A Review of Thermochemical Energy Storage Systems for Power Grid Support
Abstract
:1. Introduction
Power-to-Heat Technologies: Classification
2. Classification of Thermal Storage Systems
- is the mass of the storage medium (kg);
- is the heat capacity of the storage medium (J/(kg K));
- is the temperature difference (°C).
- is the melting or phase change enthalpy (J/kg).
Characteristics of Thermal Storage Systems
- Storage period defines how long the energy is stored (i.e., hours, days, weeks);
- Power defines how fast the energy stored in the system can be charged and discharged. In particular, power capacity (W) is the maximum amount of power that can be delivered by the storage system during discharging while Power density (W/l) is the ratio between the power capacity and the capacity of the energy storage system;
- Energy storage capacity or energy capacity is defined as the amount of energy absorbed in the storage system during the charging process under nominal conditions. The quantity of stored energy in the system after it is charged depends on the storage process, storage medium and size of the system;
- Energy density or volumetric heat capacity is defined as the ratio between the stored energy and the volume of the energy storage system;
- Charge and discharge time defines how much time is needed to charge or discharge the system. The maximum number of charge-discharge cycles in the specified conditions is defined as the cycling capacity or number of cycles;
- Self-discharge is the amount of energy initially stored and dissipated over a specified non-use time;
- Efficiency is the ratio of the energy provided to the user to the energy needed to charge the storage system. It accounts for the energy losses during the storage period and the charge/discharge cycle;
- Response time is defined as the speed with which the energy is absorbed or released [h];
- Cycle life refers to how many times the storage system releases the energy after each recharge;
- Costs are indicators to define the overall cost normalized on the total amount of capacity (€/kWh) or power (€/kW). They are capital costs, and operation and maintenance costs of the storage equipment during its lifetime;
