Evaluation for Simultaneous Removal of Anionic and Cationic Dyes onto Maple Leaf-Derived Biochar Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and methods
2.1. Chemicals
2.2. Preparation of BCs
2.3. Characterization of BCs
2.4. RSM Design and Statistical Analysis
2.5. Adsorption Experiments of Methylene Blue and Congo Red onto BCs
2.6. Effect of Adsorption of Dye Mixtures onto BC550
3. Results and Discussion
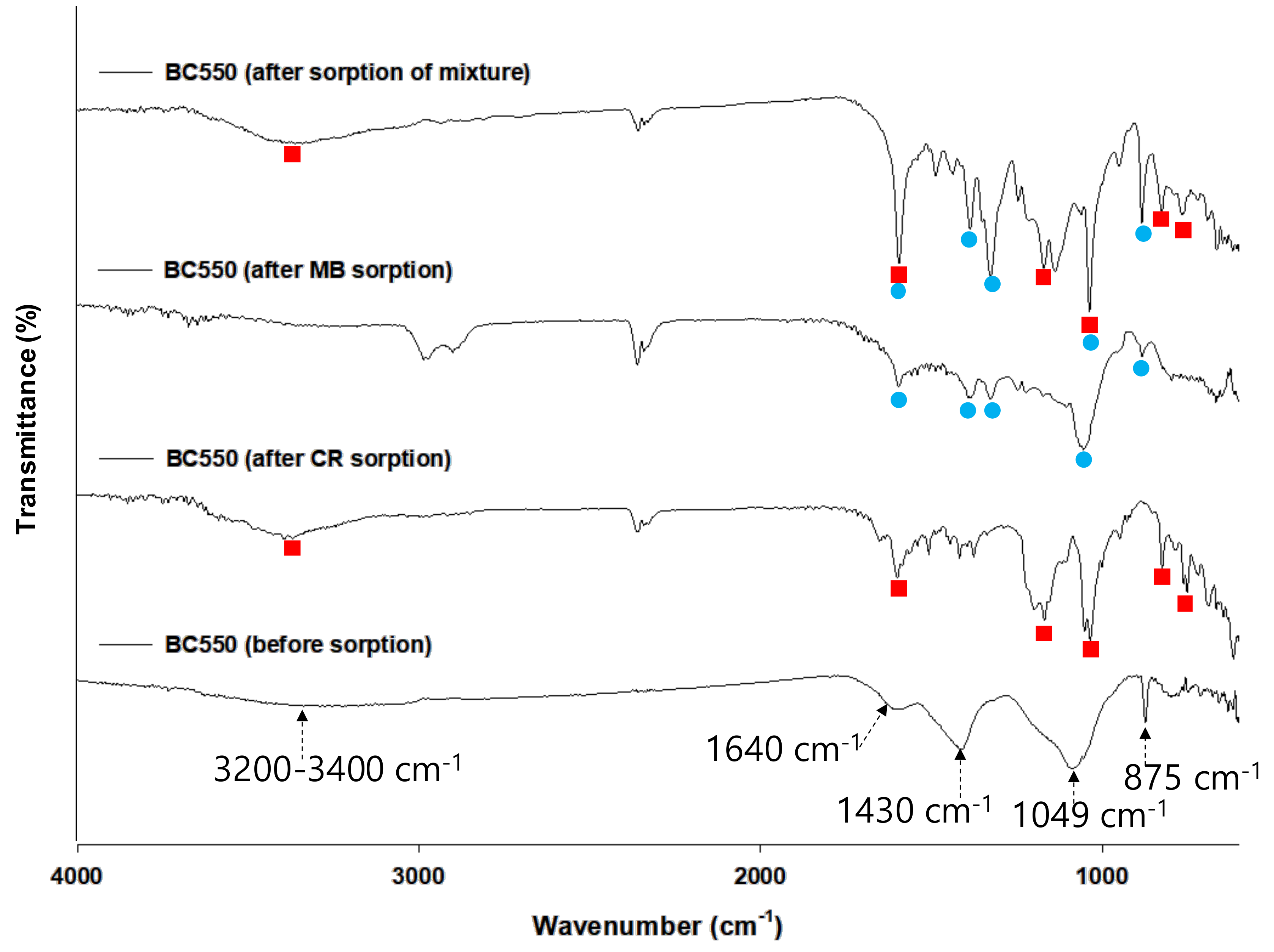
3.1. Characterization of Biochar
3.2. Effects of pH, Pyrolysis Temperature, and Adsorption Temperature on Adsorption Efficiency from RSM Results
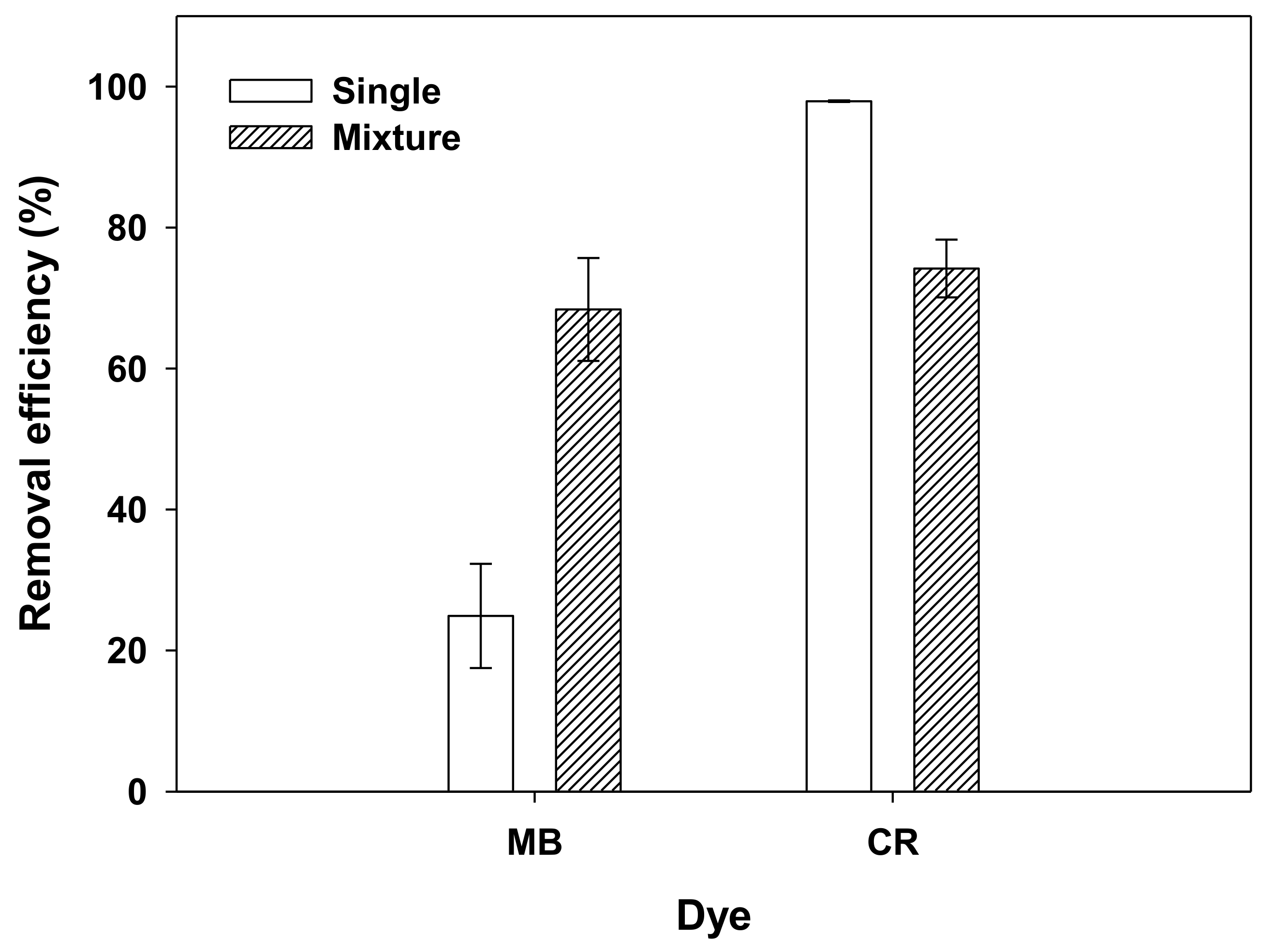
3.3. Effects of Single Dye and Dye Mixtures on Adsorption Efficiency at the Chosen Conditions
3.4. Possible Mechanisms for Dye Adsorption onto BC550
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; Zhao, J. Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption 2009, 15, 381–389. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Xian, Q.; Shen, F.; Wu, J.; Zhang, Y. Removal of congo red and methylene blue from aqueous solutions by vermicompost-derived biochars. PLoS ONE 2016, 11, e0154562. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Woo, S.H. Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour. Technol. 2017, 224, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.B.; Araújo, R.S.; Oton, L.F.; Oliveira, A.C.; Soares, J.M.; Medeiros, S.N.; Rodríguez-Castellón, E.; Rodríguez-Aguado, E. Acid Red 66 dye removal from aqueous solution by Fe/C-based composites: Adsorption, kinetics and thermodynamic studies. Materials 2020, 13, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedher, M.; Mossad, M.; El-Etriby, H.K. Enhancement of electrocoagulation process for dye removal using powdered residuals from water purification plants (PRWPP). Water Air Soil Pollut. 2017, 228, 293. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef]
- Ahmed, M.; Okoye, P.; Hummadi, E.; Hameed, B. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour. Technol. 2019, 278, 159–164. [Google Scholar] [CrossRef]
