Towards the Quantification of 5f Delocalization
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
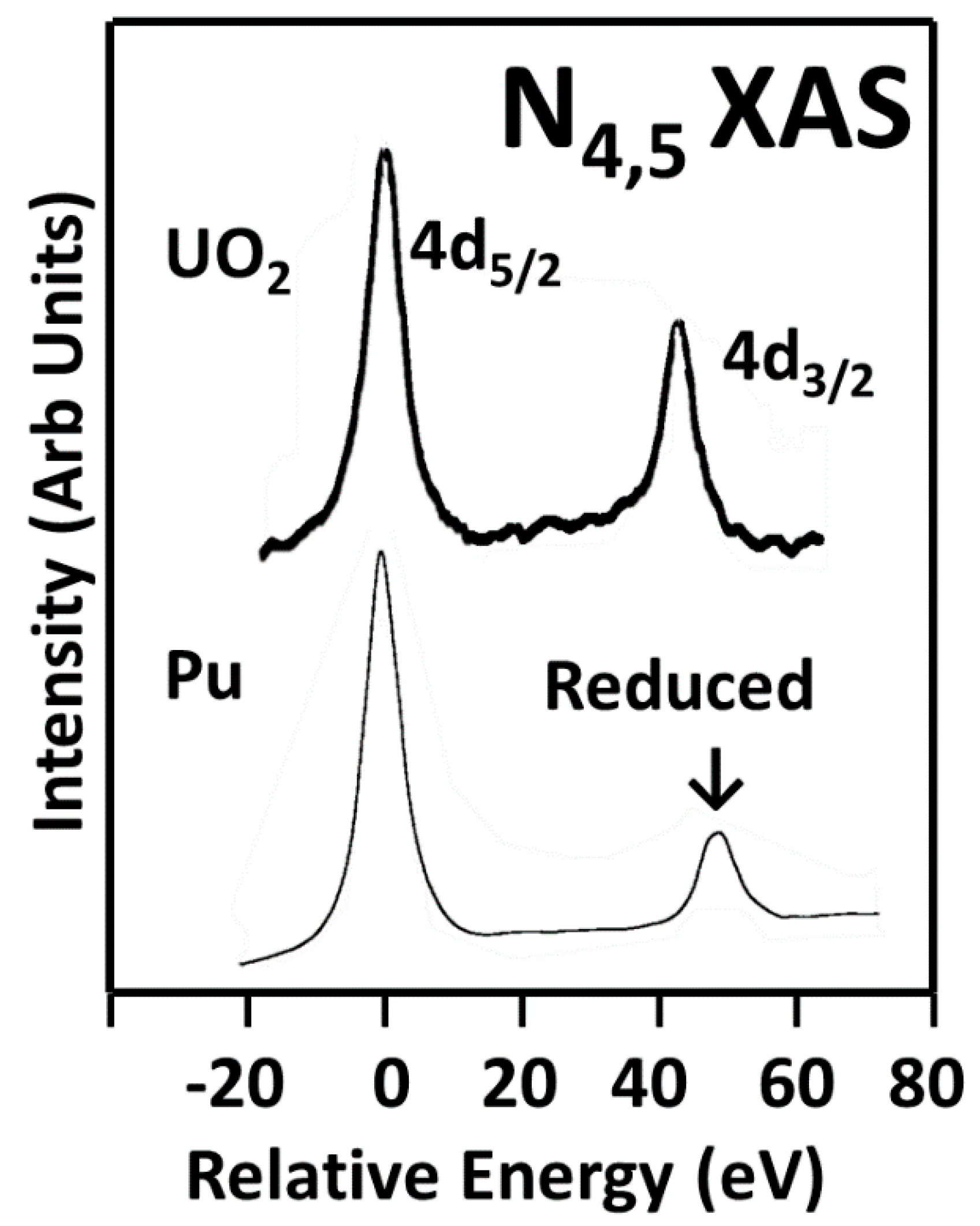
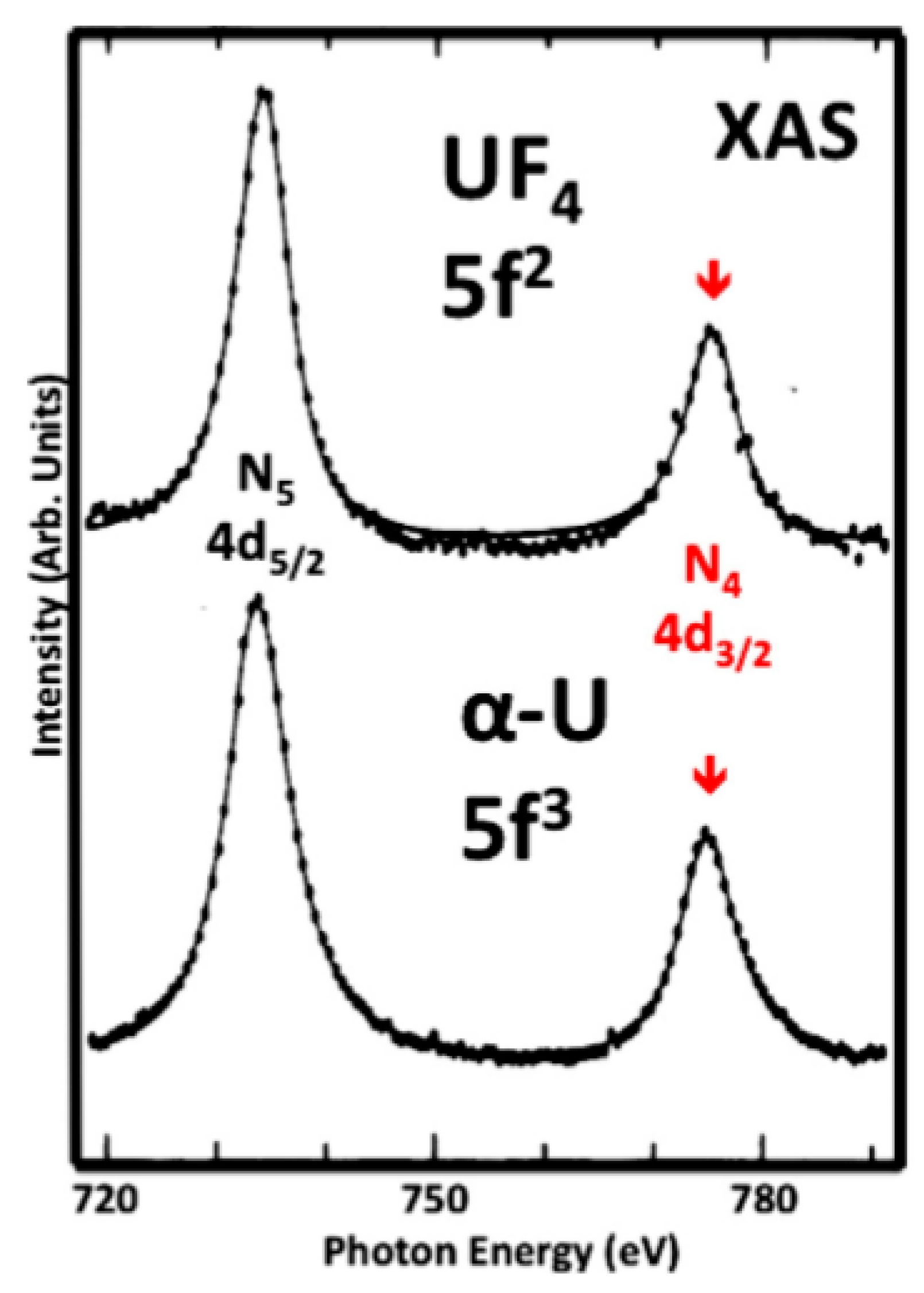
3.1. A Revisitation of XAS and the Branching Ratio
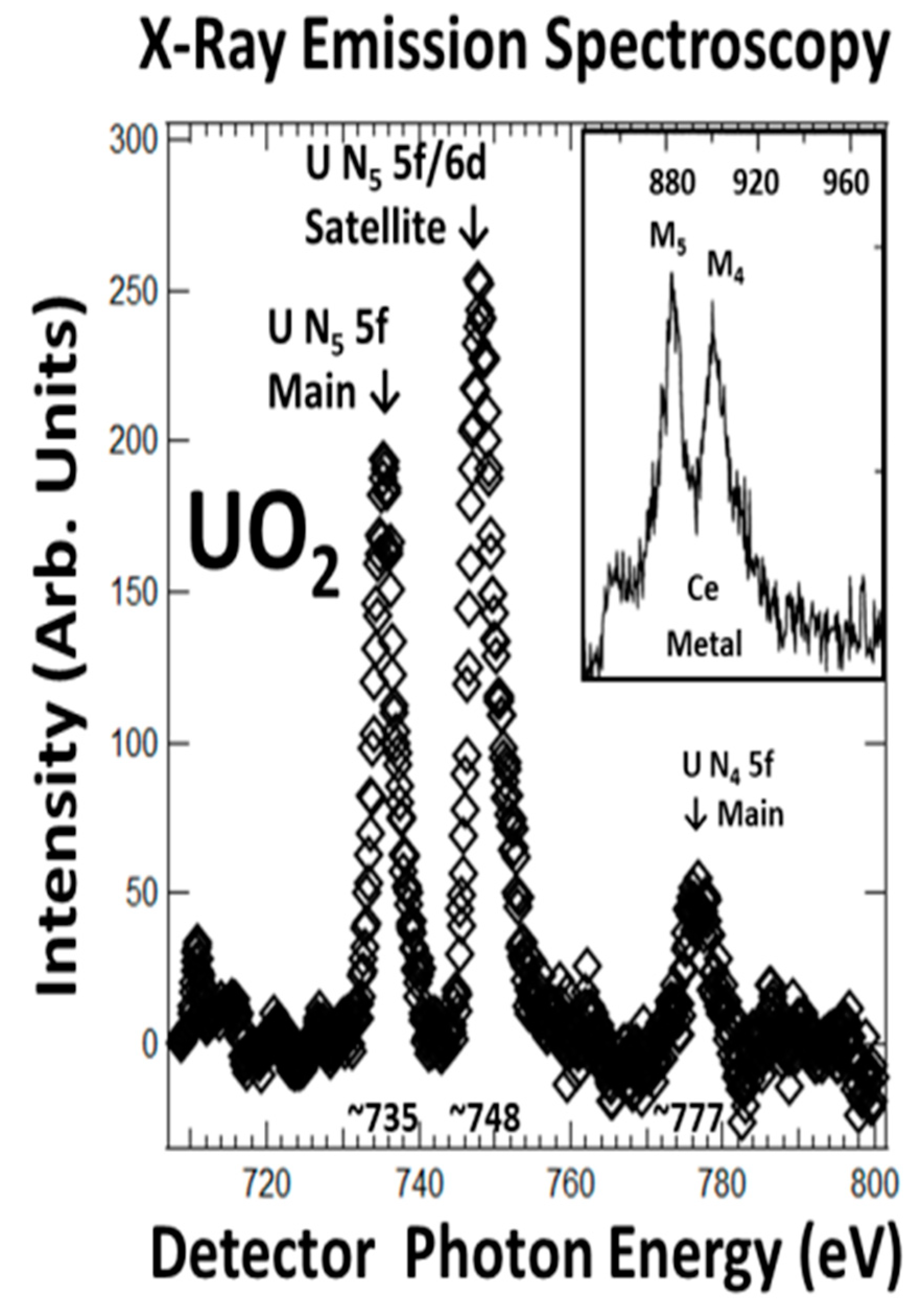
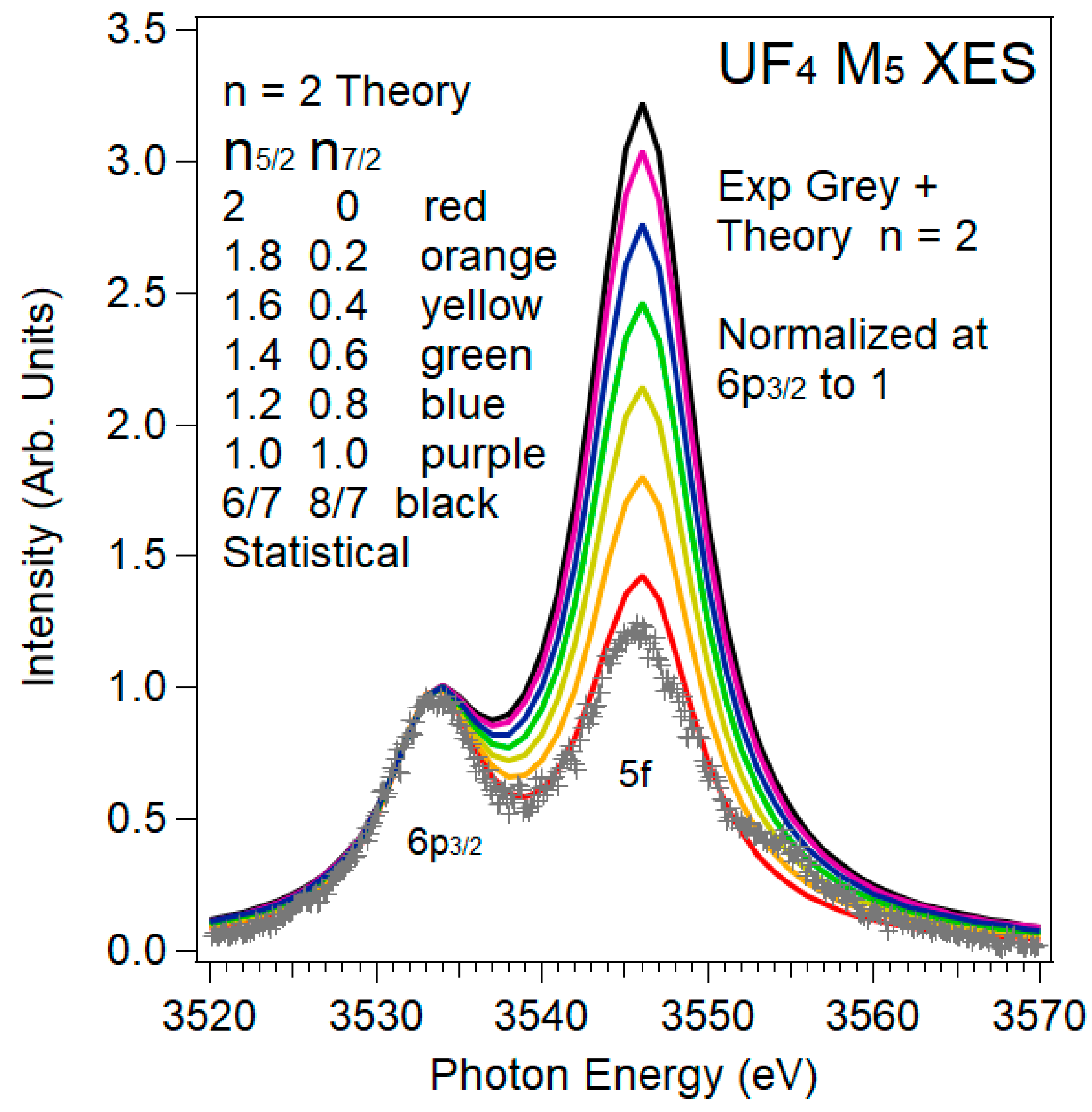
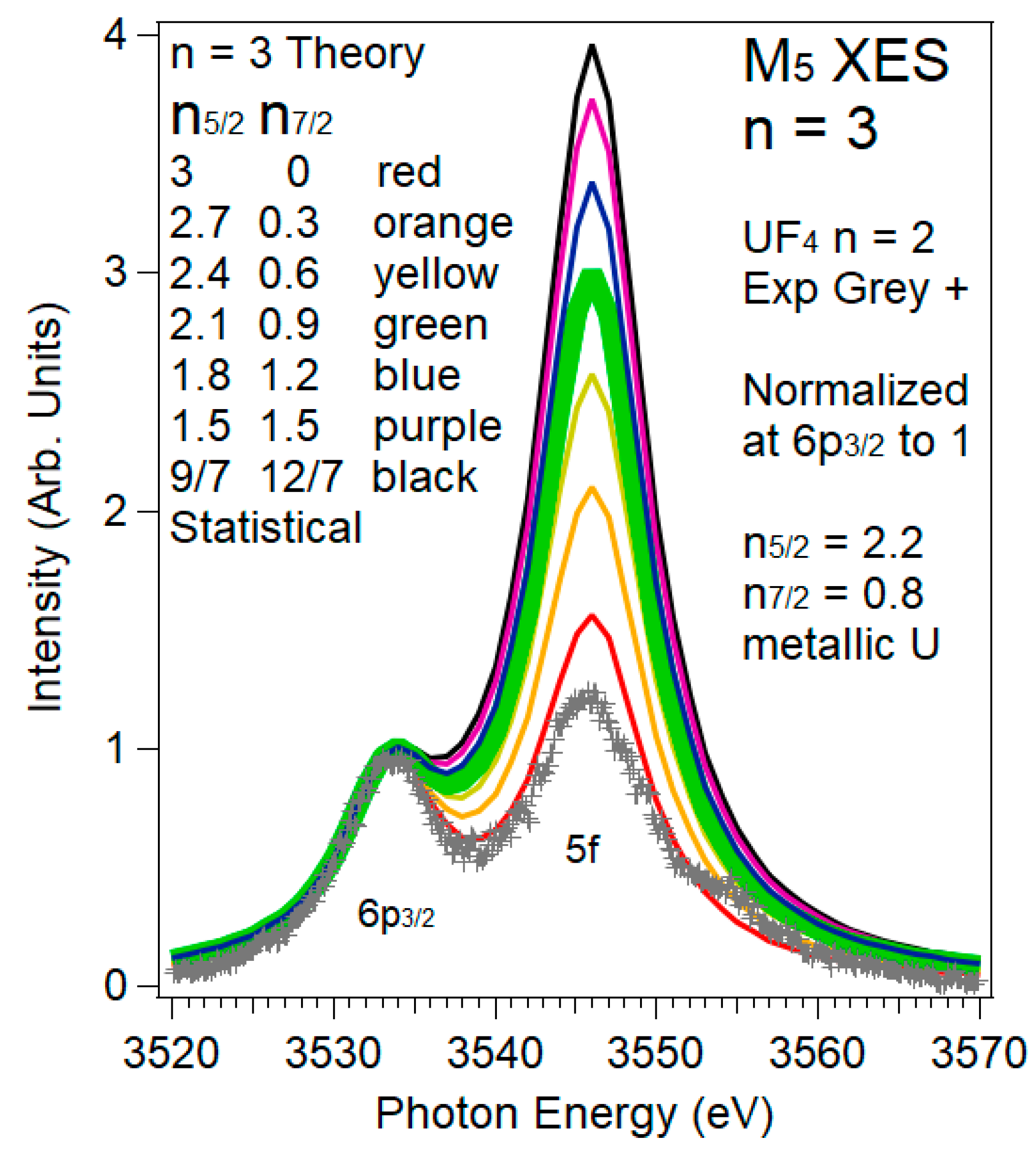
3.2. XES and 5f:5f Peak Ratios
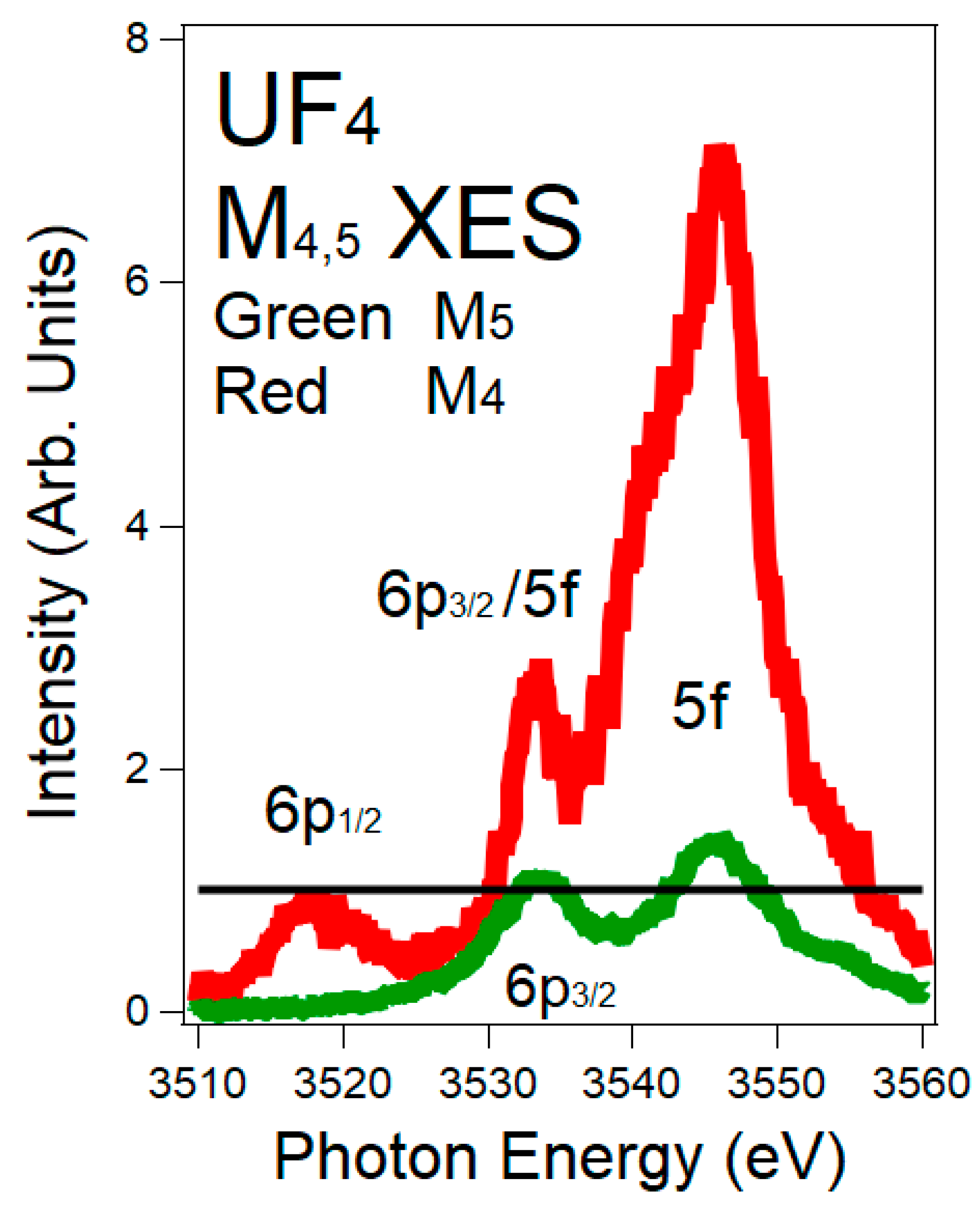
3.3. XES and 5f:6p Peak Ratios
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Tobin, J.G.; Shuh, D.K. Electron spectroscopy of the oxidation and aging of U and Pu. J. Electron. Spectrosc. Relat. Phenom. 2015, 205, 83. [Google Scholar] [CrossRef]
- Tobin, J.G.; Yu, S.-W.; Booth, C.H.; Tyliszczak, T.; Shuh, D.K.; van der Laan, G.; Sokaras, D.; Nordlund, D.; Weng, T.-C.; Bagus, P.S. Oxidation and crystal field effects in uranium. Phys. Rev. B 2015, 92, 035111. [Google Scholar] [CrossRef]
- Tobin, J.G.; Yu, S.-W.; Qiao, R.; Yang, W.L.; Booth, C.H.; Shuh, D.K.; Duffin, A.M.; Sokaras, D.; Nordlund, D.; Weng, T.-C. Covalency in oxidized uranium. Phys. Rev. B 2015, 92, 045130. [Google Scholar] [CrossRef]
