Featured Application
The goal of the paper is to direct the discussion about the spread of infectious diseases such as COVID-19, and the evolution of herd immunity to an immunity enhancement mechanism by multiple virus impacts using a simple kinetic model.
Abstract
For achieving herd immunity, the proportion of individuals who are immunized, and the proportion of susceptible individuals are normally regarded as the key factors. Here, it is discussed that the immunity is not a yes/no decision in all cases, but a limited (relative) immunity should be kept in mind. This effect would cause a dependence of infection from the level of immunity and the strength of single-infection impact events (virus load). As a result, a stepwise enhancement of low-level immunity could be achieved in case of infection contacts at low concentrations of infectious particles. This behavior is probably important for airborne infection paths. Therefore, it might play a role in the case of the recent SARS (new coronavirus) pandemic and could have a strong effect on herd immunity.
1. Introduction
The discussion of herd immunity plays a very important role in the decisions of the management of the recent SARS COVID-19 pandemic [1]. On the one hand, there is a strong hope that herd immunity can help to protect the majority of the world population in the near future, on the other hand, there is the danger of millions of victims in case of a further uncontrolled spread of the disease.
Population models for herd immunization are based, mainly, on the concept of a clear presence or absence of immunity (yes/no decision). This concept is very important and describes the effect of immunization after surviving the disease or after a successful vaccination [2,3]. Model simulations have been also applied in order to predict the evolution of the SARS-CoV-2 pandemic [4].
To calculate the spread of infectious diseases, population dynamics models are applied usually [5]. However, in contrast to the assumed yes/no decisions in the individual immune response, it has to be taken into account that a reduced response of individual immune systems could also occur in case of a low-level impact of infecting objects such as viruses [6]. The meaning behind the term “low-level impact” is the assumption that not only the strength of antibody production can vary in case of a massive infection, but there might also be a production of a lower number of antibodies or of less specific antibodies in case of an exposition against a lower concentration of antigens. Quantitative effects seem to play a crucial role in the development and reliability of immunity. Such an effect was recognized in the case of a vaccine-induced enhancement of viral infections during the development of an HIV vaccine, for example [7]. Additionally, the influence of the route of vaccination on the success of immunization as it was recently described for smallpox speaks for quantitative effects [8].
The individual response to viruses is important for the formation of herd immunity, but herd immunity is a complex principle. In the case of influenza, a mix between social distancing, vaccination of children [9], and pre-existing antibodies from vaccinations or earlier infections in older people can contribute to herd immunity [10]. An earlier study on HIV vaccines speaks for the possibility that herd immunity can be achieved even in the case of imperfect vaccines [11].
Some simple simulations will be presented which illustrate the effect of a step-wise low-level increase of immunity of an individual. The approach is related to the possible response on the level of individuals but will be discussed in their consequences for the spreading of infection in a population and for supporting the development of herd immunity as well.
2. Concept of Low-Level Multi-Step Immunization
The basic idea behind low-level multi-step immunization is that not only a massive impact of viruses but also a low-level impact causes a certain response of an individual’s immune system. It might be that this immune response has a higher or lower specificity against the impacting viruses, but this aspect will not be discussed in detail. Here, it is only assumed that in the case of each infection, a competition between the multiplication of viruses and stimulation of the immune system by the production of antibodies, for example, takes place. This can be illustrated by a simple competition model:
The increase of virus concentration v in a certain time interval is described by a virus growth rate k, a rate of antibody-dependent virus decay r and a generalized antibody concentration a:
v(t) = v(0) + k*v(0)*[1 − v(0)/v(max)] − r*v(0)*a(0)
The development of antibody concentration is described by:
a(t) = a(0) + sqrt{s*v(0)*[1 − s*a(0)/a(max)]} − r*v(0)*a(0)
This iterative formulation can be regarded as a simple system of two coupled differential equations:
dv/dt = k*v(0)*[1 − v(0)/v(max)] − r*v(0)*a(0)
da/dt = sqrt{s*v(0)*[1 − s*a(0)/a(max)]} − r*v(0)*a(0)
For
v(max) > v(t) and a(max) > a(t)
For simulation, the iterative model of Equations (1) and (2) was used with the application of arbitrarily chosen parameters k, r, s, v(max), and a(max) and a time step dt = 1.
3. Illustration of Competition between Infection and Immune Response
The competition between the multiplication of the virus and the stimulation of the immune response can be simulated using the formulated simple model above. For the demonstration, the following parameter set is applied:
- k = 0.1
- r = 0.03
- s = 0.01
- v(max) = 100
- a(max) = 10
The numbers are related to each single time step in the simulation. The obtained “concentrations of viruses and antibodies” are arbitrary units. The results are shown without any scaling to real concentrations (numbers, numbers per volumes, log (numbers), and real time scales (hours, days).
The study presented here is based on two assumptions: It is assumed that the velocity of initial antibody production may depend on the strength of antigen exposure (viral dose). And it is assumed that immunity can drift from a safe immune state into a sensitive state by lowering of the antibody concentration. Vice versa, it is taken into account that the manifestation of infection can be dependent on the virus load and the virus doses in case of multiple exposure situations.
It is clear that a certain level of antibodies can mean protection over decades or an entire life. On the other hand, it should be considered that immunity can be lost over time in some cases.
