Tympanic Membrane Collagen Expression by Dynamically Cultured Human Mesenchymal Stromal Cell/Star-Branched Poly(ε-Caprolactone) Nonwoven Constructs
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Scaffold Preparation
2.2.2. Scaffold Characterization
2.2.3. Scaffold Sterilization
2.2.4. Ethical Statement
2.2.5. Cell Isolation and Seeding on the Scaffolds
2.2.6. Construct Differentiation
2.2.7. Neutral Red Assay
2.2.8. Gene Expression Analysis
2.2.9. Sample Preparation for Morphological and Histological Analysis
2.2.10. CLSM Analysis
2.2.11. Immunohistochemistry
2.2.12. Computational Analysis of Bioreactor Fluid Dynamics
2.2.13. Statistical Analysis
3. Results
3.1. *PCL Scaffold Characterization
3.2. Viability of hMSC/*PCL Constructs
3.3. Gene Expression Evaluation in the hMSC/*PCL Constructs
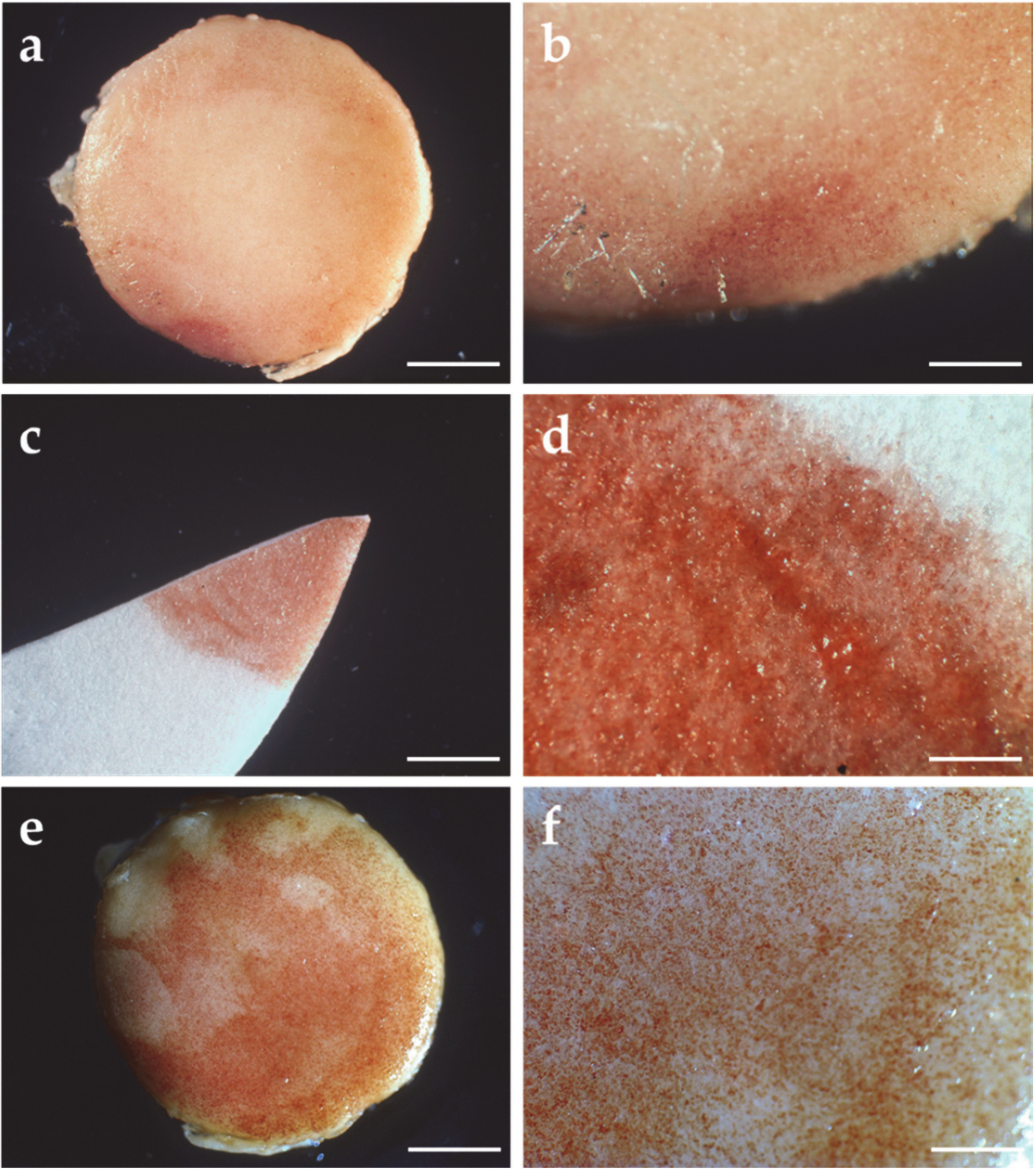
3.4. Morphological Analysis of the *PCL/MSC Constructs
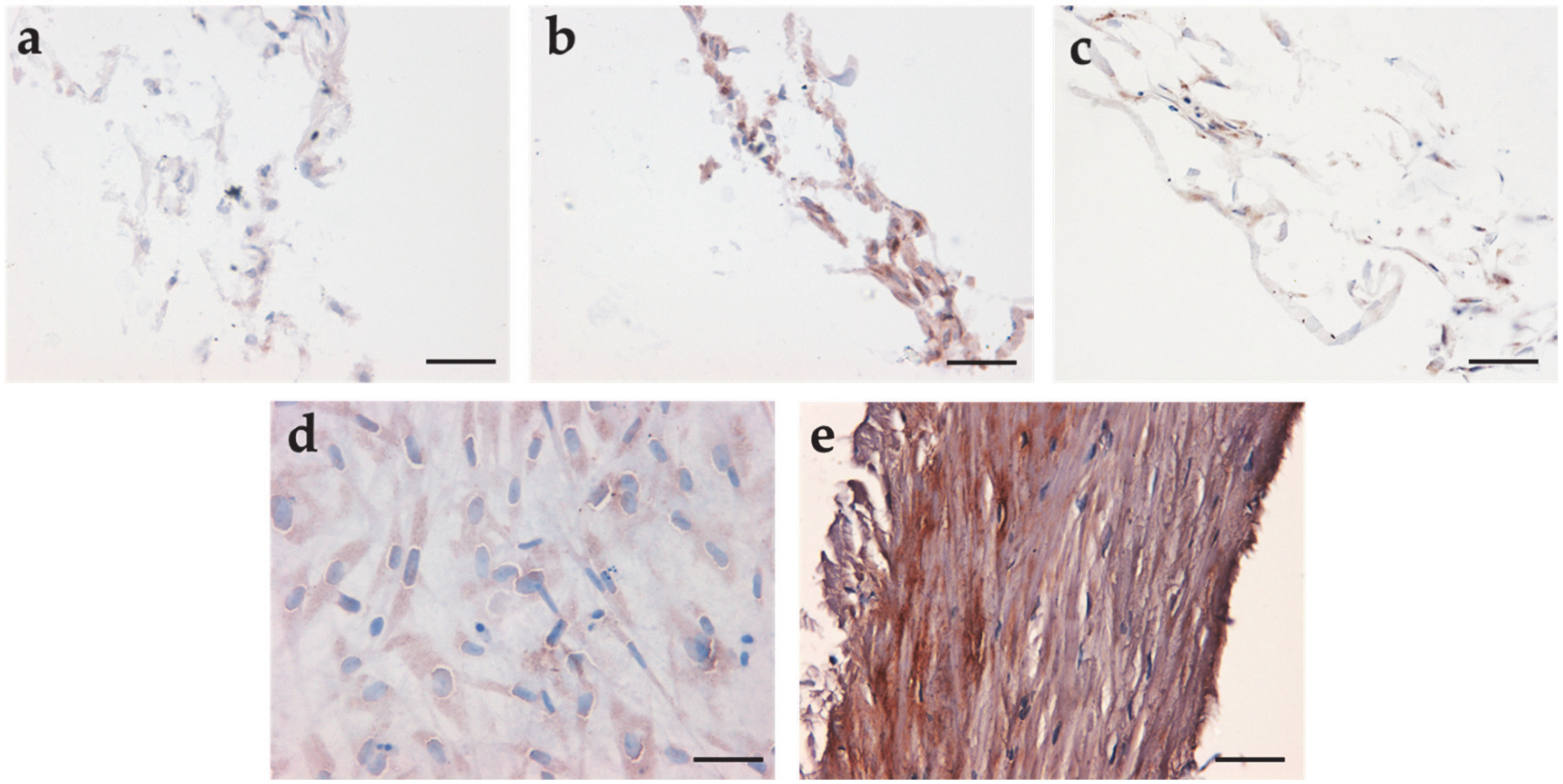
3.5. Immunohistochemical Analysis
3.6. Bioreactor Analysis
3.7. Viability Assay and Immunohistochemical Analysis in the hMSC/HA/*PCL Constructs
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lim, D.J. Structure and function of the tympanic membrane: A review. Acta Oto Rhino Laryngol. Belg. 1995, 49, 101–115. [Google Scholar]
- Milazzo, M.; Fallah, E.; Carapezza, M.; Kumar, N.S.; Lei, J.H.; Olson, E.S. The path of a click stimulus from ear canal to umbo. Hear. Res. 2017, 346, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallo, M. Embryological and genetic aspects of middle ear development. Int. J. Dev. Boil. 1998, 42, 11–22. [Google Scholar]
- Lim, D.J. Tympanic membrane: Electron microscopic observation part I: Pars tensa. Acta Oto Laryngol. 1968, 66, 181–198. [Google Scholar] [CrossRef]
- Lim, D.J. Human tympanic membrane: An ultrastructural observation. Acta Oto Laryngol. 1970, 70, 176–186. [Google Scholar] [CrossRef]
- Shimada, T.; Lim, D.J. The fiber arrangement of the human tympanic membrane. A scanning electron microscopic observation. Ann. Otol. Rhinol. Laryngol. 1971, 80, 210–217. [Google Scholar] [CrossRef]
- Fay, J.P.; Puria, S.; Steele, C.R. The discordant eardrum. Proc. Natl. Acad. Sci. USA 2006, 103, 19743–19748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutsson, J.; Bagger-Sjöbäck, D.; Von Unge, M. Collagen type distribution in the healthy human tympanic membrane. Otol. Neurotol. 2009, 30, 1225–1229. [Google Scholar] [CrossRef]