- Booth, C.H.; Jiang, Y.; Wang, D.L.; Mitchell, J.N.; Tobash, P.H.; Bauer, E.D.; Wall, M.A.; Allen, P.G.; Sokaras, D.; Nordlund, D.; et al. Multiconfigurational nature of 5f orbitals in uranium and plutonium intermetallics. Proc. Natl. Acad. Sci. USA 2012, 109, 10205–10209. [Google Scholar] [CrossRef]
- Booth, C.H.; Medling, S.A.; Jiang, Y.U.; Bauer, E.D.; Tobash, P.H.; Mitchell, J.N.; Veirs, D.K.; Wall, M.A.; Allen, P.G.; Kas, J.J.; et al. Delocalization and occupancy effects of 5f orbitals in plutonium intermetallics using L3-edge resonant X-ray emission spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 2014, 194, 57. [Google Scholar] [CrossRef]
- Booth, C.H.; Medling, S.A.; Tobin, J.G.; Baumbach, R.E.; Bauer, E.D.; Sokaras, D.; Nordlund, D.; Weng, T.C. Probing 5f-state configurations in URu2Si2 with U LIII-edge resonant x-ray emission spectroscopy. Phys. Rev. B 2016, 94, 045121. [Google Scholar] [CrossRef]
- Kvashnina, K.O.; Butorin, S.M.; Martin, P.; Glatzel, P. Chemical State of Complex Uranium Oxides. Phys. Rev. Lett. 2013, 111, 253002. [Google Scholar] [CrossRef]