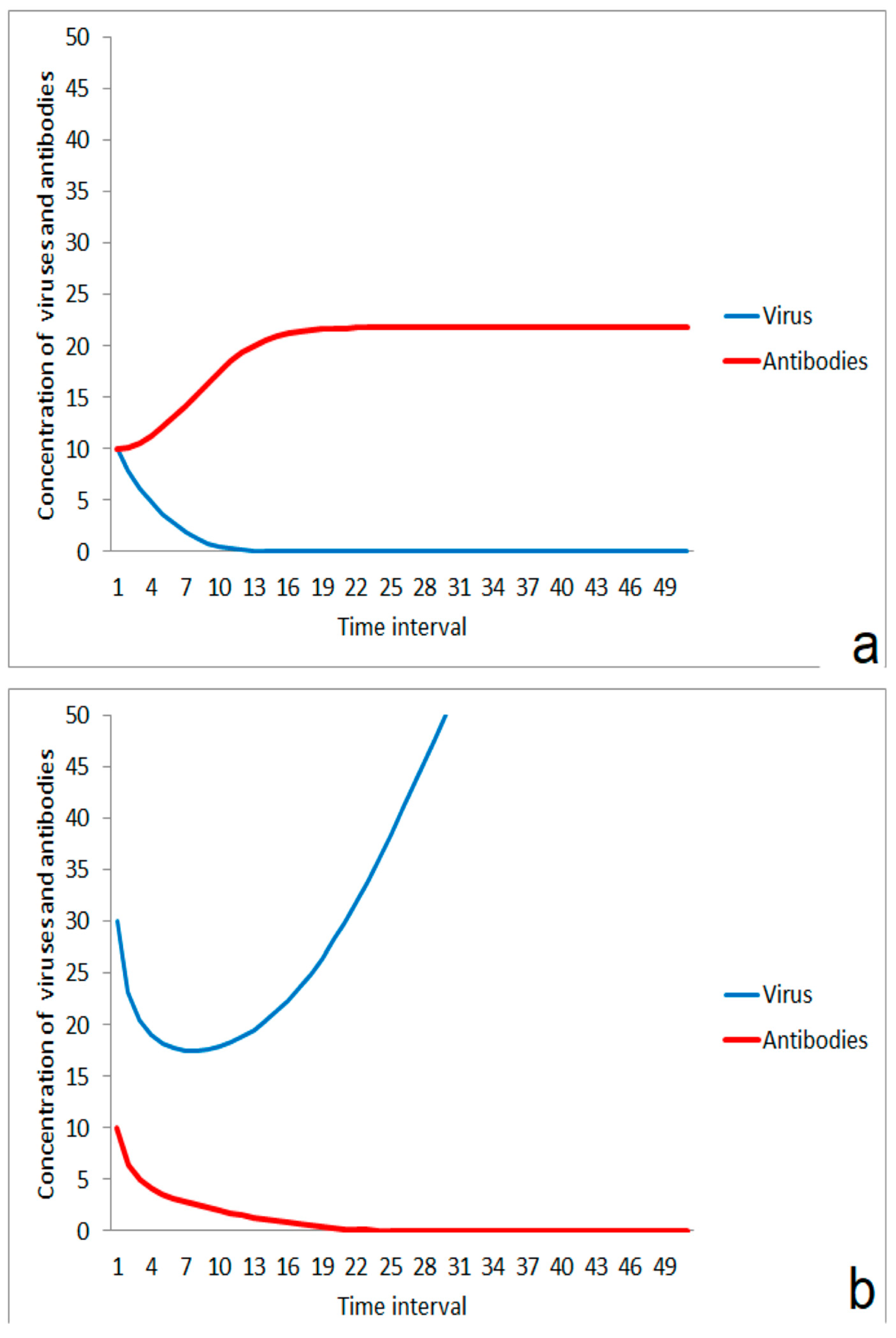
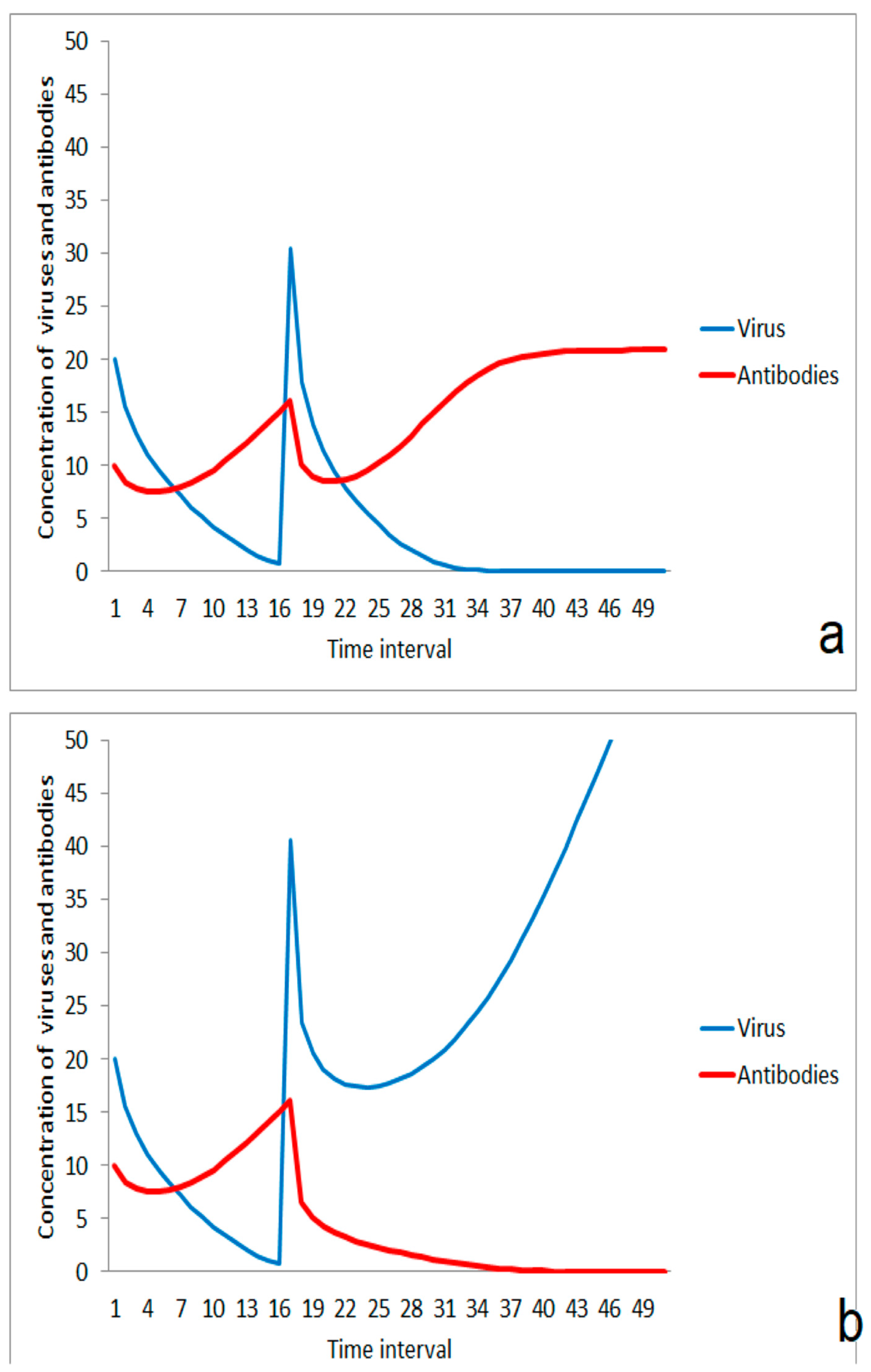
For the consequences of the impact of a virus, the virus concentration, and the strength of the individual immune system are important. A moderate impact of viruses results in the dominance of the stimulation effect of the immune system (Figure 1a). The virus concentration is then lowered and the infection is suppressed. In the case of a higher virus impact, the immune system is not able to compensate for the attack and the infection propagates (Figure 1b). These different kinetic responses could reflect the difference between the exposition by a few small aerosol droplets containing viruses on the one hand and stronger impact by massive virus-loaded aerosols on the other.