- Stenfeldt, K.; Johansson, C.; Hellström, S. The collagen structure of the tympanic membrane: Collagen types I, II, and III in the healthy tympanic membrane, during healing of a perforation, and during infection. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 293. [Google Scholar] [CrossRef] [Green Version]
- Broekaert, D. The tympanic membrane: A biochemical updating of structural components. Acta Oto Rhino Laryngol. Belg. 1995, 49, 127–137. [Google Scholar]
- Mallo, M. Formation of the middle ear: Recent progress on the developmental and molecular mechanisms. Dev. Boil. 2001, 231, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Slepecky, N.B.; Savage, J.E.; Yoo, T.J. Localization of type II, IX and V collagen in the inner ear. Acta Oto Laryngol. 1992, 112, 611–617. [Google Scholar] [CrossRef]
- Hardman, J.; Muzaffar, J.; Nankivell, P.; Coulson, C. Tympanoplasty for chronic tympanic membrane perforation in children: Systematic review and meta-analysis. Otol. Neurotol. 2015, 36, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Lerut, B.; Pfammatter, A.; Moons, J.; Linder, T. Functional correlations of tympanic membrane perforation size. Otol. Neurotol. 2012, 33, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalali, M.M.; Motasaddi, M.; Kouhi, A.; Dabiri, S.; Soleimani, R. Comparison of cartilage with temporalis fascia tympanoplasty: A meta-analysis of comparative studies. Laryngoscope 2016, 127, 2139–2148. [Google Scholar] [CrossRef]
- Lee, P.; Kelly, G.; Mills, R.P. Myringoplasty: Does the size of the perforation matter? Clin. Otolaryngol. Allied Sci. 2002, 27, 331–334. [Google Scholar] [CrossRef]
- Teh, B.M.; Marano, R.J.; Shen, Y.; Friedland, P.L.; Dilley, R.J.; Atlas, M.D. Tissue Engineering of the Tympanic Membrane. Tissue Eng. Part B Rev. 2013, 19, 116–132. [Google Scholar] [CrossRef]
- Villar-Fernandez, M.A.; Lopez-Escamez, J.A. Outlook for tissue engineering of the tympanic membrane. Audiol. Res. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Mota, C.; Danti, S.; D’Alessandro, D.; Trombi, L.; Ricci, C.; Puppi, D.; Dinucci, D.; Milazzo, M.; Stefanini, C.; Chiellini, F.; et al. Multiscale fabrication of biomimetic scaffolds for tympanic membrane tissue engineering. Biofabrication 2015, 7, 025005. [Google Scholar] [CrossRef]