- Vitova, T.; Pidchenko, I.; Fellhauer, D.; Bagus, P.S.; Joly, Y.; Pruessmann, T.; Bahl, S.; Gonzalez-Robles, E.; Rothe, J.; Altmaier, M.; et al. The role of the 5f valence orbitals of early actinides in chemical bonding. Nat. Comm. 2017, 8, 16053. [Google Scholar] [CrossRef]
- Tobin, J.G.; Yu, S.-W. Orbital Specificity in the Unoccupied States of UO2 from Resonant Inverse Photoelectron Spectroscopy. Phys. Rev. Lett. 2011, 107, 167406. [Google Scholar] [CrossRef]
- Tobin, J.G.; Moore, K.T.; Chung, B.W.; Wall, M.A.; Schwartz, A.J.; van der Laan, G.; Kutepov, A.L. Competition between delocalization and spin-orbit splitting in the actinide 5f states. Phys. Rev. B 2005, 72, 085109. [Google Scholar] [CrossRef]
- van der Laan, G.; Moore, K.T.; Tobin, J.G.; Chung, B.W.; Wall, M.A.; Schwartz, A.J. Applicability of the Spin-Orbit Sum Rule for the Actinide 5f States. Phys. Rev. Lett. 2004, 93, 097401. [Google Scholar] [CrossRef]
- Moore, K.T.; Wall, M.A.; Schwartz, A.J.; Chung, B.W.; Shuh, D.K.; Schulze, R.K.; Tobin, J.G. Failure of Russell-Saunders Coupling in the 5f States of Plutonium. Phys. Rev. Lett. 2003, 90, 196404. [Google Scholar] [CrossRef]
