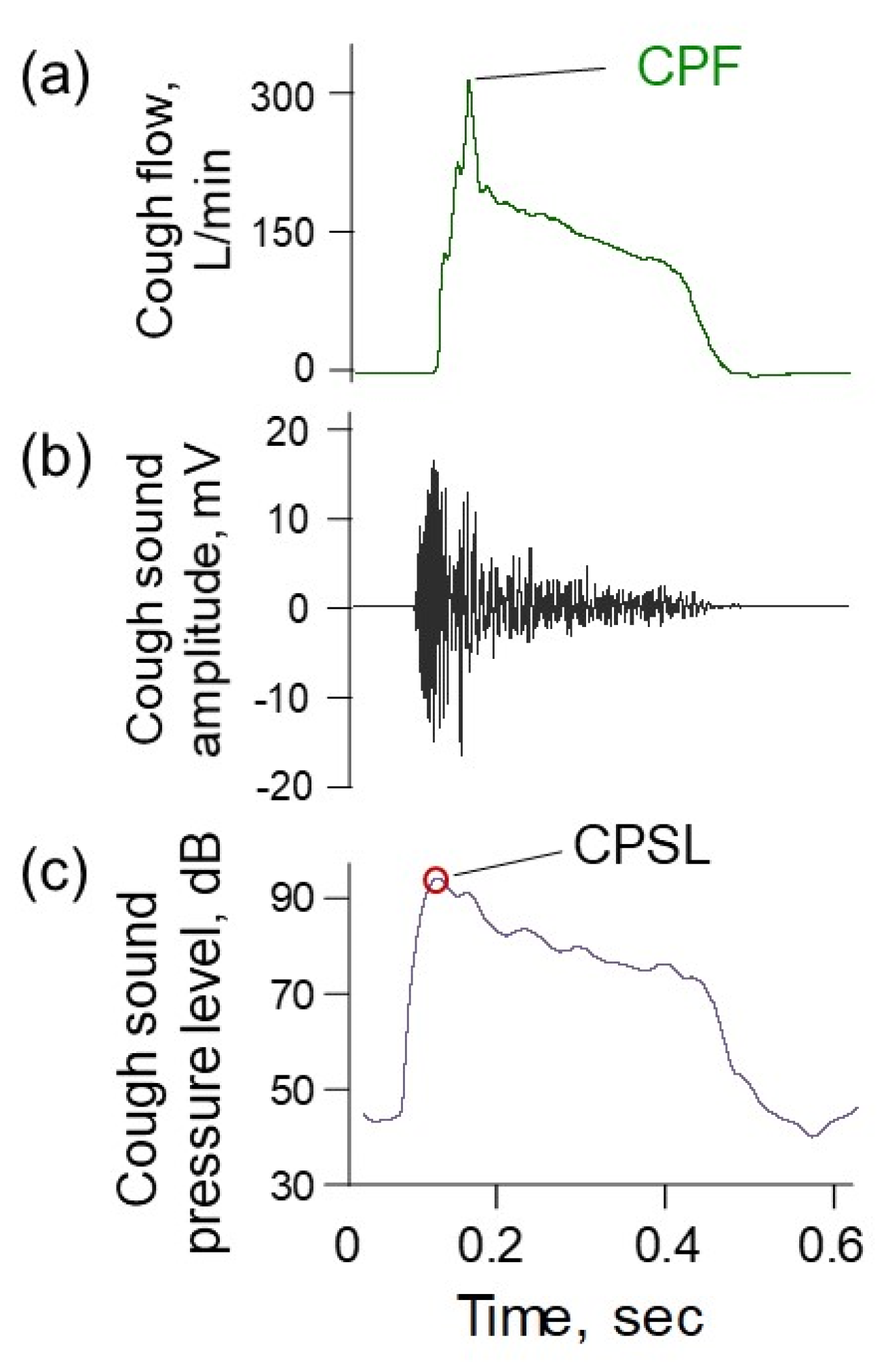
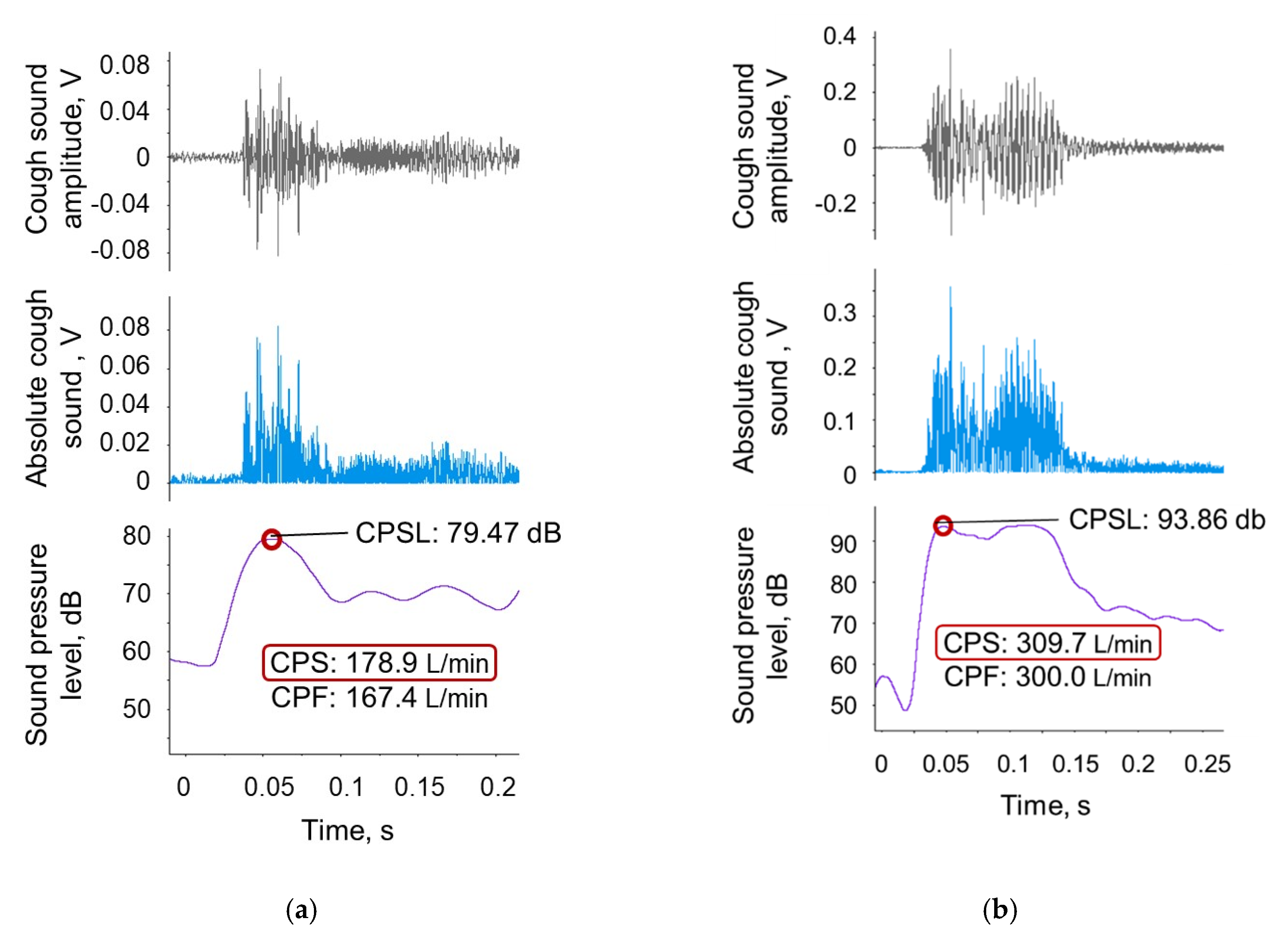
4.1. CPF Estimation Model Using Cough Sounds
4.2. Non-Contact CPF Measurement Device
4.3. Evaluation of Cough Strength Using a Smartphone in the Elderly and in a Patient with Spinal Muscular Atrophy
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Teramoto, S.; Fukuchi, Y.; Sasaki, H.; Sato, K.; Sekizawa, K.; Matsuse, T.; Japanese Study Group on Aspiration Pulmonary Disease. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: A multicenter, prospective study in Japan. J. Am. Geriatr. Soc. 2008, 56, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Baiardi, P.; Khirani, S.; Cantarella, G. Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. Am. J. Phys. Med. Rehabil. 2012, 91, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Kawamoto, K.; Shimizu, Y.; Fukuhara, T.; Koyama, S.; Kataoka, H.; Kitano, H.; Takeuchi, H. A novel reflex cough testing device. BMC Pulm. Med. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B. The physiology of cough. Paediatr. Respir. Rev. 2006, 7, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Schmit, K.M.; Coeytaux, R.R.; Goode, A.P.; McCrory, D.C.; Yancy, W.S., Jr.; Kemper, A.R.; Hasselblad, V.; Heidenfelder, B.L.; Sanders, G.D. Evaluating cough assessment tools: A systematic review. Chest 2013, 144, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, S.; Sekiya, H.; Miyagi, M.; Ebihara, T.; Okazaki, T. Dysphagia, dystussia, and aspiration pneumonia in elderly people. J. Thorac. Dis. 2016, 8, 632–639. [Google Scholar] [CrossRef]
- Smina, M.; Salam, A.; Khamiees, M.; Gada, P.; Amoateng-Adjepong, Y.; Manthous, C.A. Cough peak flows and extubation outcomes. Chest 2003, 124, 262–268. [Google Scholar] [CrossRef]
- Hull, J.; Aniapravan, R.; Chan, E.; Chatwin, M.; Forton, J.; Gallagher, J.; Gibson, N.; Gordon, J.; Hughes, I.; McCulloch, R.; et al. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax 2012, 67 (Suppl. 1), i1–i40. [Google Scholar] [CrossRef]
- Morice, A.H.; Fontana, G.A.; Belvisi, M.G.; Birring, S.S.; Chung, K.F.; Dicpinigaitis, P.V.; Kastelik, J.A.; McGarvey, L.P.; Smith, J.A.; Tatar, M.; et al. ERS guidelines on the assessment of cough. Eur. Respir. J. 2007, 29, 1256–1276. [Google Scholar] [CrossRef]