- Kozin, E.D.; Black, N.L.; Cheng, J.T.; Cotler, M.J.; McKenna, M.J.; Lee, D.J.; Lewis, J.A.; Rosowski, J.J.; Remenschneider, A.K. Design, fabrication, and in vitro testing of novel three-dimensionally printed tympanic membrane grafts. Hear. Res. 2016, 340, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Danti, S.; D’Alessandro, D.; Mota, C.; Bruschini, L.; Berrettini, S. Applications of bioresorbable polymers in skin and eardrum. In Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081002667. [Google Scholar]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef] [Green Version]
- Burchard, W. Solution Properties of Branched Macromolecules. In Branched Polymers II; Springer: Berlin/Heidelberg, Germany, 1999; ISBN 978-3-540-65005-8. [Google Scholar]
- Puppi, D.; Detta, N.; Piras, A.M.; Chiellini, F.; Clarke, D.A.; Reilly, G.C.; Chiellini, E. Development of electrospun three-arm star poly(ε-caprolactone) meshes for tissue engineering applications. Macromol. Biosci. 2010, 10, 887–897. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Gardella, L.; Forouharshad, M.; Pellegrino, T.; Monticelli, O. Star poly(ε-caprolactone)-based electrospun fibers as biocompatible scaffold for doxorubicin with prolonged drug release activity. Colloids Surf. B Biointerfaces 2018, 161, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danti, S.; Mota, C.; D’alessandro, D.; Trombi, L.; Ricci, C.; Redmond, S.L.; De Vito, A.; Pini, R.; Dilley, R.J.; Moroni, L.; et al. Tissue engineering of the tympanic membrane using electrospun PEOT/PBT copolymer scaffolds: A morphological in vitro study. Hear. Balance Commun. 2015, 13, 133–147. [Google Scholar] [CrossRef]
- Lee, C.H.; Moioli, E.K.; Mao, J.J. Fibroblastic differentiation of human mesenchymal stem cells using connective tissue growth factor. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, New York, NY, USA, 30 August–3 September 2006. [Google Scholar]
- Tew, S.R.; Murdoch, A.D.; Rauchenberg, R.P.; Hardingham, T.E. Cellular methods in cartilage research: Primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods 2008, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.A.; Gomide, V.; Monteiro, F.J. The role of perfusion bioreactors in bone tissue engineering. Biomatter 2013, 2, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Puppi, D.; Piras, A.M.; Detta, N.; Dinucci, D.; Chiellini, F. Poly (lactic-co-glycolic acid) electrospun fibrous meshes for the controlled release of retinoic acid. Acta Biomater. 2010, 6, 1258–1268. [Google Scholar] [CrossRef]