- Veal, B.W.; Lam, D.J.; Diamond, H.; Hoekstra, H.R. X-ray photoelectron-spectroscopy study of oxides of the transuranium elements Np, Pu, Am, Cm, Bk, and Cf. Phys. Rev. B 1977, 15, 2929. [Google Scholar] [CrossRef]
- Veal, B.W.; Lam, D.J.; Carnall, W.T.; Hoekstra, H.R. X-ray photoemission spectroscopy study of hexavalent uranium compounds. Phys. Rev. B 1975, 12, 5651. [Google Scholar] [CrossRef]
- Veal, B.W.; Lam, D.J. X-ray photoelectron studies of thorium, uranium, and their dioxides. Phys. Rev. B 1974, 10, 4902. [Google Scholar] [CrossRef]
- Veal, B.W.; Lam, D.J. Bonding in uranium oxides: The role of 5f electrons. Phys. Lett. 1974, 49A, 466–468. [Google Scholar] [CrossRef]
- Naegele, J.R. Actinides and some of their alloys and compounds, Electronic Structure of Solids: Photoemission Spectra and Related Data, Landolt-Bornstein Numerical Data and Functional Relationships in Science and Technology, ed. A Goldmann Group III 1994, 23, 183–327. [Google Scholar]
- Baer, Y.; Lang, J.K. High-energy spectroscopic study of the occupied and unoccupied 5f and valence states in Th and U metals. Phys. Rev. B 1980, 21, 2060. [Google Scholar] [CrossRef]
- Baer, Y.; Schoenes, J. Electronic structure and Coulomb correlation energy UO2 single crystal. Solid State Commun. 1980, 33, 885. [Google Scholar] [CrossRef]