- Korpas, J.; Tomori, Z. Cough and Other Respiratory Reflexes; Karger: Basel, Switzerland, 1979. [Google Scholar] [CrossRef]
- Cherniack, R.M.; Naimark, A.; Cherniack, L. Respiration in Health and Disease; WB Saunders: Philadelphia, PA, USA, 1972; p. 496. [Google Scholar]
- Collier, J.G.; Fuller, R.W. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br. J. Pharmacol. 1984, 81, 113–117. [Google Scholar] [CrossRef]
- Bickerman, H.A.; Barach, A.L. The experimental production of cough in human subjects induced by citric acid aerosols; preliminary studies on the evaluation of antitussive agents. Am. J. Med. Sci. 1954, 228, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bickerman, H.A.; German, E.; Cohen, B.M.; Itkin, S.E. The cough response of healthy human subjects stimulated by citric acid aerosol. II. Evaluation of antitussive agents. Am. J. Med. Sci. 1957, 234, 191–206. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Aliverti, A. Respiratory muscle activation and action during voluntary cough in healthy humans. J. Electromyogr. Kinesiol. 2019, 49, 102359. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Rao, P.; Reilly, C.C.; Rafferty, G.F.; Polkey, M.I.; Kalra, L.; Moxham, J. Poor cough flow in acute stroke patients is associated with reduced functional residual capacity and low cough inspired volume. BMJ Open Respir. Res. 2017, 4, e000230. [Google Scholar] [CrossRef]
- Smith, J.A.; Aliverti, A.; Quaranta, M.; McGuinness, K.; Kelsall, A.; Earis, J.; Calverley, P.M. Chest wall dynamics during voluntary and induced cough in healthy volunteers. J. Physiol. 2012, 590, 563–574. [Google Scholar] [CrossRef]
- Kang, S.W.; Shin, J.C.; Park, C.I.; Moon, J.H.; Rha, D.W.; Cho, D.H. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord 2006, 44, 242–248. [Google Scholar] [CrossRef]
- Bahat, G.; Tufan, A.; Ozkaya, H.; Tufan, F.; Akpinar, T.S.; Akin, S.; Bahat, Z.; Kaya, Z.; Kiyan, E.; Erten, N.; et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male Off. J. Int. Soc. Study Aging Male 2014, 17, 136–140. [Google Scholar] [CrossRef]
- Lavietes, M.H.; Smeltzer, S.C.; Cook, S.D.; Modak, R.M.; Smaldone, G.C. Airway dynamics, oesophageal pressure and cough. Eur. Respir. J. 1998, 11, 156–161. [Google Scholar] [CrossRef]
- Singh, P.; Murty, G.E.; Mahajan, R.P.; Knights, D.; Aitkenhead, A.R. The tussometer: Accuracy and reproducibility. Br. J. Anaesth. 1994, 73, 145–148. [Google Scholar] [CrossRef]