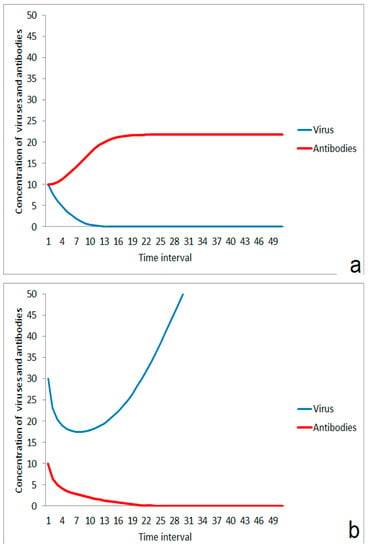
Figure 1.
Competition between immune system and virus after impact: (a) suppression of infection after moderate virus impact, (b) outbreak of disease after a stronger impact by viruses.
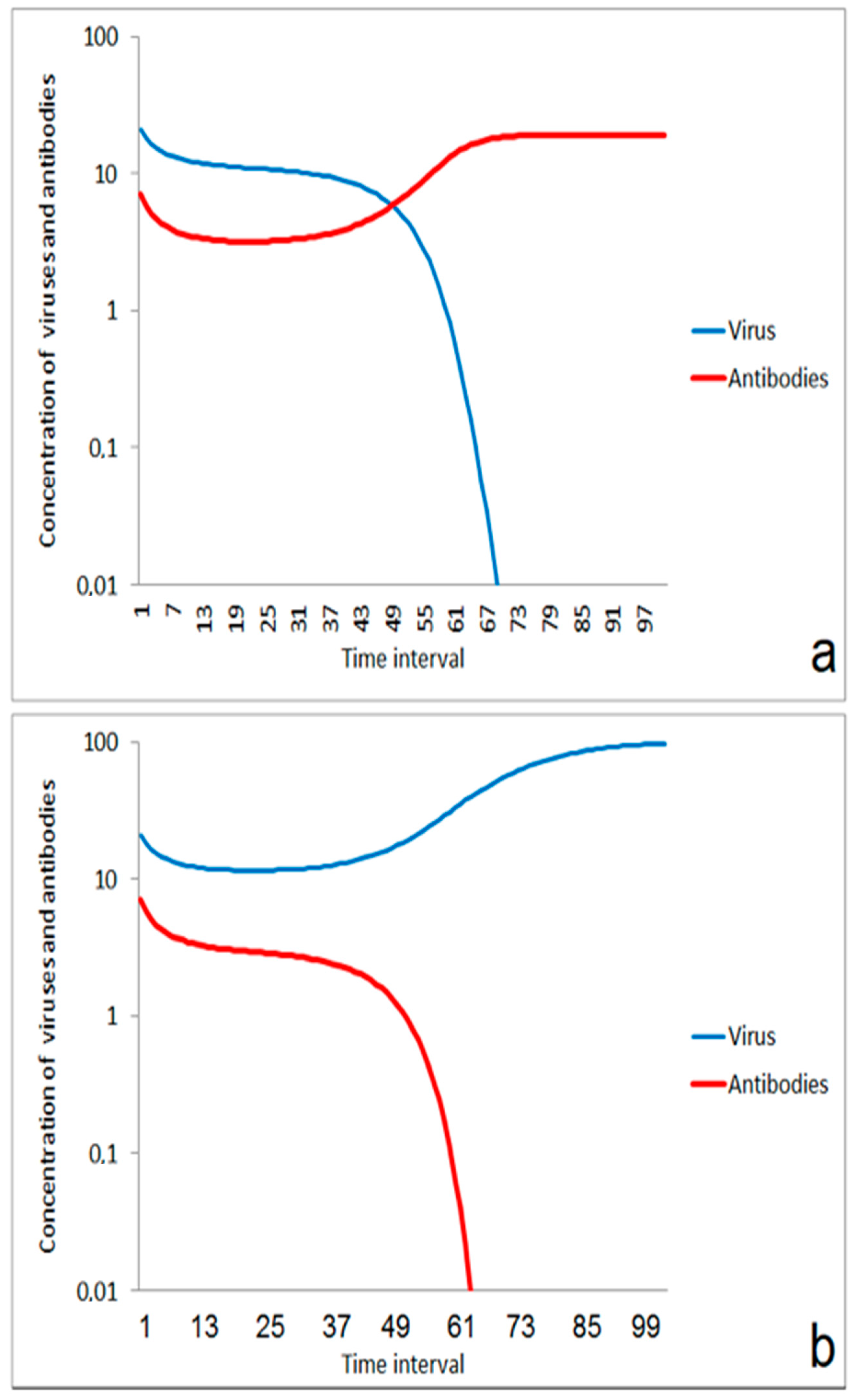
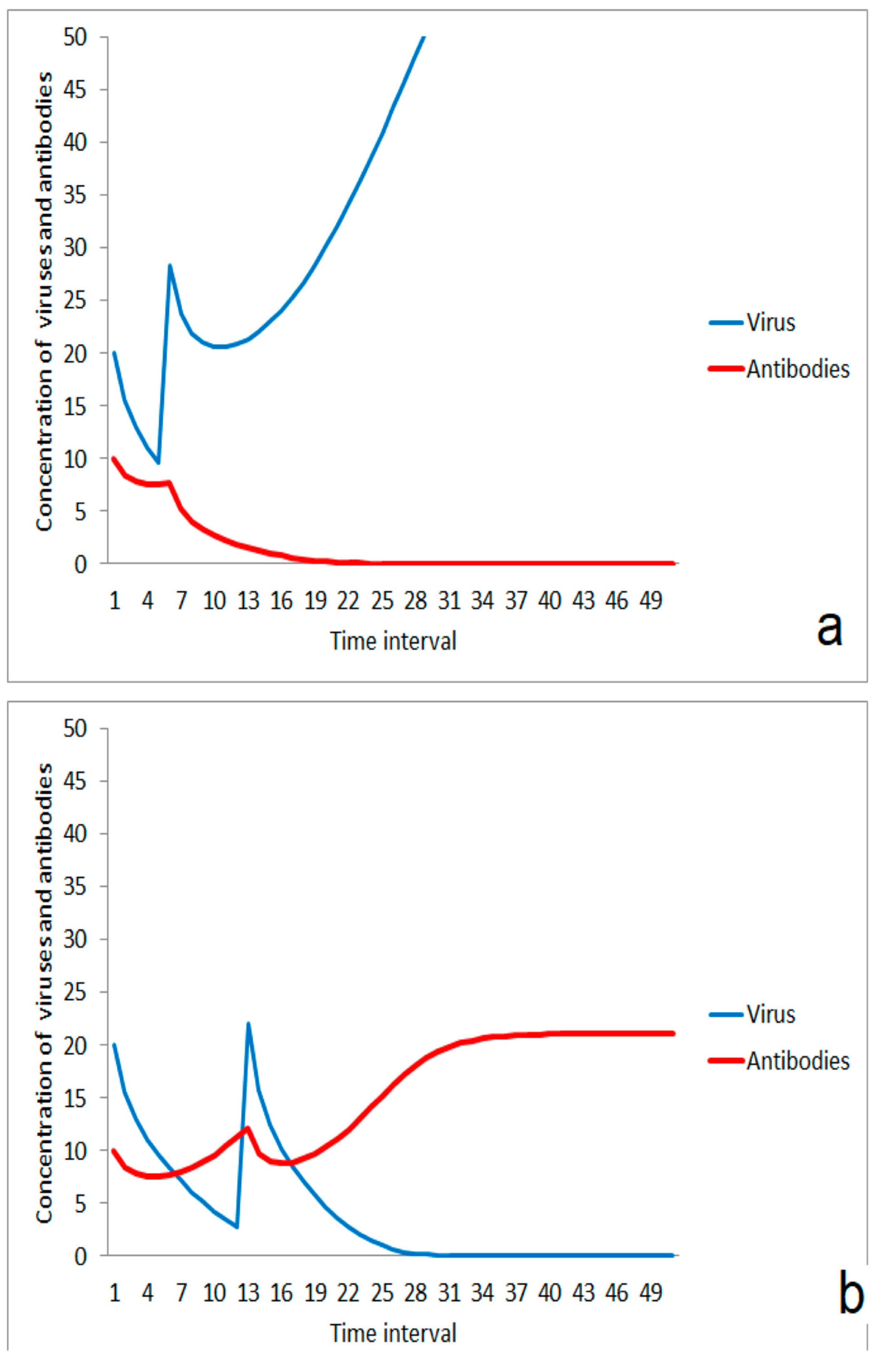
In a critical range of ratios between immune competence and virus impact, transient states could be expected. For a certain time, the order of magnitude of virus and antibody concentration is only slightly changed. But after the transition period, either the immune system (Figure 2a) or the infection (Figure 2b) wins the game. The duration of the transient period is very sensitive against small differences in the ratio between viruses and antibodies. This effect could be responsible for larger differences in the incubation time as it was sometimes observed for infections caused by the new coronavirus.
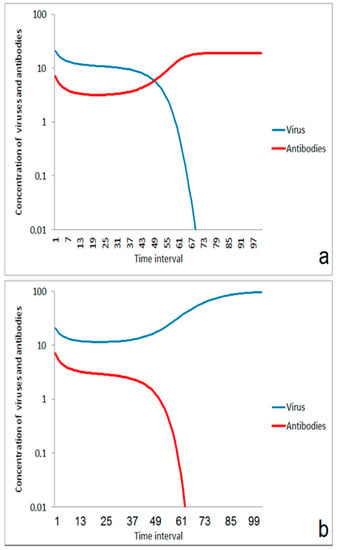
Figure 2.
Transient behavior in the competition between virus and immune system in a critical range of start ratio of viruses and antibodies (concentration axis as log scale): (a) the transient phase results in a suppression of infection; start conditions: v(0)/a(0) = 20/9; (b) The infection propagates after the transient phase; start conditions: v(0)/a(0) = 20/8.
The scenario of Figure 2 was obtained by the assumption of a certain concentration of antibodies that could bind to the infecting viruses. These antibodies can either be residual of an earlier specific immune response with a low remaining concentration of antibodies, or it can be regarded as a less specific immune response which was formerly induced by exposure to a related class of viruses. The initial decrease in the antibody concentration can be interpreted by a quick consumption of antibodies from reactions between antibodies and viruses. The later increase of antibodies is caused by the stimulating effect of the virus exposure on antibody production. The simple model presented here does not distinguish between a higher concentration of antibodies with lower specificity and a lower concentration of antibodies with higher specificity.