- Kim, J.E.; Bhatia, S.K.; Song, H.J.; Yoo, E.; Jeon, H.J.; Yoon, J.-Y.; Yang, Y.; Gurav, R.; Yang, Y.-H.; Kim, H.J. Adsorptive removal of tetracycline from aqueous solution by maple leaf-derived biochar. Bioresour. Technol. 2020, 123092. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Choi, T.-R.; Gurav, R.; Bhatia, S.K.; Park, Y.-L.; Kim, H.J.; Kan, E.; Yang, Y.-H. Adsorption behavior of tetracycline onto Spirulina sp.(microalgae)-derived biochars produced at different temperatures. Sci. Total Environ. 2020, 710, 136282. [Google Scholar] [CrossRef]
- Kharel, G.; Sacko, O.; Feng, X.; Morris, J.R.; Phillips, C.L.; Trippe, K.; Kumar, S.; Lee, J.W. Biochar surface oxygenation by ozonization for super high cation exchange capacity. ACS Sustain. Chem. Eng. 2019, 7, 16410–16418. [Google Scholar] [CrossRef]
- Huff, M.D.; Marshall, S.; Saeed, H.A.; Lee, J.W. Surface oxygenation of biochar through ozonization for dramatically enhancing cation exchange capacity. Bioresour. Bioprocess. 2018, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Park, Y.-L.; Park, J.Y.; Han, Y.-H.; Vyavahare, G.; Jadhav, J.; Song, H.-S.; Yang, P.; et al. Treatment of furazolidone contaminated water using banana pseudostem biochar engineered with facile synthesized magnetic nanocomposites. Bioresour. Technol. 2020, 297, 122472. [Google Scholar] [CrossRef]
- Li, G.; Zhu, W.; Zhang, C.; Zhang, S.; Liu, L.; Zhu, L.; Zhao, W. Effect of a magnetic field on the adsorptive removal of methylene blue onto wheat straw biochar. Bioresour. Technol. 2016, 206, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Sorptive removal of methylene blue from simulated wastewater using biochars derived from pulp and paper sludge. Environ. Technol. Inno. 2017, 8, 132–140. [Google Scholar] [CrossRef]