- Freitas, F.S.; Ibiapina, C.C.; Alvim, C.G.; Britto, R.R.; Parreira, V.F. Relationship between cough strength and functional level in elderly. Rev. Bras. Fisioter. (Sao Carlos (Sao PauloBraz.)) 2010, 14, 470–476. [Google Scholar] [CrossRef]
- Sancho, J.; Servera, E.; Diaz, J.; Marin, J. Predictors of ineffective cough during a chest infection in patients with stable amyotrophic lateral sclerosis. Am. J. Respir. Crit. Care Med. 2007, 175, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Sancho, J.; Servera, E.; Diaz, J.; Marin, J. Comparison of peak cough flows measured by pneumotachograph and a portable peak flow meter. Am. J. Phys. Med. Rehabil. 2004, 83, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Duan, J. Use of Cough Peak Flow Measured by a Ventilator to Predict Re-Intubation When a Spirometer Is Unavailable. Respir. Care 2017, 62, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Satake, M.; Kimoto, Y.; Iwasawa, S.; Suzuki, R.; Kobayashi, M.; Wada, C.; Shioya, T. Approaches to Cough Peak Flow Measurement With Duchenne Muscular Dystrophy. Respir. Care 2018, 63, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.R.; Saporito, L.R. Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure. A different approach to weaning. Chest 1996, 110, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Mellies, U.; Goebel, C. Optimum insufflation capacity and peak cough flow in neuromuscular disorders. Ann. Am. Thorac. Soc. 2014, 11, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.P.; Carnaby-Mann, G.; Pitts, T.; Davenport, P.; Okun, M.S.; Sapienza, C. Concordance and discriminatory power of cough measurement devices for individuals with Parkinson disease. Chest 2014, 145, 1089–1096. [Google Scholar] [CrossRef][Green Version]
- Suarez, A.A.; Pessolano, F.A.; Monteiro, S.G.; Ferreyra, G.; Capria, M.E.; Mesa, L.; Dubrovsky, A.; De Vito, E.L. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am. J. Phys. Med. Rehabil. 2002, 81, 506–511. [Google Scholar] [CrossRef]
- Tzani, P.; Chiesa, S.; Aiello, M.; Scarascia, A.; Catellani, C.; Elia, D.; Marangio, E.; Chetta, A. The value of cough peak flow in the assessment of cough efficacy in neuromuscular patients. A cross sectional study. Eur. J. Phys. Rehabil. Med. 2014, 50, 427–432. [Google Scholar] [PubMed]