- Chauvet, G.; Baptist, R. Inverse photoemission study of uranium dioxide. Solid State Commun. 1982, 43, 793. [Google Scholar] [CrossRef]
- Kalkowski, G.; Kaindl, G.K.; Brewer, W.D.; Krone, W. Near-edge x-ray-absorption fine structure in uranium compounds. Phys. Rev. B 1987, 35, 2667–2677. [Google Scholar] [CrossRef]
- van der Laan, G.; Thole, B.T. X-ray-absorption sum rules in jj-coupled operators and ground-state moments of actinide ions. Phys. Rev. B 1996, 53, 14458. [Google Scholar] [CrossRef]
- Zachariasen, W.H. Metallic radii and electron configurations of the 5f−6d metals. J. Inorg. Nucl. Chem. 1973, 35, 3487. [Google Scholar] [CrossRef]
- Skriver, H.L.; Andersen, O.K.; Johansson, B. Calculated Bulk Properties of the Actinide Metals. Phys. Rev. Lett. 1978, 41, 42. [Google Scholar] [CrossRef]
- Ryzhkov, M.V.; Mirmelstein, A.; Yu, S.-W.; Chung, B.W.; Tobin, J.G. Probing actinide electronic structure through pu cluster calculations. Int. J. Quantum Chem. 2013, 113, 1957. [Google Scholar] [CrossRef]