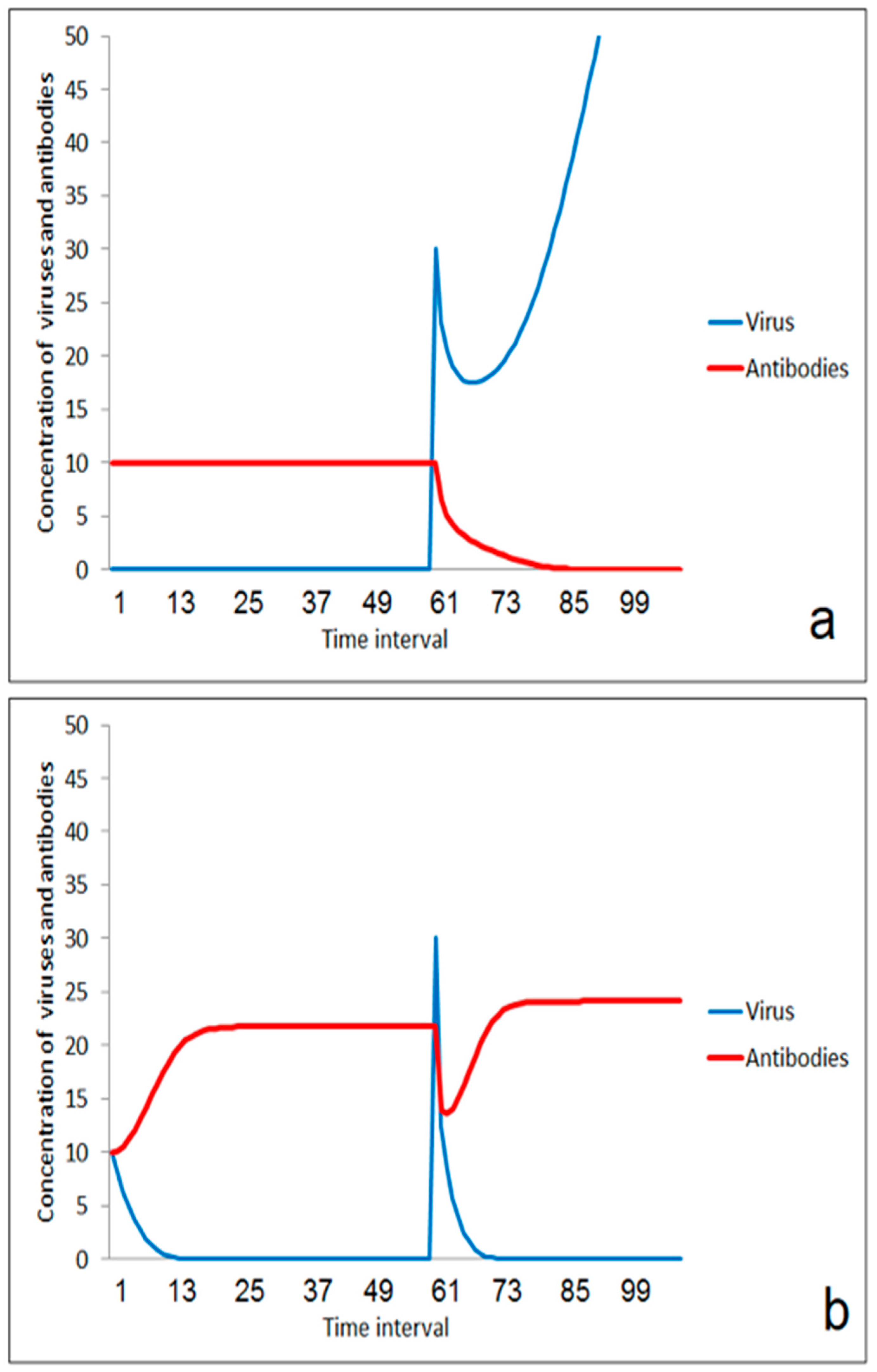
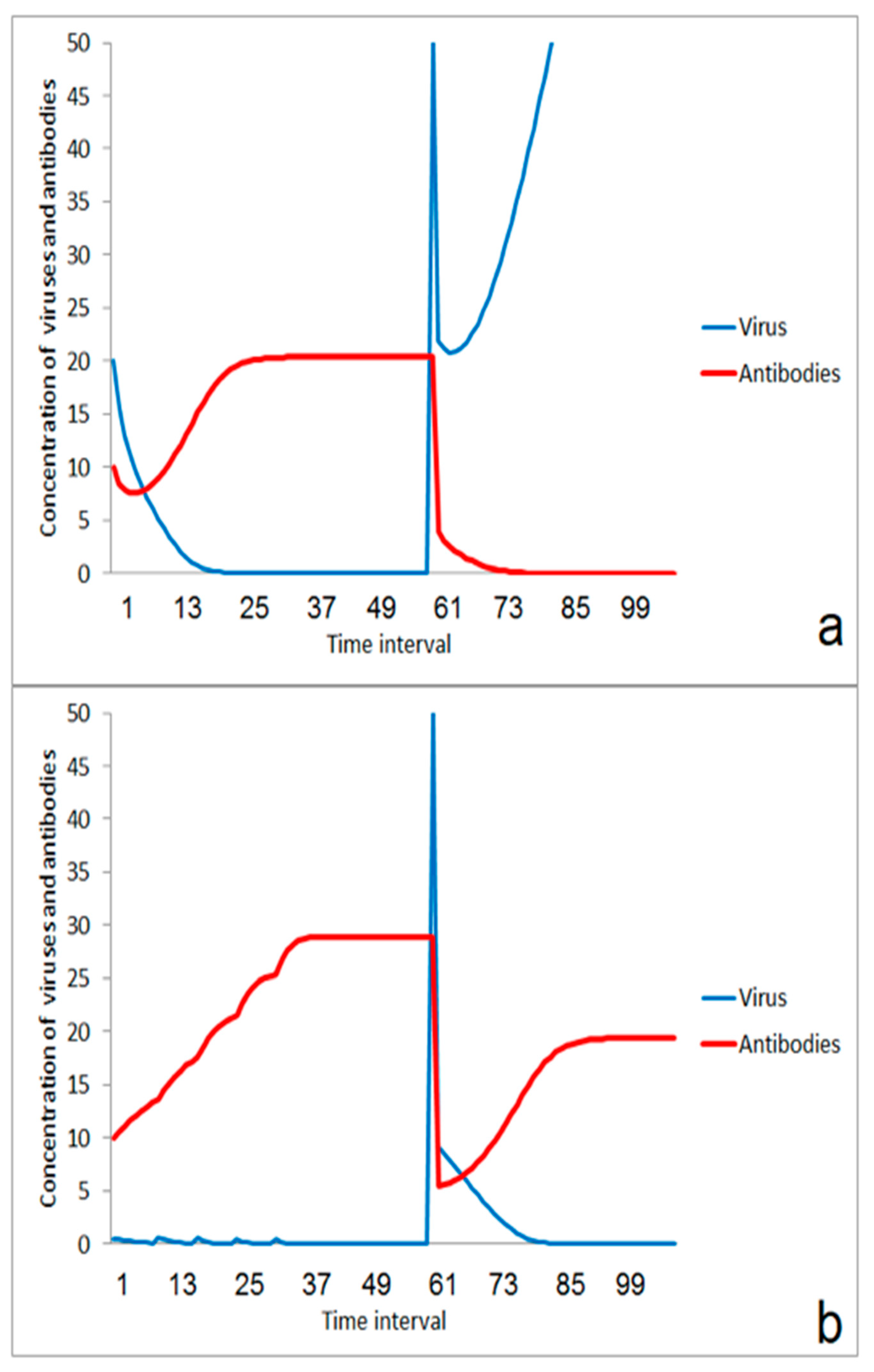
The immune system is overstressed in the case of a larger virus impact (Figure 3a). However, moderate stimulation of the immune system in an earlier phase could help to enhance the threshold for the outbreak of the disease (Figure 3b). This effect is very important for herd immunity. Individuals become more robust against viruses if their immune system is stimulated to a certain extent by smaller doses of the virus. If the number of such individuals in a population increases, the number of new infections decreases, and the risk of massive impacts by aerosols from ill individuals decrease. The improvement of immune response for avoiding the outbreak of disease depends on the effect of the second virus impact. If the impact is moderate, the immune system will win (Figure 4a). If the second virus impact is too strong, then even a previously stimulated immune system is overstressed and the disease goes on (Figure 4b).