- Ayari, F.; Khelifi, S.; Othman, A.B.; Ayadi, M.T. Case studies focusing on the most successful advanced methods/approach for the treatment of nanomaterials in wastewater. In Emerging and Nanomaterial Contaminants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2019; pp. 311–353. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Kan, E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere 2019, 218, 741–748. [Google Scholar] [CrossRef]
- Usman, A.R.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrol. 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Cherifi, H.; Fatiha, B.; Salah, H. Kinetic studies on the adsorption of methylene blue onto vegetal fiber activated carbons. Appl. Surf. Sci. 2013, 282, 52–59. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mole Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Leng, L.; Yuan, X.; Huang, H.; Shao, J.; Wang, H.; Chen, X.; Zeng, G. Bio-char derived from sewage sludge by liquefaction: Characterization and application for dye adsorption. Appl. Surf. Sci. 2015, 346, 223–231. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environ. Monit. Assess. 2019, 191, 735. [Google Scholar] [CrossRef] [PubMed]
- David, N.; Anavi, D.; Milanovich, M.; Popowski, Y.; Frid, L.; Amir, E. Preparation and properties of electro-conductive fabrics based on polypyrrole: Covalent vs. non-covalent attachment. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Corfu, Greece, 29–31 May 2017; p. 032002. [Google Scholar] [CrossRef]



| Dye | Empirical Formula | Color Index | Molecular Weight (g/mol) | λmax (nm) | pKa | Molecular Structure |
|---|---|---|---|---|---|---|
| Methylene blue | C16H18ClN3S | 52015 | 319.85 | 670 | 2.6, 11.2 | 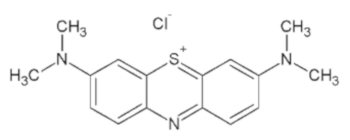 |
| Congo red | C32H22N6Na2O6S2 | 22120 | 696.66 | 500 | 4.1 | 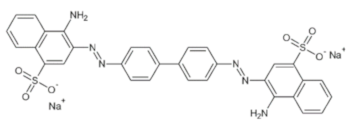 |
| Factor | Variables | Levels of Variables | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | Solution pH | 4 | 7 | 10 |
| X2 | Pyrolysis temperature (°C) | 350 | 550 | 750 |
| X3 | Adsorption temperature (°C) | 20 | 30 | 40 |
| Run | Independent Variables | Response (Adsorption Efficiency, %) | |||
|---|---|---|---|---|---|
| X1 | X2 (°C) | X3 (°C) | Methylene Blue | Congo Red | |