- Trombi, L.; Danti, S.; Savelli, S.; Moscato, S.; D’Alessandro, D.; Ricci, C.; Giannotti, S.; Petrini, M. Mesenchymal Stromal Cell Culture and Delivery in Autologous Conditions: A Smart Approach for Orthopedic Applications. J. Vis. Exp. 2016, e54845. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT--PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Lou, Z.-C.; Lou, Z.-H.; Zhang, Q.-P. Traumatic tympanic membrane perforations: A study of etiology and factors affecting outcome. Am. J. Otolaryngol. 2012, 33, 549–555. [Google Scholar] [CrossRef]
- Milazzo, M.; Jung, G.S.; Danti, S.; Buehler, M.J. Wave propagation and energy dissipation in collagen molecules. ACS Biomater. Sci. Eng. 2020, 6, 1367–1374. [Google Scholar] [CrossRef]
- Fukuta, S.; Oyama, M.; Kavalkovich, K.; Fu, F.H.; Niyibizi, C. Identification of types II, IX and X collagens at the insertion site of the bovine achilles tendon. Matrix Biol. 1998, 17, 65–73. [Google Scholar] [CrossRef]
- Andriamanalijaona, R. Cell therapies for articular cartilage repair: Chondrocytes and mesenchymal stem cells. In Regenerative Medicine and Biomaterials for the Repair of Connective Tissues; Elsevier: Amsterdam, The Netherlands, 2010; pp. 266–300. [Google Scholar]
- Thalmann, I. Collagen of accessory structures of organ of Corti. Connect. Tissue Res. 1993, 29, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.J.; Day, R.M.; Dilley, R.J. Tympanic membrane organ culture using cell culture well inserts engrafted with tympanic membrane tissue explants. BioTechniques 2017, 62, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Dumas, M.; Chaudagne, C.; Bonté, F.; Meybeck, A. In vitro biosynthesis of type I and III collagens by human dermal fibroblasts from donors of increasing age. Mech. Ageing Dev. 1994, 73, 179–187. [Google Scholar] [CrossRef]
- Danti, S.; D’Acunto, M.; Trombi, L.; Berrettini, S.; Pietrabissa, A. A Micro/Nanoscale Surface Mechanical Study on Morpho-Functional Changes in Multilineage-Differentiated Human Mesenchymal Stem Cells. Macromol. Biosci. 2007, 7, 589–598. [Google Scholar] [CrossRef]
- Ho, S.T.B.; Tanavde, V.M.; Hui, J.H.; Lee, E.H. Upregulation of adipogenesis and chondrogenesis in MSC serum-free culture. Cell Med. 2011, 2, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Von Der Mark, K.; Gauss, V.; Von Der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Fay, J.; Puria, S.; Decraemer, W.F.; Steele, C. Three approaches for estimating the elastic modulus of the tympanic membrane. J. Biomech. 2005, 38, 1807–1815. [Google Scholar] [CrossRef]