- Cost per output (useful) energy is the ratio of the cost per unit energy divided by the storage efficiency;
- Cost per cycle is defined as the cost per unit energy divided by the cycle life.
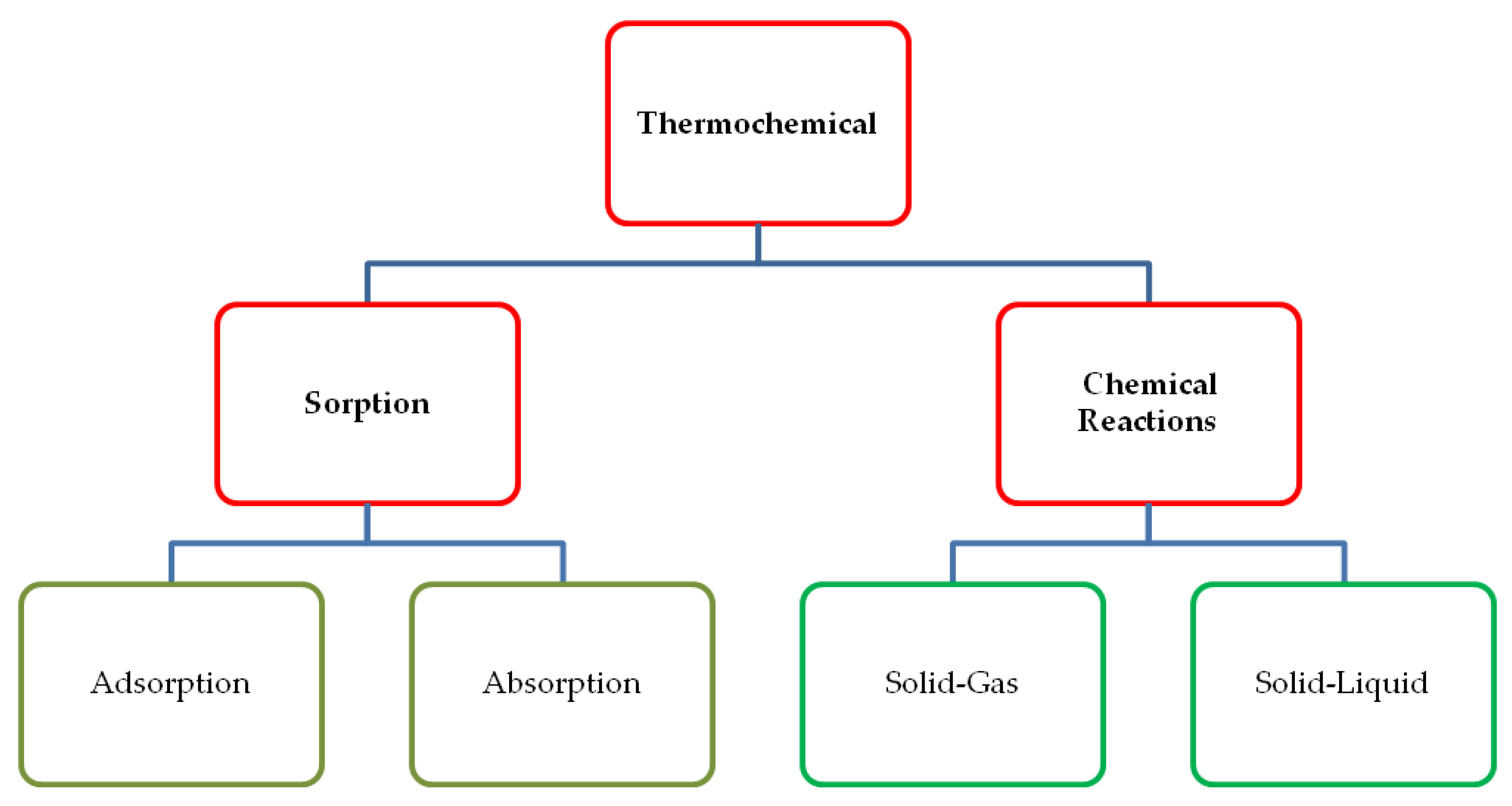
3. Thermochemical Heat Storage: Description of Materials and Processes
3.1. Thermochemical Processes and Materials
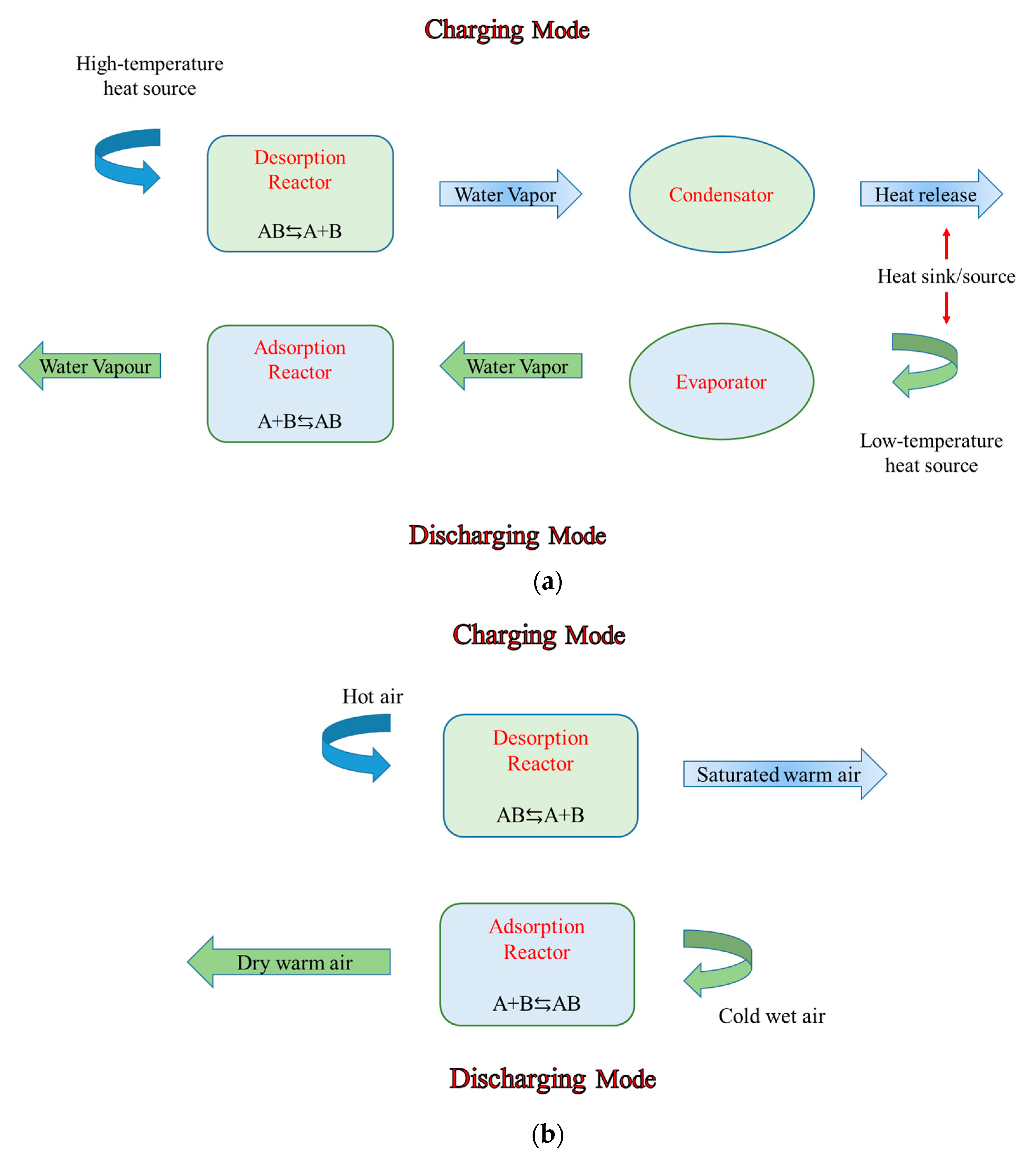
3.2. Thermochemical Heat Storage Systems
4. Thermochemical Storage in Power-to-Heat Applications
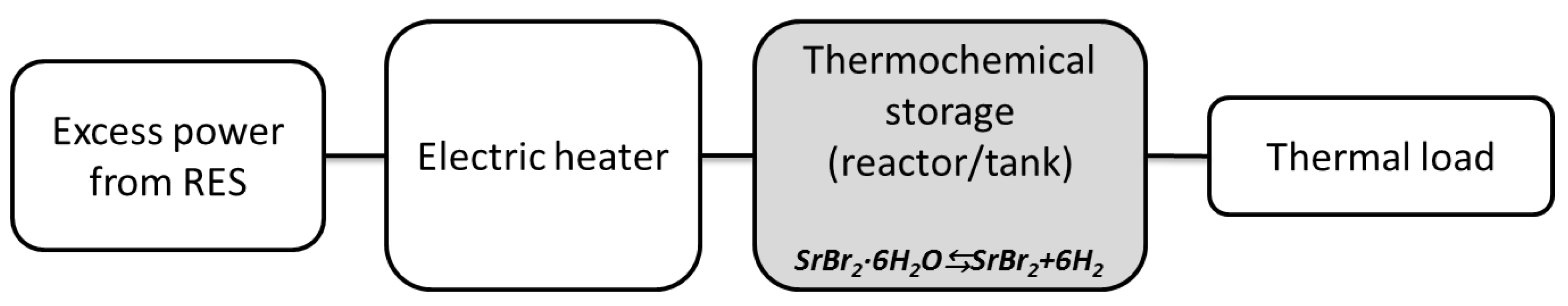
4.1. Thermochemical Storage Energy Systems in Power-to-Heat Applications: Case Studies
4.2. Discussion and Outlook
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AB | Storage material |
| A,B | Reaction products |
| ALPOs | Aluminophosphates |
| CAES | Compressed air energy storage |
| Cp | Heat capacity (J/(kg K)) |
| CHP | Combined heat and power |
| COP | Coefficient of Performance |
| CSP | Collectors and Concentrating Solar Plant |
| DHS | District heating systems |
| DSM | Demand-side management |
| Δh | Phase change enthalpy (°C) |
| ΔH | Standard reaction enthalpy (J/mol) |
| ΔS | Standard reaction entropy (J/(°C mol)) |
| ΔT | Temperature difference (°C) |
| GHG | Greenhouse gases |
| HCTSR | Hybrid compression thermochemical refrigeration system |
| HPs | Heat pumps |
| HtP | Heat to power |
| LTES | Latent thermal energy storage |
| m | Mass (kg) |
| MVC | Mechanical vapor compression |
| ORC | Organic Rankine cycle |
| PCM | Phase change materials |
| PCR | Phase change redox |
| PtH | Power-to-heat |
| PV | Photovoltaic |
| PV-CaL | Photovoltaic Calcium looping |
| Ql | Latent energy stored (J) |
| Qs | Sensible energy stored (J) |
| RES | Renewable energy sources |
| SAPOs | Silico-aluminophosphates |
| STES | Sensible heat storage |
| T | Turbine |
| Tc | Charging temperature (°C) |
| Td | Discharging temperature (°C) |
| TCTES | Thermochemical thermal energy storage |
| TES | Thermal energy storage |
| TESs | Thermal energy storage systems |
| VRE | Variable renewable electricity |
References
- Intergovernmental Panel on Climate Change Climate Change 2014: Mitigation of Climate Change: Working Group III Contribution to the IPCC Fifth Assessment Report. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ipcc_wg3_ar5_frontmatter.pdf (accessed on 23 April 2020).