| pH | Pyrolysis Temperature | Adsorption Temperature | Experimental | Experimental | |
| 1 | 4 | 350 | 30 | 11.38 ± 0.54 | 52.61 ± 3.37 |
| 2 | 10 | 350 | 30 | 20.54 ± 0.75 | 15.01 ± 0.01 |
| 3 | 4 | 750 | 30 | 13.84 ± 1.64 | 98.70 ± 2.40 |
| 4 | 10 | 750 | 30 | 23.88 ± 4.40 | 23.27 ± 1.79 |
| 5 | 4 | 550 | 20 | 10.04 ± 0.06 | 16.19 ± 1.14 |
| 6 | 10 | 550 | 20 | 16.96 ± 8.43 | 14.84 ± 1.18 |
| 7 | 4 | 550 | 40 | 25.45 ± 0.78 | 98.75 ± 0.35 |
| 8 | 10 | 550 | 40 | 45.09 ± 4.11 | 97.79 ± 0.29 |
| 9 | 7 | 350 | 20 | 4.46 ± 2.07 | 4.38 ± 0.87 |
| 10 | 7 | 750 | 20 | 10.04 ± 0.06 | 16.36 ± 2.32 |
| 11 | 7 | 350 | 40 | 14.29 ± 1.81 | 6.24 ± 0.33 |
| 12 | 7 | 750 | 40 | 39.06 ± 1.32 | 98.87 ± 1.23 |
| 13 | 7 | 550 | 30 | 20.98 ± 0.02 | 97.99 ± 1.40 |
| 14 | 7 | 550 | 30 | 33.48 ± 0.73 | 97.77 ± 0.31 |
| 15 | 7 | 550 | 30 | 20.31 ± 3.80 | 97.99 ± 1.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-K.; Gurav, R.; Kim, H.J.; Yang, Y.-H.; Bhatia, S.K. Evaluation for Simultaneous Removal of Anionic and Cationic Dyes onto Maple Leaf-Derived Biochar Using Response Surface Methodology. Appl. Sci. 2020, 10, 2982. https://doi.org/10.3390/app10092982
Choi Y-K, Gurav R, Kim HJ, Yang Y-H, Bhatia SK. Evaluation for Simultaneous Removal of Anionic and Cationic Dyes onto Maple Leaf-Derived Biochar Using Response Surface Methodology. Applied Sciences. 2020; 10(9):2982. https://doi.org/10.3390/app10092982
Chicago/Turabian StyleChoi, Yong-Keun, Ranjit Gurav, Hyung Joo Kim, Yung-Hun Yang, and Shashi Kant Bhatia. 2020. "Evaluation for Simultaneous Removal of Anionic and Cationic Dyes onto Maple Leaf-Derived Biochar Using Response Surface Methodology" Applied Sciences 10, no. 9: 2982. https://doi.org/10.3390/app10092982
APA StyleChoi, Y.-K., Gurav, R., Kim, H. J., Yang, Y.-H., & Bhatia, S. K. (2020). Evaluation for Simultaneous Removal of Anionic and Cationic Dyes onto Maple Leaf-Derived Biochar Using Response Surface Methodology. Applied Sciences, 10(9), 2982. https://doi.org/10.3390/app10092982