- Ryzhkov, M.V.; Mirmelstein, A.; Delley, B.; Yu, S.-W.; Chung, B.W.; Tobin, J.G. The Effects of Mesoscale Confinement in Pu Clusters. J. Electron. Spectrosc. and Relat. Phenom. 2014, 194, 45. [Google Scholar] [CrossRef]
- Tobin, J.G. The apparent absence of chemical sensitivity in the X-ray absorption spectroscopy of uranium compounds. J. Electron. Spectrosc. Relat. Phenom. 2014, 194, 14. [Google Scholar] [CrossRef]
- Opeil, C.P.; Schulze, R.K.; Volz, H.M.; Lashley, J.C.; Manley, M.E.; Hults, W.L.; Hanrahan, R.J., Jr.; Smith, J.L.; Mihaila, B.; Blagoev, K.B.; et al. Angle-resolved photoemission and first-principles electronic structure of single-crystalline α-U(001). Phys. Rev. B 2007, 75, 045120. [Google Scholar] [CrossRef]
- Butterfield, M.T.; Tobin, J.G.; Teslich, N.E., Jr.; Bliss, R.A.; Wall, M.A.; McMahan, A.K.; Chung, B.W.; Schwartz, A.J.; Kutepov, A.L. Utilizing Nano-focussed Bremstrahlung Isochromat Spectroscopy (nBIS) to Determine the Unoccupied Electronic Structure of Pu. Matl. Res. Soc. Symp. Proc. 2006, 893, 95. [Google Scholar]
- Tobin, J.G.; Nowak, S.; Yu, S.-W.; Alonso-Mori, R.; Kroll, T.; Nordlund, D.; Weng, T.-C.; Sokaras, D. Observation of 5f intermediate coupling in uranium x-ray emission spectroscopy. J. Phys. Commun. 2020, 4, 015013. [Google Scholar] [CrossRef]