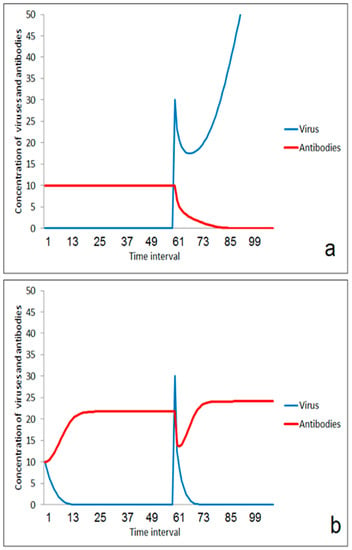
Figure 3.
Effect of moderate first virus impact on the reaction on a second impact: (a) without immunization by the first impact, (b) after enhancement of immune-reaction after the first impact.
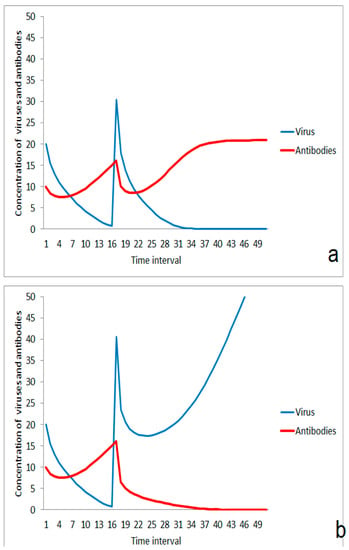
Figure 4.
Effect of the strength of second virus impact after induced enhancement of immunization by a first moderate impact: (a) suppression of infection after lower second virus impact, (b) ongoing disease after higher second virus impact.
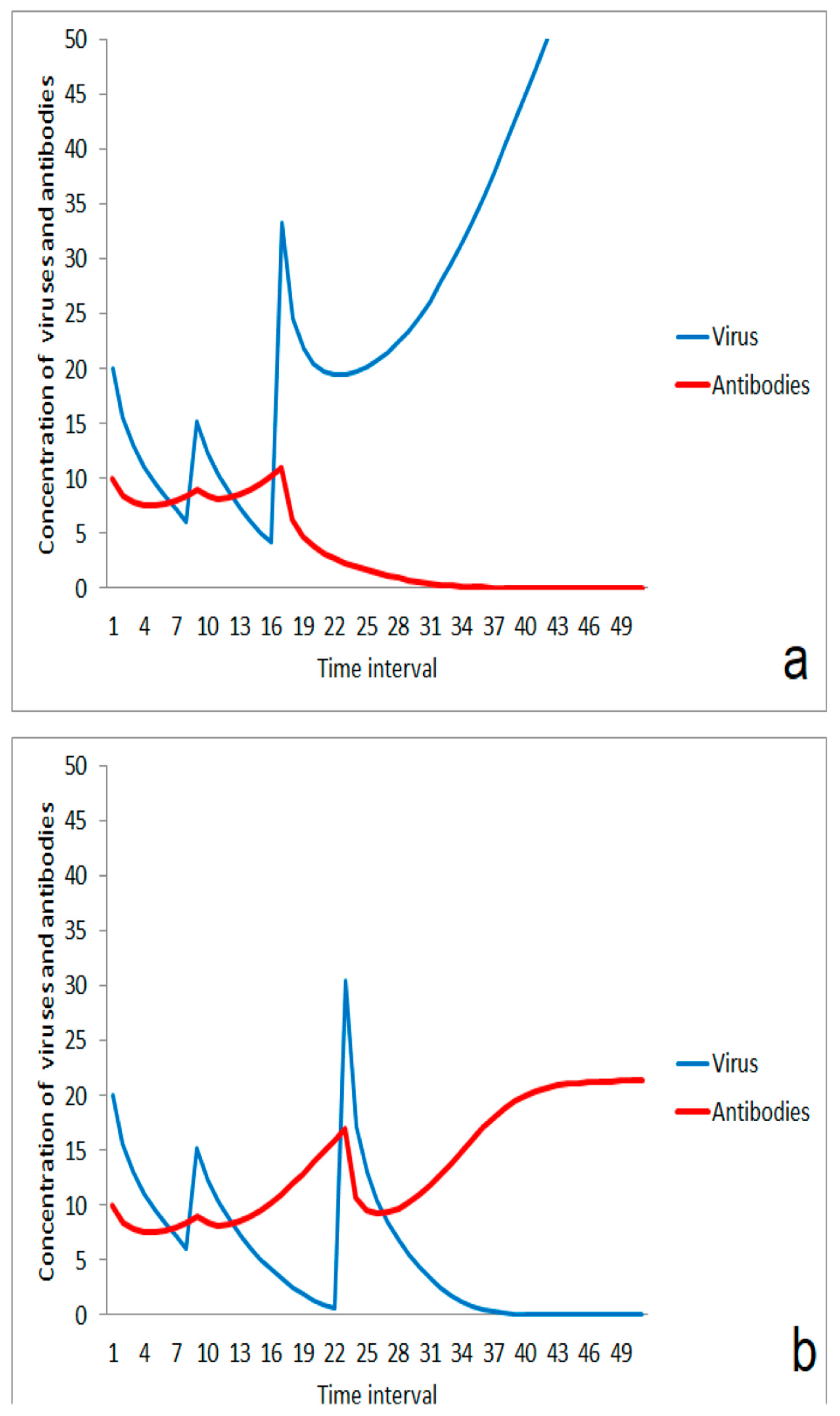
Besides strength, the timing of the second virus impact is important for the efficiency of the first antigen stimulation for enhancement of immune response. If the second impact comes too early, an additive effect takes place resulting in dominance of the infection over the immune system (Figure 5a). An impact of the same strength occurring a little later can be compensated by the immune system when it is regenerated after the first impact and the stimulation effect contributes to the immune robustness (Figure 5b). An analog effect can also happen if the immune response was stimulated by two or more moderate virus impacts. The infection wins in case of a small duration between second and third impact (Figure 6a), but is suppressed in case of a little longer relaxation time between both of the last impacts (Figure 6b).