- Climate Change 2014: Mitigation of Climate Change. Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.buildup.eu/en/practices/publications/ipcc-2014-climate-change-2014-mitigation-climate-change-contribution-working (accessed on 23 April 2020).
- Dagoumas, A.S.; Koltsaklis, N.E. Review of models for integrating renewable energy in the generation expansion planning. Appl. Energy 2019, 242, 1573–1587. [Google Scholar] [CrossRef]
- Iovine, A.; Rigaut, T.; Damm, G.; De Santis, E.; Di Benedetto, M.D. Power management for a DC MicroGrid integrating renewables and storages. Control Eng. Pract. 2019, 85, 59–79. [Google Scholar] [CrossRef]
- Matamala, C.; Moreno, R.; Sauma, E. The value of network investment coordination to reduce environmental externalities when integrating renewables: Case on the Chilean transmission network. Energy Policy 2019, 126, 251–263. [Google Scholar] [CrossRef]
- Arteconi, A.; Hewitt, N.J.; Polonara, F. Domestic demand-side management (DSM): Role of heat pumps and thermal energy storage (TES) systems. Appl. Therm. Eng. 2013, 51, 155–165. [Google Scholar] [CrossRef]
- Fang, J.; Liu, Q.; Guo, S.; Lei, J.; Jin, H. Spanning solar spectrum: A combined photochemical and thermochemical process for solar energy storage. Appl. Energy 2019, 247, 116–126. [Google Scholar] [CrossRef]
- Hsieh, E.; Anderson, R. Grid flexibility: The quiet revolution. Electr. J. 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Huber, M.; Dimkova, D.; Hamacher, T. Integration of wind and solar power in Europe: Assessment of flexibility requirements. Energy 2014, 69, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Denholm, P.; Margolis, R.M. Evaluating the limits of solar photovoltaics (PV) in traditional electric power systems. Energy Policy 2007, 35, 2852–2861. [Google Scholar] [CrossRef]
- DeCesaro, J.; Porter, K.; Milligan, M. Wind Energy and Power System Operations: A Review of Wind Integration Studies to Date. Electr. J. 2009, 22, 34–43. [Google Scholar]
- Lund, P.D.; Lindgren, J.; Mikkola, J.; Salpakari, J. Review of energy system flexibility measures to enable high levels of variable renewable electricity. Renew. Sustain. Energy Rev. 2015, 45, 785–807. [Google Scholar] [CrossRef] [Green Version]