- Kimura, Y.; Takahashi, M.; Wada, F.; Hachisuka, K. Differences in the Peak Cough Flow among Stroke Patients With and Without Dysphagia. J. Uoeh 2013, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, A.; LoMauro, A.; Santi, M.; Biffi, E.; D’Angelo, M.G.; Aliverti, A. Acute Effects of Mechanical Insufflation-Exsufflation on the Breathing Pattern in Stable Subjects With Duchenne Muscular Dystrophy. Respir. Care 2018, 63, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Kulnik, S.T.; MacBean, V.; Birring, S.S.; Moxham, J.; Rafferty, G.F.; Kalra, L. Accuracy of portable devices in measuring peak cough flow. Physiol. Meas. 2015, 36, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.R.; Ishikawa, Y.; Kim, H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest 1997, 112, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, C.; Shimizu, T.; Nakayama, Y.; Haraguchi, M. Cough peak flow decline rate predicts survival in patients with amyotrophic lateral sclerosis. Muscle Nerve 2019, 59, 168–173. [Google Scholar] [CrossRef]
- Beuret, P.; Roux, C.; Auclair, A.; Nourdine, K.; Kaaki, M.; Carton, M.J. Interest of an objective evaluation of cough during weaning from mechanical ventilation. Intensive. Care Med. 2009, 35, 1090–1093. [Google Scholar] [CrossRef]
- Duan, J.; Zhou, L.; Xiao, M.; Liu, J.; Yang, X. Semiquantitative cough strength score for predicting reintubation after planned extubation. Am. J. Crit. Care Off. Publ. Am. Assoc. Crit. Care Nurses 2015, 24, e86–e90. [Google Scholar] [CrossRef]
- Finder, J.D.; Birnkrant, D.; Carl, J.; Farber, H.J.; Gozal, D.; Iannaccone, S.T.; Kovesi, T.; Kravitz, R.M.; Panitch, H.; Schramm, C.; et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am. J. Respir. Crit. Care Med. 2004, 170, 456–465. [Google Scholar] [CrossRef]
- Bach, J.R.; Bianchi, C.; Vidigal-Lopes, M.; Turi, S.; Felisari, G. Lung inflation by glossopharyngeal breathing and “air stacking” in Duchenne muscular dystrophy. Am. J. Phys. Med. Rehabil. 2007, 86, 295–300. [Google Scholar] [CrossRef]
- Bach, J.R.; Martinez, D. Duchenne muscular dystrophy: Continuous noninvasive ventilatory support prolongs survival. Respir. Care 2011, 56, 744–750. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Miura, T.; Ishikawa, Y.; Aoyagi, T.; Ogata, H.; Hamada, S.; Minami, R. Duchenne muscular dystrophy: Survival by cardio-respiratory interventions. Neuromuscul. Disord. 2011, 21, 47–51. [Google Scholar] [CrossRef]