- Luo, H.; Dai, C.; Gan, R.Z.; Lu, H. Measurement of Young’s modulus of human tympanic membrane at high strain rates. J. Biomech. Eng. 2009, 131, 064501. [Google Scholar] [CrossRef] [PubMed]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-actin cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatamochi, A.; Aumailley, M.; Mauch, C.; Chu, M.L.; Timpl, R.; Krieg, T. Regulation of collagen VI expression in fibroblasts. Effects of cell density, cell-matrix interactions, and chemical transformation. J. Biol. Chem. 1989, 264, 3494–3499. [Google Scholar] [PubMed]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Levita, G.; Marchetti, A.; Lazzeri, A. Fracture of ultrafine calcium carbonate/polypropylene composites. Polym. Compos. 1989, 10, 39–43. [Google Scholar] [CrossRef]
- Spadaccio, C.; Rainer, A.; Trombetta, M.; Vadalá, G.; Chello, M.; Covino, E.; Denaro, V.; Toyoda, Y.; Genovese, J.A. Poly-L-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann. Biomed. Eng. 2009, 37, 1376–1389. [Google Scholar] [CrossRef]
- Yeatts, A.B.; Geibel, E.M.; Fears, F.F.; Fisher, J.P. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol. Bioeng. 2012, 109, 2381–2391. [Google Scholar] [CrossRef] [Green Version]
- Limongi, T.; Tirinato, L.; Pagliari, F.; Giugni, A.; Allione, M.; Perozziello, G.; Candeloro, P.; Di Fabrizio, E. Fabrication and applications of micro/nanostructured devices for tissue engineering. Nano Micro Lett. 2017, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jang, C.H.; Kim, G.H. A polycaprolactone/silk-fibroin nanofibrous composite combined with human umbilical cord serum for subacute tympanic membrane perforation; an in vitro and in vivo study. J. Mater. Chem. B 2014, 2, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.; Barsotti, G.; Puppi, D.; Coli, A.; Briganti, A.; Giannessi, E.; Miragliotta, V.; Mota, C.; Pirosa, A.; Stornelli, M.R.; et al. Tailored star poly (ε-caprolactone) wet-spun scaffolds for in vivo regeneration of long bone critical size defects. J. Bioact. Compat. Polym. 2016, 31, 15–30. [Google Scholar] [CrossRef]