- Salpakari, J.; Mikkola, J.; Lund, P.D. Improved flexibility with large-scale variable renewable power in cities through optimal demand side management and power-to-heat conversion. Energy Convers. Manag. 2016, 126, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, J.; Growitsch, C.; Lorenczik, S.; Nagl, S. Flexibility in Europe’s power sector—An additional requirement or an automatic complement. Energy Econ. 2016, 53, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Denholm, P.; Hand, M. Grid flexibility and storage required to achieve very high penetration of variable renewable electricity. Energy Policy 2011, 39, 1817–1830. [Google Scholar] [CrossRef]
- Reynders, G.; Amaral Lopes, R.; Marszal-Pomianowska, A.; Aelenei, D.; Martins, J.; Saelens, D. Energy flexible buildings: An evaluation of definitions and quantification methodologies applied to thermal storage. Energy Build. 2018, 166, 372–390. [Google Scholar] [CrossRef]
- Vigna, I.; Pernetti, R.; Pasut, W.; Lollini, R. New domain for promoting energy efficiency: Energy Flexible Building Cluster. Sustain. Cities Soc. 2018, 38, 526–533. [Google Scholar] [CrossRef]
- Petersen, M.K.; Edlund, K.; Hansen, L.H.; Bendtsen, J.; Stoustrup, J. A taxonomy for modeling flexibility and a computationally efficient algorithm for dispatch in Smart Grids. In Proceedings of the American Control Conference, Washington, DC, USA, 17–19 June 2013. [Google Scholar]
- Graditi, G.; Di Silvestre, M.L.; Gallea, R.; Sanseverino, E.R. Heuristic-based shiftable loads optimal management in smart micro-grids. IEEE Trans. Ind. Inform. 2015, 11, 271–280. [Google Scholar] [CrossRef]
- Ferruzzi, G.; Cervone, G.; Delle Monache, L.; Graditi, G.; Jacobone, F. Optimal bidding in a Day-Ahead energy market for Micro Grid under uncertainty in renewable energy production. Energy 2016, 106, 194–202. [Google Scholar] [CrossRef]
- Enescu, D.; Chicco, G.; Porumb, R.; Seritan, G. Thermal energy storage for grid applications: Current status and emerging trends. Energies 2020, 13. [Google Scholar] [CrossRef] [Green Version]
- van der Roest, E.; Snip, L.; Fens, T.; van Wijk, A. Introducing Power-to-H3: Combining renewable electricity with heat, water and hydrogen production and storage in a neighbourhood. Appl. Energy 2020, 257, 114024. [Google Scholar] [CrossRef]