- Bach, J.R.; Upadhyaya, N. Association of Need for Tracheotomy With Decreasing Mechanical In-Exsufflation Flows in Amyotrophic Lateral Sclerosis. Am. J. Phys. Med. Rehabil. 2018, 97, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.R.; Giménez, G.C.; Chiou, M. Mechanical In-exsufflation-Expiratory Flows as Indication for Tracheostomy Tube Decannulation: Case Studies. Am. J. Phys. Med. Rehabil. 2019, 98, e18–e20. [Google Scholar] [CrossRef] [PubMed]
- Omari, T.I.; Kritas, S.; Cock, C.; Besanko, L.; Burgstad, C.; Thompson, A.; Rommel, N.; Heddle, R.; Fraser, R.J.L. Swallowing dysfunction in healthy older people using pharyngeal pressure-flow analysis. Neurogastroenterol. Motil. 2014, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Ohira, M.; Yokokawa, Y. Cough Strength Is an Indicator of Aspiration Risk When Restarting Food Intake in Elderly Subjects With Community-Acquired Pneumonia. Respir. Care 2020, 65, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-T.; Yu, C.-J. Conventional Weaning Parameters Do Not Predict Extubation Outcome in Intubated Subjects Requiring Prolonged Mechanical Ventilation. Respir. Care 2013, 58, 1307–1314. [Google Scholar] [CrossRef]
- Thille, A.W.; Boissier, F.; Ben Ghezala, H.; Razazi, K.; Mekontso-Dessap, A.; Brun-Buisson, C. Risk factors for and prediction by caregivers of extubation failure in ICU patients: A prospective study. Crit. Care Med. 2015, 43, 613–620. [Google Scholar] [CrossRef]
- Birring, S.S.; Matos, S.; Patel, R.B.; Prudon, B.; Evans, D.H.; Pavord, I.D. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir. Med. 2006, 100, 1105–1109. [Google Scholar] [CrossRef]
- Birring, S.S.; Fleming, T.; Matos, S.; Raj, A.A.; Evans, D.H.; Pavord, I.D. The Leicester Cough Monitor: Preliminary validation of an automated cough detection system in chronic cough. Eur. Respir. J. 2008, 31, 1013–1018. [Google Scholar] [CrossRef]
- Spinou, A.; Birring, S.S. An update on measurement and monitoring of cough: What are the important study endpoints? J. Thorac. Dis. 2014, 6, S728–S734. [Google Scholar] [CrossRef]
- Kraman, S.S. The relationship between airflow and lung sound amplitude in normal subjects. Chest 1984, 86, 225–229. [Google Scholar] [CrossRef]
