Performance of a Trickling-Bed Biocathode Microbial Electrochemical System Treating Domestic Wastewater and Functional Microbial Community Characteristics
Abstract
1. Introduction
2. Materials and Methods
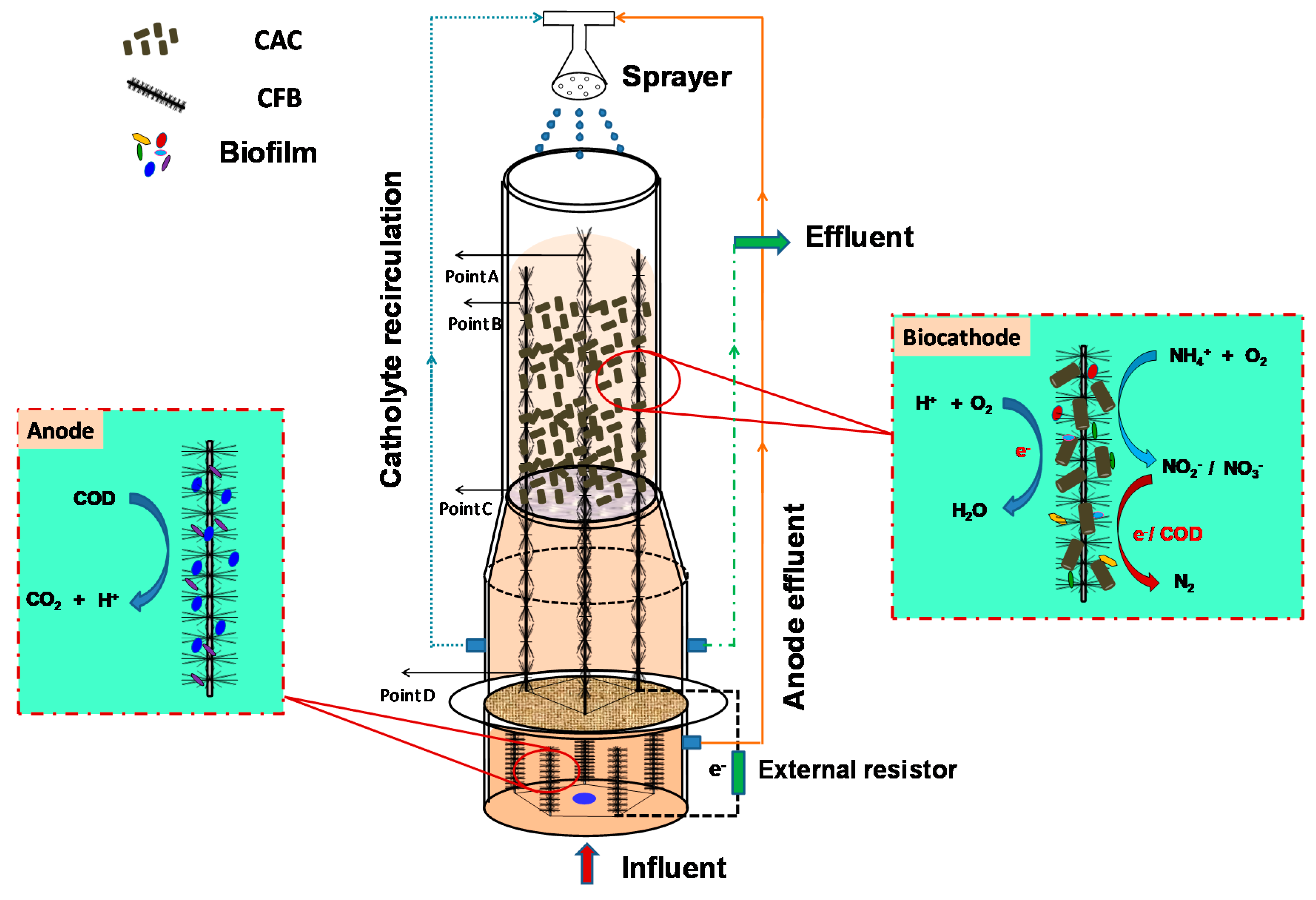
2.1. Configuration of TB-MES
2.2. Inoculation and Operation
2.3. Measurements and Calculation
2.4. Microbial Community Analysis
3. Results
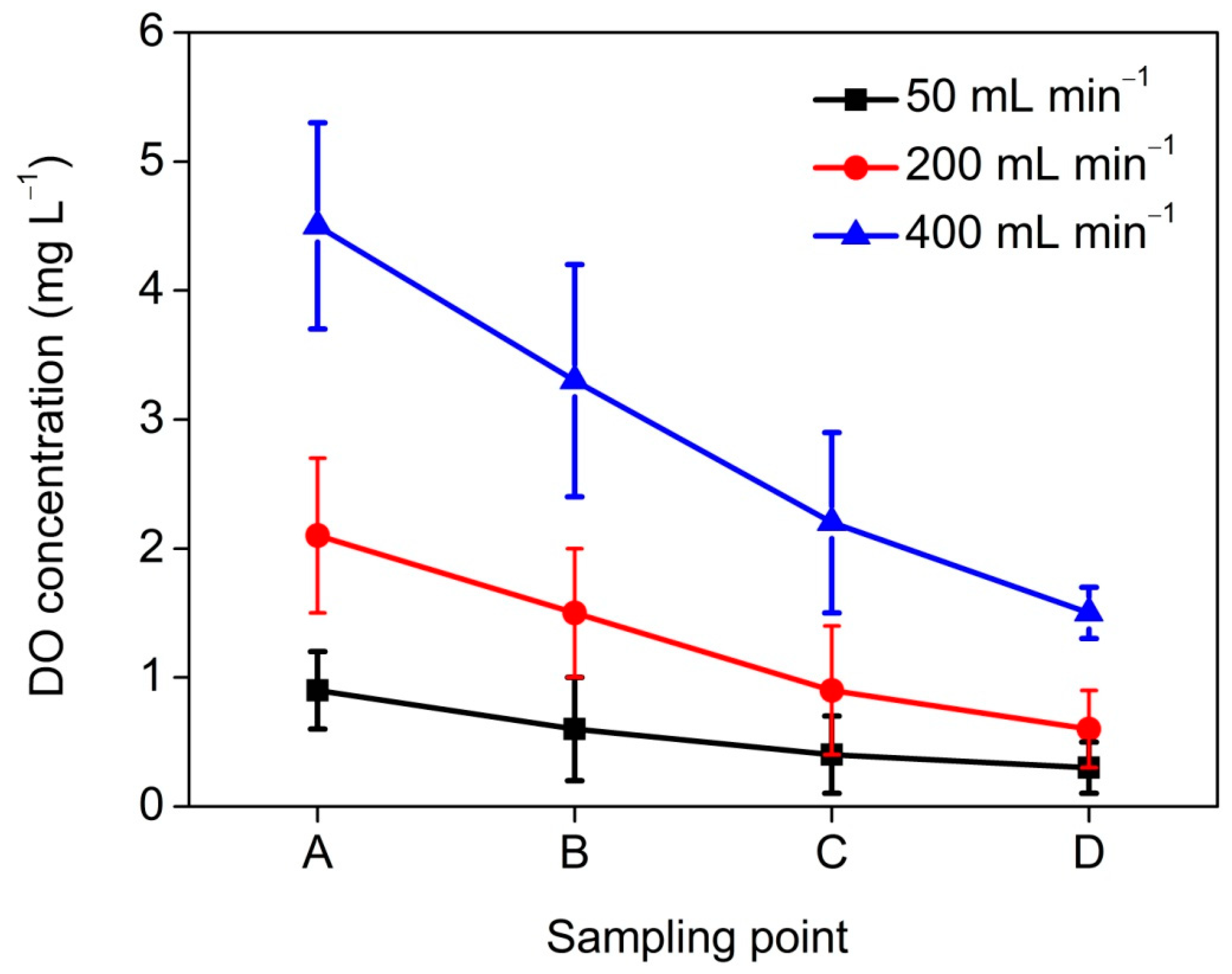
3.1. Effects of Catholyte Recirculation Rate
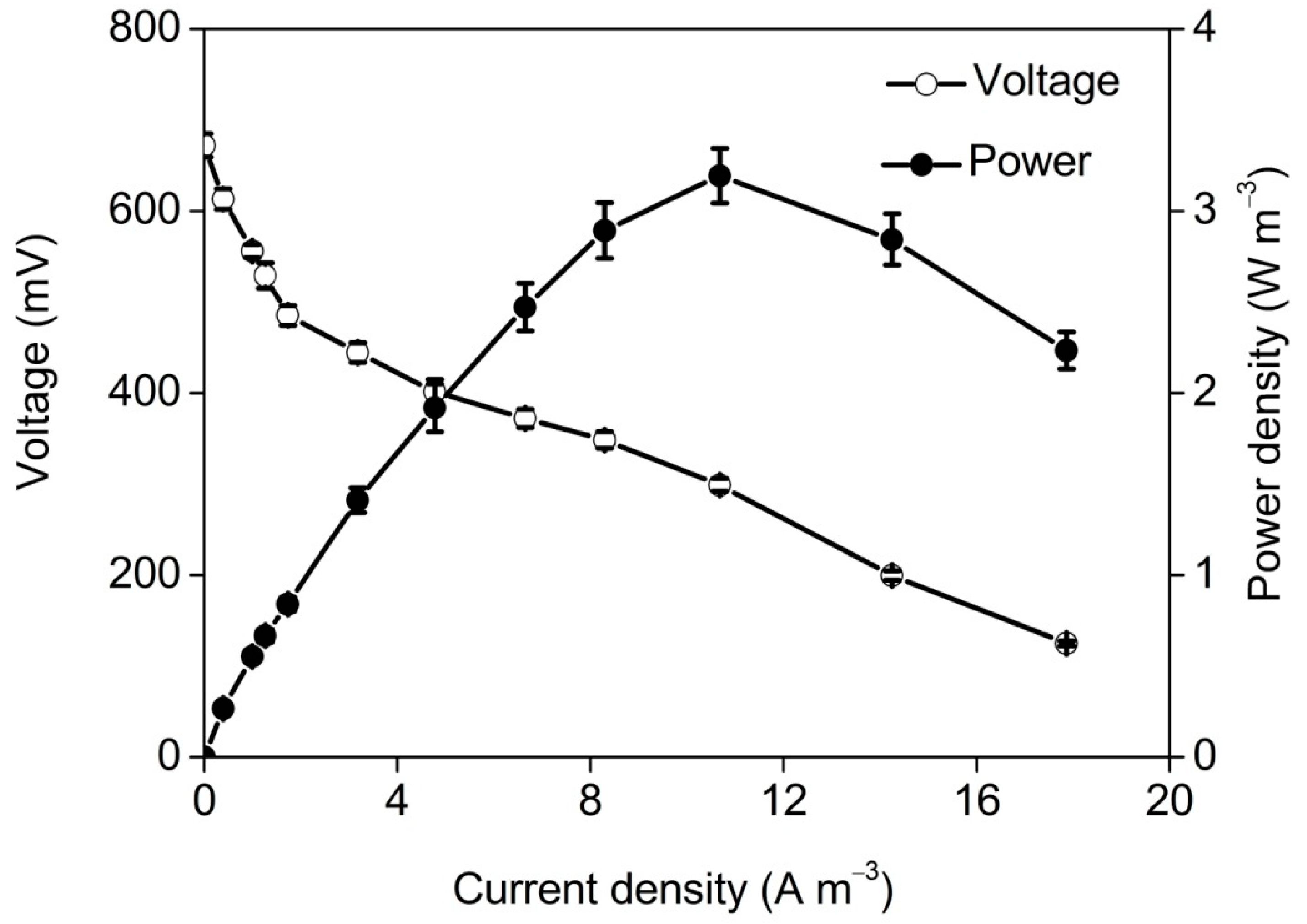
3.2. Performance of TB-MES with Domestic Wastewater
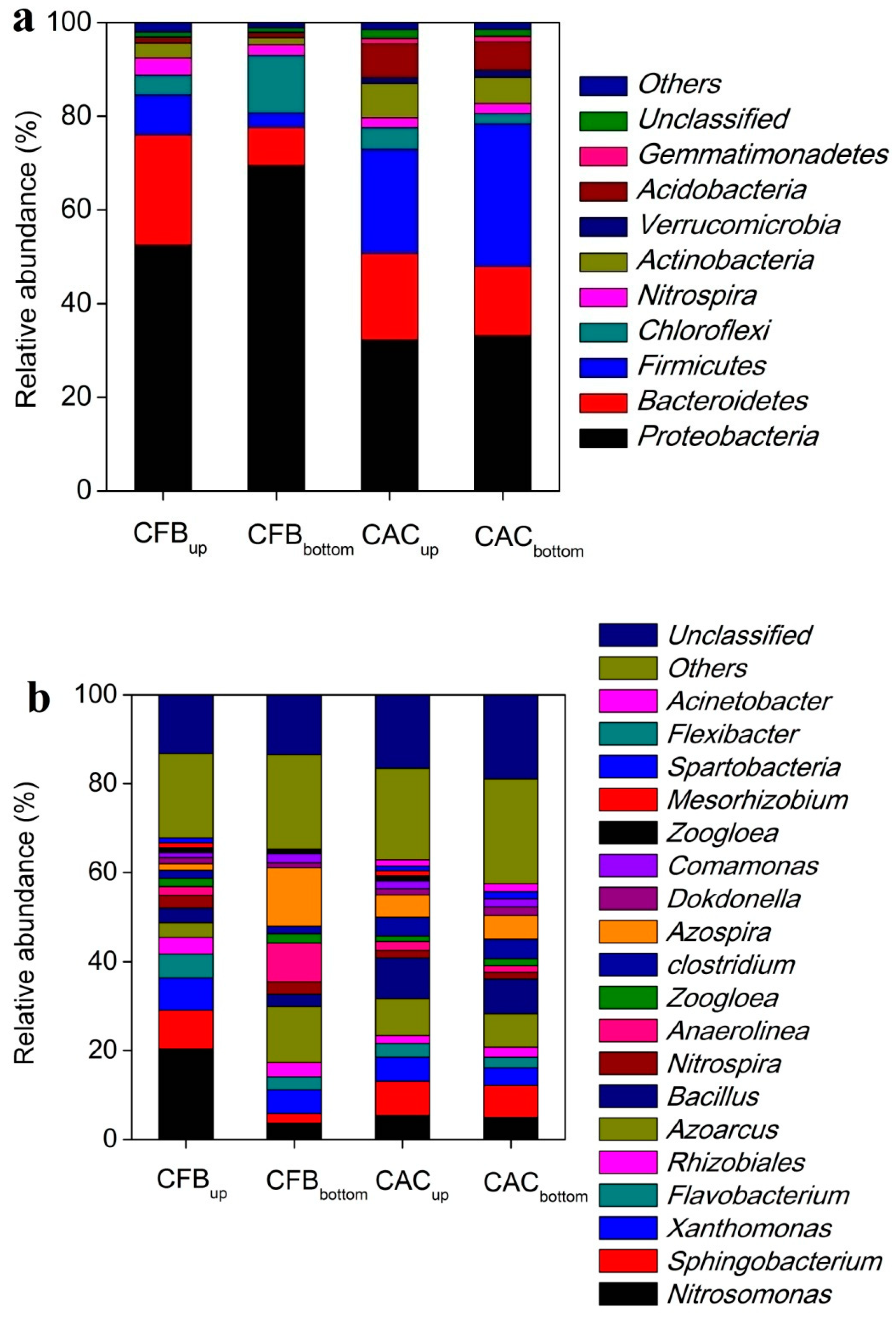
3.3. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mccarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer-Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Ni, B.J. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 2018, 639, 910–920. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Abdallah, M.; Feroz, S.; Alani, S.; Sayed, E.T.; Shanableh, A. Continuous and scalable applications of microbial fuel cells: A critical review. Rev. Environ. Sci. Biotechnol. 2019, 18, 543–578. [Google Scholar] [CrossRef]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Clauwaert, P.; van der Ha, D.; Boon, N.; Verbeken, K.; Verhaege, M.; Rabaey, K.; Verstraete, W. Open air biocathode enables effective electricity generation with microbial fuel cells. Environ. Sci. Technol. 2007, 41, 7564–7569. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Regan, J.M.; Quan, X. Electron transfer mechanisms, new applications, and performance of biocathode microbial fuel cells. Bioresour. Technol. 2011, 102, 316–323. [Google Scholar] [CrossRef]