- Kohlhepp, P.; Harb, H.; Wolisz, H.; Waczowicz, S.; Müller, D.; Hagenmeyer, V. Large-scale grid integration of residential thermal energy storages as demand-side flexibility resource: A review of international field studies. Renew. Sustain. Energy Rev. 2019, 101, 527–547. [Google Scholar] [CrossRef]
- Kiviluoma, J.; Meibom, P. Influence of wind power, plug-in electric vehicles, and heat storages on power system investments. Energy 2010, 35, 1244–1255. [Google Scholar] [CrossRef]
- Stadler, I. Power grid balancing of energy systems with high renewable energy penetration by demand response. Util. Policy 2008, 16, 90–98. [Google Scholar] [CrossRef]
- Bloess, A.; Schill, W.; Zerrahn, A. Power-to-heat for renewable energy integration: A review of technologies, modeling approaches, and fl exibility potentials. Appl. Energy 2018, 212, 1611–1626. [Google Scholar] [CrossRef]
- Hasan, K.N.; Preece, R.; Milanović, J.V. Existing approaches and trends in uncertainty modelling and probabilistic stability analysis of power systems with renewable generation. Renew. Sustain. Energy Rev. 2019, 101, 168–180. [Google Scholar] [CrossRef]
- Steffen, B.; Weber, C. Efficient storage capacity in power systems with thermal and renewable generation. Energy Econ. 2013, 36, 556–567. [Google Scholar] [CrossRef]
- Beccali, M.; Cellura, M.; Mistretta, M. Environmental effects of energy policy in sicily: The role of renewable energy. Renew. Sustain. Energy Rev. 2007, 11, 282–298. [Google Scholar] [CrossRef]
- Navarro, L.; de Gracia, A.; Colclough, S.; Browne, M.; McCormack, S.J.; Griffiths, P.; Cabeza, L.F. Thermal energy storage in building integrated thermal systems: A review. Part 1. active storage systems. Renew. Energy 2016, 88, 526–547. [Google Scholar] [CrossRef] [Green Version]
- De Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Palizban, O.; Kauhaniemi, K. Energy storage systems in modern grids—Matrix of technologies and applications. J. Energy Storage 2016, 6, 248–259. [Google Scholar] [CrossRef]
- Palomba, V.; Ferraro, M.; Frazzica, A.; Vasta, S.; Sergi, F.; Antonucci, V. Experimental and numerical analysis of a SOFC-CHP system with adsorption and hybrid chillers for telecommunication applications. Appl. Energy 2018, 216, 620–633. [Google Scholar] [CrossRef]