- Nowak, S.H.; Armenta, R.; Schwartz, C.P.; Gallo, A.; Abraham, B.; Garcia-Esparza, A.T.; Biasin, E.; Prado, A.; Maciel, A.; Zhang, D.; et al. A versatile Johansson-type tender x-ray emission spectrometer. Rev. Sci. Instrum. 2020, 91, 033101. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.G.; Nowak, S.; Booth, C.H.; Bauer, E.D.; Yu, S.-W.; Alonso-Mori, R.; Kroll, T.; Nordlung, D.; Weng, T.-C.; Sokaras, D. Separate Measurement of the 5f5/2 and 5f7/2 Unoccupied Density of States of UO2. J. Electron. Spectrosc. Relat. Phenom. 2019, 232, 100. [Google Scholar] [CrossRef]
- Yu, S.-W.; Tobin, J.G. Multi-electronic effects in uranium dioxide from X-ray Emission Spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 2013, 187, 15. [Google Scholar] [CrossRef]
- Tobin, J.G.; Yu, S.W.; Chung, B.W.; Waddill, G.D.; Duda, L.; Nordgren, J. Observation of strong resonant behavior in the inverse photoelectron spectroscopy of Ce oxide. Phys. Rev. B 2011, 83, 085104. [Google Scholar] [CrossRef]
- Courtesy of Emiliana Damian, L. Duda, and J. Nordgren. Please see Ref. [34].