- Song, H.-L.; Zhu, Y.; Li, J. Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells—A mini review. Arab. J. Chem. 2019, 12, 2236–2243. [Google Scholar] [CrossRef]
- Clauwaert, P.; Rabaey, K.; Aelterman, P.; De Schamphelaire, L.; Ham, T.H.; Boeckx, P.; Boon, N.; Verstraete, W. Biological denitrification in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3354–3360. [Google Scholar] [CrossRef]
- Desloover, J.; Puig, S.; Virdis, B.; Clauwaert, P.; Boeckx, P.; Verstraete, W.; Boon, N. Biocathodic nitrous oxide removal in bioelectrochemical systems. Environ. Sci. Technol. 2011, 45, 10557–10566. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.R.; Kuntke, P.; Jeremiasse, A.W.; Sleutels, T.H.J.A.; Buisman, C.J.N.; Ter Heijne, A. Bioelectrochemical systems for nitrogen removal and recovery from wastewater. Environ. Sci. Water Res. Technol. 2015, 1, 22–33. [Google Scholar] [CrossRef]
- Virdis, B.; Rabaey, K.; Yuan, Z.; Keller, J. Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res. 2008, 42, 3013–3024. [Google Scholar] [CrossRef]
- Virdis, B.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification and carbon removal in microbial fuel cells. Water Res. 2010, 44, 2970–2980. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- He, Z.; Shao, H.; Angenent, L.T. Increased power production from a sediment microbial fuel cell with a rotating cathode. Biosens. Bioelectron. 2007, 22, 3252–3255. [Google Scholar] [CrossRef]
- He, Z.; Kan, J.; Wang, Y.; Huang, Y.; Mansfeld, F.; Nealson, K.H. Electricity production coupled to ammonium in a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 3391–3397. [Google Scholar] [CrossRef]