- Vasta, S.; Brancato, V.; La Rosa, D.; Palomba, V.; Restuccia, G.; Sapienza, A.; Frazzica, A. Adsorption heat storage: State-of-the-art and future perspectives. Nanomaterials 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Energy density and storage capacity cost comparison of conceptual solid and liquid sorption seasonal heat storage systems for low-temperature space heating. Renew. Sustain. Energy Rev. 2017, 76, 1314–1331. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Qiu, L.; Li, P.; Zang, Y.; Zou, H.; Yu, Z.; Zhang, X. Review on nanoporous composite phase change materials: Fabrication, characterization, enhancement and molecular simulation. Renew. Sustain. Energy Rev. 2019, 109, 578–605. [Google Scholar] [CrossRef]
- Badenhorst, H. A review of the application of carbon materials in solar thermal energy storage. Sol. Energy 2019, 192, 35–68. [Google Scholar] [CrossRef]
- Bott, C.; Dressel, I.; Bayer, P. State-of-technology review of water-based closed seasonal thermal energy storage systems. Renew. Sustain. Energy Rev. 2019, 113, 109241. [Google Scholar] [CrossRef]
- Palacios, A.; Cong, L.; Navarro, M.E.; Ding, Y.; Barreneche, C. Thermal conductivity measurement techniques for characterizing thermal energy storage materials—A review. Renew. Sustain. Energy Rev. 2019, 108, 32–52. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Ümitcan, H.; Keles, D.; Chiodi, A.; Hartel, R.; Mikuli, M. Analysis of the power-to-heat potential in the European energy system. Energy Strategy Rev. 2018, 20, 6–19. [Google Scholar]
- Tarroja, B.; Mueller, F.; Eichman, J.D.; Samuelsen, S. Metrics for evaluating the impacts of intermittent renewable generation on utility load-balancing. Energy 2012, 42, 546–562. [Google Scholar] [CrossRef]
- Geyer, P.; Buchholz, M.; Buchholz, R.; Provost, M. Hybrid thermo-chemical district networks—Principles and technology. Appl. Energy 2017, 186, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Federal Energy Regulatory Commission. FERC Benefits of Demand Response in Electricity Markets and Recommendations for Achieving Them. Available online: https://eetd.lbl.gov/sites/all/files/publications/report-lbnl-1252d.pdf (accessed on 23 April 2020).