- Günday, C.; Anand, S.; Gencer, H.B.; Munafò, S.; Moroni, L.; Fusco, A.; Donnarumma, G.; Ricci, C.; Hatir, P.C.; Türeli, N.G.; et al. Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv. Transl. Res. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Valente, F.; Astolfi, L.; Simoni, E.; Danti, S.; Franceschini, V.; Chicca, M.; Martini, A. Nanoparticle drug delivery systems for inner ear therapy: An overview. J. Drug Deliv. Sci. Technol. 2017, 39, 28–35. [Google Scholar] [CrossRef]






| Gene | Acronym | Primer Sequence (5′—3′) | Product (bp) | Tm (°C) |
|---|---|---|---|---|
| Collagen type I-α1 | COL1A1 | AGACATCCCACCAATCAC GTCATCGCACAACACCTT | 118 | 60.5 62.1 |
| Collagen type II-α1 | COL2A1 | GAGCAGCAAGAGCAAGGA GACAGCAGGCGTAGGAAG | 135 | 63.9 64.0 |
| Collagen type IV-α1 | COL4A1 | CGCAAGTTCAGCACAATG CACAGCACACCTACTAATAAATG | 168 | 61.5 61.3 |
| Collagen type IV-α2 | COL4A2 | ACTGGTGATTTCGGTGAC CCTTCTGTTCCCTTCTCTC | 125 | 61.1 60.6 |
| Ribosomal protein large, P0 | RPLP0 | CCTCATATCCGGGGGAATGTG GCAGCAGCTGGCACCTTATTG | 95 | 63.6 63.3 |
| Time (s) | 0 | 0.5 | 1.0 |
|---|---|---|---|
| *PCL | 0.0112 | 0.0626 | 0.0896 |
| HA/*PCL | 0.0070 | 0.0308 | 0.0411 |
| Collagens | *PCL, Static | *PCL, Dynamic | HA/*PCL, Static | HA/*PCL, Dynamic |
|---|---|---|---|---|
| Type I | +++ | +++ | - | - |
| Type II | + | ++ | + | + |
| Type III | + | + | - | - |
| Type IV | ++/+++ | ++ | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscato, S.; Rocca, A.; D’Alessandro, D.; Puppi, D.; Gramigna, V.; Milazzo, M.; Stefanini, C.; Chiellini, F.; Petrini, M.; Berrettini, S.; et al. Tympanic Membrane Collagen Expression by Dynamically Cultured Human Mesenchymal Stromal Cell/Star-Branched Poly(ε-Caprolactone) Nonwoven Constructs. Appl. Sci. 2020, 10, 3043. https://doi.org/10.3390/app10093043
Moscato S, Rocca A, D’Alessandro D, Puppi D, Gramigna V, Milazzo M, Stefanini C, Chiellini F, Petrini M, Berrettini S, et al. Tympanic Membrane Collagen Expression by Dynamically Cultured Human Mesenchymal Stromal Cell/Star-Branched Poly(ε-Caprolactone) Nonwoven Constructs. Applied Sciences. 2020; 10(9):3043. https://doi.org/10.3390/app10093043
Chicago/Turabian StyleMoscato, Stefania, Antonella Rocca, Delfo D’Alessandro, Dario Puppi, Vera Gramigna, Mario Milazzo, Cesare Stefanini, Federica Chiellini, Mario Petrini, Stefano Berrettini, and et al. 2020. "Tympanic Membrane Collagen Expression by Dynamically Cultured Human Mesenchymal Stromal Cell/Star-Branched Poly(ε-Caprolactone) Nonwoven Constructs" Applied Sciences 10, no. 9: 3043. https://doi.org/10.3390/app10093043