- Sayess, R.R.; Saikaly, P.E.; El-Fadel, M.; Li, D.; Semerjian, L. Reactor performance in terms of COD and nitrogen removal and bacterial community structure of a three-stage rotating bioelectrochemical contactor. Water Res. 2013, 47, 881–894. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; He, W.; Qu, Y.; Li, D.; Feng, Y. Energy-positive nitrogen removal from reject water using a tide-type biocathode microbial electrochemical system. Bioresour. Technol. 2016, 222, 317–325. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; He, W.; Qu, Y.; Li, D.; Jiang, Q.; Feng, Y. Enhanced power generation of oxygen-reducing biocathode with an alternating hydrophobic and hydrophilic surface. ACS Appl. Mater. Interfaces 2016, 8, 31995–32003. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Shih, J.-C.; Lin, C.-W. Continuous production of power using microbial fuel cells with integrated biotrickling filter for ethyl acetate-contaminated air stream treatment. Int. J. Hydrog. Energy 2016, 41, 21945–21954. [Google Scholar] [CrossRef]
- Lin, C.-W.; Tsao, C.-Y.; Jiang, S.-L.; Liu, S.-H. Enhanced gaseous ethyl acetate degradation and power generation by a bioelectrochemical system. Chem. Eng. J. 2018, 344, 270–276. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, H.-H.; Wen, S.; Lin, C.-W. Performance of trickling bed microbial fuel cell treating isopropyl alcohol vapor: Effects of shock-load and shut-down episodes. Chemosphere 2019, 224, 168–175. [Google Scholar] [CrossRef]