- Gottfried, K. Quantum Mechanics, Volume I: Fundamentals; Benjamin-Cummings: Reading, MA, USA, 1966. [Google Scholar]
- Cohen-Tannoudji, C.; Diu, B.; Laloë, F. Quantum Mechanics; Wiley: New York, NY, USA, 1973; Volumes I & II. [Google Scholar]
- Fujimori, S.-I.; Kobata, M.; Takeda, Y.; Okane, T.; Saitoh, Y.; Fujimori, A.; Yamagami, H.; Haga, Y.; Yamamoto, E.; Onuki, Y. Manifestation of electron correlation effect in 5f states of uranium compounds revealed by 4d–5f resonant photoelectron spectroscopy. Phys. Rev. B 2019, 99, 035109. [Google Scholar] [CrossRef]
- Fujimori, S.-I. Band structures of 4f and 5f materials studied by angle-resolved photoelectron spectroscopy. J. Phys. Condens. Matter 2016, 28, 153002. [Google Scholar] [CrossRef]
- Fujimori, S.-I.; Ohkochi, T.; Kawasaki, I.; Yasui, A.; Takeda, Y.; Okane, T.; Satoh, Y.; Fujimori, A.; Haga, Y.; Yamamoto, E.; et al. Electronic Structure of Heavy Fermion Uranium Compounds Studied by Core-Level Photoelectron Spectroscopy. Phys. Soc. Jpn. 2012, 81, 014703. [Google Scholar] [CrossRef][Green Version]
- Tobin, J.G. Beyond spin-orbit: Probing electron correlation in the Pu 5f states using spin-resolved photoelectron spectroscopy. J. Alloys Cmpds. 2007, 444–445, 154–161. [Google Scholar] [CrossRef]
- Tobin, J.G. 5f states with spin-orbit and crystal field splittings. J. Vac. Sci. Technol. A 2019, 37, 031201. [Google Scholar] [CrossRef]
- Pollmann, F.; Zwicknagl, G. Spectral functions for strongly correlated 5f electrons. Phys. Rev. B 2006, 73, 035121. [Google Scholar] [CrossRef]
- Runge, E.; Fulde, P.; Efremov, D.V.; Hasselmann, N.; Zwicknagl, G. Approximative treatment of 5f-systems with partial localization due to intra-atomic correlations. Phys. Rev. B 2004, 69, 155110. [Google Scholar] [CrossRef]



| 5f5/2 Empty (Full) | 5f7/2 Empty (Full) | ||
|---|---|---|---|
| N5 (M5) | d5/2 Full (Empty) | ||
| N4 (M4) | d3/2 Full (Empty) | 0 |
| n | BR | n5/2 | n7/2 | N | N5/2 | N7/2 | N5/2/N | N7/2/N | |
|---|---|---|---|---|---|---|---|---|---|
| Int. Coupling, UO2 and UF4 | 2 | 0.68 | 1.96 | 0.04 | 12 | 4.04 | 7.96 | 0.337~0.34 | 0.663~0.66 |
| U metal | 3 | 0.68 | 2.23 | 0.77 | 11 | 3.77 | 7.23 | 0.343~0.34 | 0.657~0.66 |
| 5f5/2 Full (Empty) | 5f7/2 Full (Empty) | 5f5/2 Full | 5f7/2 Full | |||
|---|---|---|---|---|---|---|
| N5 (M5) | d5/2 Empty (Full) | d5/2 1 Hole | ||||
| N4 (M4) | d3/2 Empty (Full) | 0 | d3/2 1 Hole | 0 |
| 6p1/2 Full | 6p3/2 Full | 6p1/2 Full | 6p3/2 Full | |||
|---|---|---|---|---|---|---|
| M5 | 3d5/2 Empty | 0 | 12/5 | 3d5/2 1 Hole | 0 | 2/5 |
| M4 | 3d3/2 Empty | 4/3 | 4/15 | 3d3/2 1 Hole | 1/3 | 1/15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobin, J.G.; Nowak, S.; Yu, S.-W.; Alonso-Mori, R.; Kroll, T.; Nordlund, D.; Weng, T.-C.; Sokaras, D. Towards the Quantification of 5f Delocalization. Appl. Sci. 2020, 10, 2918. https://doi.org/10.3390/app10082918
Tobin JG, Nowak S, Yu S-W, Alonso-Mori R, Kroll T, Nordlund D, Weng T-C, Sokaras D. Towards the Quantification of 5f Delocalization. Applied Sciences. 2020; 10(8):2918. https://doi.org/10.3390/app10082918
Chicago/Turabian StyleTobin, J. G., S. Nowak, S.-W. Yu, R. Alonso-Mori, T. Kroll, D. Nordlund, T.-C. Weng, and D. Sokaras. 2020. "Towards the Quantification of 5f Delocalization" Applied Sciences 10, no. 8: 2918. https://doi.org/10.3390/app10082918
APA StyleTobin, J. G., Nowak, S., Yu, S.-W., Alonso-Mori, R., Kroll, T., Nordlund, D., Weng, T.-C., & Sokaras, D. (2020). Towards the Quantification of 5f Delocalization. Applied Sciences, 10(8), 2918. https://doi.org/10.3390/app10082918