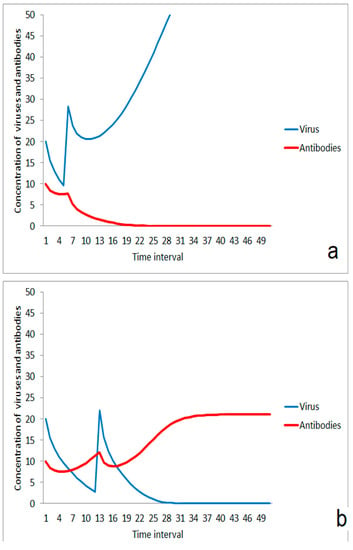
Figure 5.
Role of the duration between first moderate virus impact and second impact: (a) outbreak of disease after a short time span between both impacts, (b) suppression of infection after a longer duration between both virus impacts.
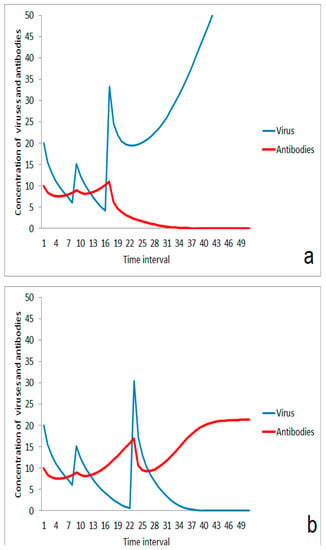
Figure 6.
Role of the duration between a second and a third virus impact in case of a two-step immunization on immune response: (a) outbreak of disease after a short time span; (b) suppression of infection after a longer duration between last virus impacts.
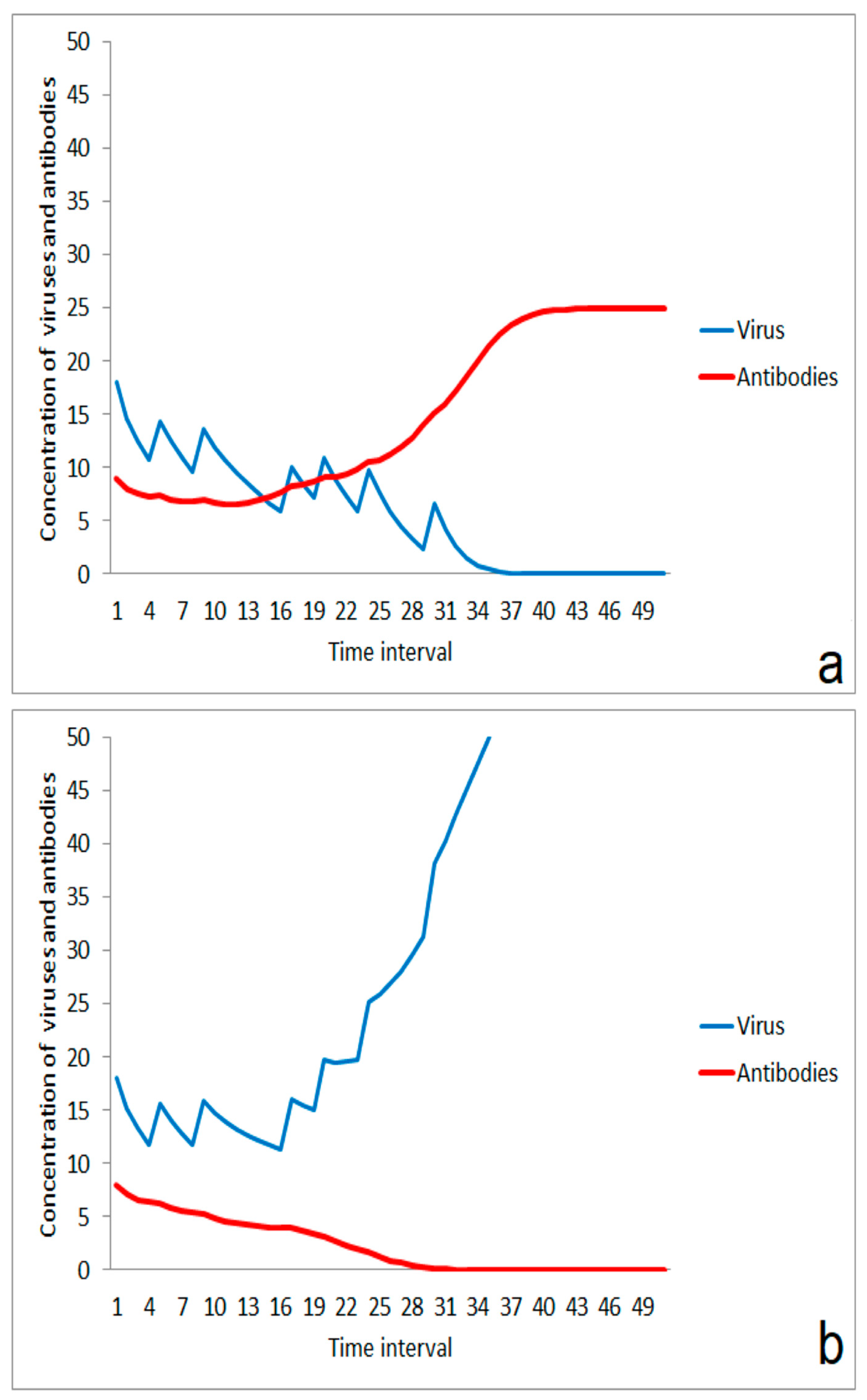
With increasing virus infections in a population, there is a rising risk of infection by an increasing number of impact events for each individual. Careful behavior can help to lower the massiveness of an impact, but it might be difficult to reduce the number of impact events after a certain duration of an epidemic. In this situation, the immune system can successfully respond to a series of impact events if the starting conditions of the immune system are suited (Figure 7a). The situation is completely changed if the starting performance is low. Even a small reduction in the immune situation at the beginning can lead to an outbreak of the disease (Figure 7b). This proposed mechanism is a strong argument for managing a slow increase in the number of infected people in a population and for the reduction of the massiveness of single-impact events if repeated contacts with infected people cannot be excluded. On the other hand, this effect of moderate multi-event exposures speaks for an acceleration of herd immunity in a developed phase of an epidemic process if the massiveness of impacts can be controlled.