- Bao, T.; Chen, T.; Tan, J.; Wille, M.-L.; Zhu, D.; Chen, D.; Xi, Y. Synthesis and performance of iron oxide-based porous ceramsite in a biological aerated filter for the simultaneous removal of nitrogen and phosphorus from domestic wastewater. Sep. Purif. Technol. 2016, 167, 154–162. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [CrossRef]
- Ahn, Y.; Logan, B.E. A multi-electrode continuous flow microbial fuel cell with separator electrode assembly design. Appl. Microbiol. Biotechnol. 2012, 93, 2241–2248. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Ren, N.; Logan, B.E. Syntrophic interactions drive the hydrogen production from glucose at low temperature in microbial electrolysis cells. Bioresour. Technol. 2012, 124, 68–76. [Google Scholar] [CrossRef]
- Pham, H.T.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Van Oostveldt, P.; Verbeken, K.; Rabaey, K.; Verstraete, W. High shear enrichment improves the performance of the anodophilic microbial consortium in a microbial fuel cell. Microb. Biotechnol. 2008, 1, 487–496. [Google Scholar] [CrossRef]
- Zhang, F.; Jacobson, K.S.; Torres, P.; He, Z. Effects of anolyte recirculation rates and catholytes on electricity generation in a litre-scale upflow microbial fuel cell. Energy Environ. Sci. 2010, 3, 1347–1352. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Zhou, L. Effect of carbon source, C/N ratio, nitrate and dissolved oxygen concentration on nitrite and ammonium production from denitrification process by Pseudomonas stutzeri D6. Bioresour. Technol. 2012, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Silverstein, J. Oxygen inhibition of activated sludge denitrification. Water Res. 1999, 33, 1925–1937. [Google Scholar] [CrossRef]