- Dosani, R.; Kraman, S.S. Lung sound intensity variability in normal men. A contour phonopneumographic study. Chest 1983, 83, 628–631. [Google Scholar] [CrossRef]
- Shykoff, B.E.; Ploysongsang, Y.; Chang, H.K. Airflow and normal lung sounds. Am. Rev. Respir. Dis. 1988, 137, 872–876. [Google Scholar] [CrossRef]
- Umayahara, Y.; Soh, Z.; Ozaki, T.; Murakami, T.; Otsuka, A.; Tsuji, T. Ability to cough can be evaluated through cough sounds: An experimental investigation of effects of microphone type on accuracy. In Proceedings of the 2017 IEEE/SICE International Symposium on System Integration (SII), Taipei, Taiwan, 11–14 December 2017; pp. 936–941. [Google Scholar]
- Umayahara, Y.; Soh, Z.; Sekikawa, K.; Kawae, T.; Otsuka, A.; Tsuji, T. Estimation of Cough Peak Flow Using Cough Sounds. Sensors 2018, 18, 2381. [Google Scholar] [CrossRef]
- Smith Hammond, C.A.; Goldstein, L.B.; Zajac, D.J.; Gray, L.; Davenport, P.W.; Bolser, D.C. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology 2001, 56, 502–506. [Google Scholar] [CrossRef]
- Umayahara, Y.; Soh, Z.; Sekikawa, K.; Kawae, T.; Otsuka, A.; Tsuji, T. A Mobile Cough Strength Evaluation Device Using Cough Sounds. Sensors 2018, 18, 3810. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umayahara, Y.; Soh, Z.; Sekikawa, K.; Kawae, T.; Otsuka, A.; Tsuji, T. Clinical Significance of Cough Peak Flow and Its Non-Contact Measurement via Cough Sounds: A Narrative Review. Appl. Sci. 2020, 10, 2782. https://doi.org/10.3390/app10082782
Umayahara Y, Soh Z, Sekikawa K, Kawae T, Otsuka A, Tsuji T. Clinical Significance of Cough Peak Flow and Its Non-Contact Measurement via Cough Sounds: A Narrative Review. Applied Sciences. 2020; 10(8):2782. https://doi.org/10.3390/app10082782
Chicago/Turabian StyleUmayahara, Yasutaka, Zu Soh, Kiyokazu Sekikawa, Toshihiro Kawae, Akira Otsuka, and Toshio Tsuji. 2020. "Clinical Significance of Cough Peak Flow and Its Non-Contact Measurement via Cough Sounds: A Narrative Review" Applied Sciences 10, no. 8: 2782. https://doi.org/10.3390/app10082782
APA StyleUmayahara, Y., Soh, Z., Sekikawa, K., Kawae, T., Otsuka, A., & Tsuji, T. (2020). Clinical Significance of Cough Peak Flow and Its Non-Contact Measurement via Cough Sounds: A Narrative Review. Applied Sciences, 10(8), 2782. https://doi.org/10.3390/app10082782