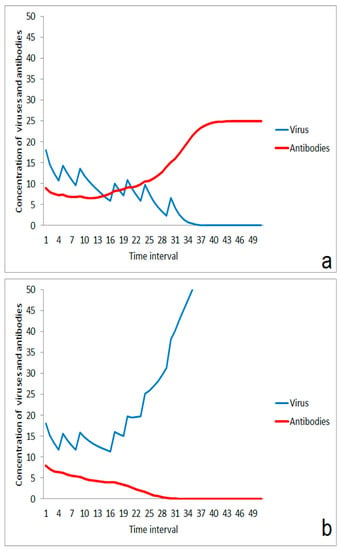
Figure 7.
Multi-event stimulation of the immune system and sensitivity on start situation: (a) successful stimulation in case of a sufficient starting performance, (b) failing of the immune system after a series of moderate impact events (same impact strengths and times as in (a)) in case of a slightly lowered starting performance of the immune system.
The simulations also suggest that a series of small impacts could be helpful for single individuals. The immune system can fail in case of a massive second impact even in the case of a relatively strong first impact (Figure 8a). In addition, such a strong first impact includes the risk of a direct harmful infection by the first event. In contrast, the chosen parameter set for the simulation illustrates the possibility that a series of small impacts which are low enough for avoiding the risk of a direct infection could improve the immune response significantly (Figure 8b). The idea is that immunity is “pumped up” by a lot of small impacts. This situation could be typical for an infection path with dried viruses or virus aggregates after partial or complete drying of aerosol droplets, resulting in small particles of very low sedimentation rate in the air. This situation might become important for higher numbers of infected persons and for the spreading of viruses in larger rooms such as lecture halls, railway stations, and shopping markets.
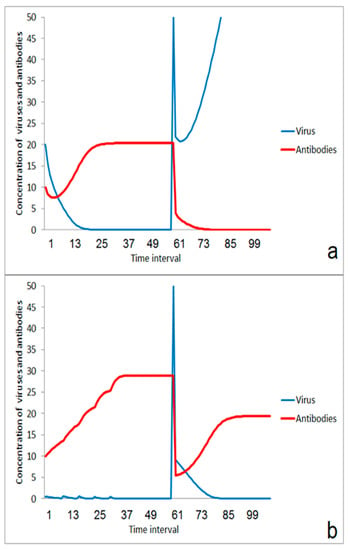
Figure 8.
Effect of a series of low-level multi-impact events for improvement of immune response: (a) outbreak of disease after a strong stimulation at the beginning, (b) suppression of the infection after a series of small impact events.
4. Conclusions
The simple simulation results presented above are based on the assumption of the presence of a gradual immune protection and the possibility of gradual immune response. It is related to individual differences in the robustness and susceptibility of the immune system against stimulation. These assumptions supply illustrations for several effects which take place during the spreading of an epidemic. The described situations could support a better understanding of the role of multi-impact events. Such scenarios could be particularly important for risk management of medical staff in frequent contact with infected persons and in case of viruses which are mainly spread by air. Despite the qualitative character of the presented model, it gives arguments for estimating the strong importance of individual behavior for the spreading of an epidemic and for achieving herd immunity.
Funding
This research received no external funding. The work was supported by the State of Thuringia in the frame of the regular professional activity of the author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tian, S.; Hu, N.; Lou, J.; Chen, K.; Kang, X.; Xiang, Z.; Chen, H.; Wang, D.; Liu, N.; Liu, D.; et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020, 80, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Eames, K.; Heymann, D.L. ‘Herd immunity’: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.J.; Gani, J. Epidemic Modeling: An Introduction; Cambridge University Press: Cambridge, UK; New, York, NY, USA, 2005. [Google Scholar]
- Richard, A.N.; Robert, D.; Valentin, D.; Emma, B.H.; Jan, A. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss. Med. Wkly. 2020, 150, w20224. [Google Scholar]
- Brauer, F.; Castillo-Chávez, C. Mathematical Models in Population Biology and Epidemiology; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2001. [Google Scholar]
- Reyes-Silveyra, J.; Mikler, A.R. Modeling immune response and its effect on infectious disease outbreak dynamics. Theor. Biol. Med. Model. 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Huisman, W.; Martina, B.E.E.; Rimmelzwaan, G.F.; Gruters, W.; Osterhaus, A.D.M.E. Vaccine-induced enhancement of viral infections. Vaccine 2009, 27, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.J.; Townsend, M.B.; Gallardo-Romero, N.; Hutson, C.L.; Patel, N.; Doty, J.B.; Salzer, J.S.; Damon, I.K.; Carroll, D.S.; Satheshkumar, P.S.; et al. Magnitude and diversity of immune response to vaccinia virus is dependent on route of administration. Virology 2020, 544, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.A.; Sugaya, N.; Fedson, D.S.; Glezen, W.P.; Tashiro, M. The Japanese experience with vaccinating schoolchildren against influenza. N. Engl. J. Med. 2001, 344, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Hoschler, K.; Hardelid, P.; Stanford, E.; Andrews, N.; Zambon, N. Incidence of 2009 pandemic influenca A H1N1 infection in England: A cross-sectional serological study. Lancet 2010, 375, 1100–1108. [Google Scholar] [CrossRef]
- McLean, A.R.; Blower, S.M. Imperfect vaccines and herd-immunity to HIV. Proc. R. Soc. B 1993, 253, 9–13. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).