- Nam, J.Y.; Kim, H.-W.; Lim, K.-H.; Shin, H.-S.; Logan, B.E. Variation of power generation at different buffer types and conductivities in single chamber microbial fuel cells. Biosens. Bioelectron. 2010, 25, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, J.; Samimi, A. Steady state electric power generation in up-flow microbial fuel cell using the estimated time span method for bacteria growth domestic wastewater. Biomass Bioenergy 2012, 45, 65–76. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, Z.; Grimaud, J.; Hurst, J.; He, Z. In situ investigation of tubular microbial fuel cells deployed in an aeration tank at a municipal wastewater treatment plant. Bioresour. Technol. 2013, 136, 316–321. [Google Scholar] [CrossRef]
- Liu, X.W.; Wang, Y.-P.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zeng, R.J.; Li, F.; Dong, F.; Wang, S.-G.; Tong, Z.-H. Integration of a microbial fuel cell with activated sludge process for energy-saving wastewater treatment: Taking a sequencing batch reactor as an example. Biotechnol. Bioeng. 2011, 108, 1260–1267. [Google Scholar] [CrossRef]
- Xia, X.; Tokash, J.C.; Zhang, F.; Liang, P.; Logan, B.E. Oxygen-reducing biocathodes operating with passive oxygen transfer in microbial fuel cells. Environ. Sci. Technol. 2013, 47, 2085–2091. [Google Scholar] [CrossRef]
- Kim, J.R.; Zuo, Y.; Regan, J.M.; Logan, B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol. Bioeng. 2008, 99, 1120–1127. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Virdis, B.; Clauwaert, P.; Read, S.T.; Daly, R.A.; Boon, N.; Piceno, Y.; Andersen, G.L.; Coates, J.D.; Rabaey, K. Bacterial community structure corresponds to performance during cathodic nitrate reduction. ISME J. Emultidisciplinary J. Microb. Ecol. 2010, 4, 1443–1455. [Google Scholar] [CrossRef]
- Li, C.; Ding, L.; Cui, H.; Zhang, L.; Xu, K.; Ren, H. Application of conductive polymers in biocathode of microbial fuel cells and microbial community. Bioresour. Technol. 2012, 116, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, J.; Li, B.; Wang, J.; Chu, P. Performance and microbial community in the biocathode of microbial fuel cells under different dissolved oxygen concentrations. J. Electroanal. Chem. 2019, 833, 433–440. [Google Scholar] [CrossRef]
- Chung, K.; Fujiki, I.; Okabe, S. Effect of formation of biofilms and chemical scale on the cathode electrode on the performance of a continuous two-chamber microbial fuel cell. Bioresour. Technol. 2011, 102, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Hurek, T. Reassessment of the taxonomic structure of the diazotrophic genus Azoarcus sensu lato and description of three new genera and new species, Azovibrio restrictus gen. nov., sp. nov., Azospira oryzae gen. nov., sp. nov. and Azonexus fungiphilus gen. nov., sp. Int. J. Syst. Evol. Microbiol. 2000, 50, 649–659. [Google Scholar] [CrossRef]
- Narihiro, T.; Sekiguchi, Y. Microbial communities in anaerobic digestion processes for waste and wastewater treatment: A microbiological update. Curr. Opin. Biotechnol. 2007, 18, 273–278. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, J.; Liang, P.; Huang, X. Microbial community analysis in biocathode microbial fuel cells packed with different materials. Amb Express 2012, 2, 21. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]



| CFBup | CFBbottom | CACup | CACbottom | |
|---|---|---|---|---|
| Sequencing reads OUT | 14706 1120 | 18726 1011 | 34133 1808 | 32657 1775 |
| Shanon | 3.373733 | 3.020138 | 4.785163 | 4.323434 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Miao, Z.; Chao, L.; Li, Y.; Wang, G. Performance of a Trickling-Bed Biocathode Microbial Electrochemical System Treating Domestic Wastewater and Functional Microbial Community Characteristics. Appl. Sci. 2020, 10, 2989. https://doi.org/10.3390/app10092989
Wang H, Miao Z, Chao L, Li Y, Wang G. Performance of a Trickling-Bed Biocathode Microbial Electrochemical System Treating Domestic Wastewater and Functional Microbial Community Characteristics. Applied Sciences. 2020; 10(9):2989. https://doi.org/10.3390/app10092989
Chicago/Turabian StyleWang, Haiman, Zhuang Miao, Lei Chao, Yafeng Li, and Guiqiang Wang. 2020. "Performance of a Trickling-Bed Biocathode Microbial Electrochemical System Treating Domestic Wastewater and Functional Microbial Community Characteristics" Applied Sciences 10, no. 9: 2989. https://doi.org/10.3390/app10092989
APA StyleWang, H., Miao, Z., Chao, L., Li, Y., & Wang, G. (2020). Performance of a Trickling-Bed Biocathode Microbial Electrochemical System Treating Domestic Wastewater and Functional Microbial Community Characteristics. Applied Sciences, 10(9), 2989. https://doi.org/10.3390/app10092989