- Khudhair, A.M.; Farid, M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Hauer, A.; Fischer, S.; Heinemann, U.; Schreiner, M.S.W. Thermochemical energy storage and heat transformation of district heat for balancing of power in a district heat network (TCS II) Final report. In Proceedings of the Final Report, Bayerisches Zentrum fuer Angewandte Energieforschung e.V., Wuerzburg, Germany, 1 February 1999. [Google Scholar]
- Hauer, A.; Avemann, E.L. Open Absorption Systems for Air Conditioning and Thermal Energy Storage. In Thermal Energy Storage for Sustainable Energy Consumption; Springer: Dordrecht, The Netherlands, 2007; pp. 429–444. [Google Scholar]
- Wang, W.; Hu, Y.; Yan, J.; Nyström, J.; Dahlquist, E. Combined heat and power plant integrated with mobilized thermal energy storage (M-TES) system. Front. Energy Power Eng. China 2010, 4, 469–474. [Google Scholar] [CrossRef]
- De Coninck, R.; Helsen, L. Quantification of flexibility in buildings by cost curves—Methodology and application. Appl. Energy 2016, 162, 653–665. [Google Scholar] [CrossRef]
- Hachem-Vermette, C.; Guarino, F.; La Rocca, V.; Cellura, M. Towards achieving net-zero energy communities: Investigation of design strategies and seasonal solar collection and storage net-zero. Sol. Energy 2019, 192, 169–185. [Google Scholar] [CrossRef]
- Shkatulov, A.; Ryu, J.; Kato, Y.; Aristov, Y. Composite material “Mg(OH)2/vermiculite”: A promising new candidate for storage of middle temperature heat. Energy 2012, 44, 1028–1034. [Google Scholar] [CrossRef]
- Aristov, Y.I. Challenging offers of material science for adsorption heat transformation: A review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- Christidis, A.; Koch, C.; Pottel, L.; Tsatsaronis, G. The contribution of heat storage to the profitable operation of combined heat and power plants in liberalized electricity markets. Energy 2012, 41, 75–82. [Google Scholar] [CrossRef]
- Aghaei, J.; Alizadeh, M.I. Multi-objective self-scheduling of CHP (combined heat and power)-based microgrids considering demand response programs and ESSs (energy storage systems). Energy 2013, 55, 1044–1054. [Google Scholar] [CrossRef]
- Meroueh, L.; Chen, G. Thermal energy storage radiatively coupled to a supercritical Rankine cycle for electric grid support. Renew. Energy 2020, 145, 604–621. [Google Scholar] [CrossRef]
- Fischer, D.; Bernhardt, J.; Madani, H.; Wittwer, C. Comparison of control approaches for variable speed air source heat pumps considering time variable electricity prices and PV. Appl. Energy 2017, 204, 93–105. [Google Scholar] [CrossRef]
- Fischer, D.; Toral, T.R.; Lindberg, K.B.; Wille-Haussmann, B.; Madani, H. Investigation of Thermal Storage Operation Strategies with Heat Pumps in German Multi Family Houses. Energy Procedia 2014, 58, 137–144. [Google Scholar] [CrossRef]
- Battaglia, M.; Haberl, R.; Bamberger, E.; Haller, M. Increased self-consumption and grid flexibility of PV and heat pump systems with thermal and electrical storage. Energy Procedia 2017, 135, 358–366. [Google Scholar] [CrossRef]
- Oudalov, A.; Cherkaoui, R.; Beguin, A. Sizing and optimal operation of battery energy storage system for peak shaving application. In Proceedings of the 2007 IEEE Lausanne POWERTECH, Lausanne, Switzerland, 1–5 July 2007; pp. 621–625. [Google Scholar]
- Levron, Y.; Shmilovitz, D. Power systems’ optimal peak-shaving applying secondary storage. Electr. Power Syst. Res. 2012, 89, 80–84. [Google Scholar] [CrossRef]
- Péan, T.Q.; Salom, J.; Costa-Castelló, R. Review of control strategies for improving the energy flexibility provided by heat pump systems in buildings. J. Process Control 2019, 74, 35–49. [Google Scholar] [CrossRef]
- Pilpola, S.; Lund, P.D. Different flexibility options for better system integration of wind power. Energy Strateg. Rev. 2019, 26, 100368. [Google Scholar] [CrossRef]
- Stinner, S.; Huchtemann, K.; Müller, D. Quantifying the operational flexibility of building energy systems with thermal energy storages. Appl. Energy 2016, 181, 140–154. [Google Scholar] [CrossRef]
- Le, K.X.; Huang, M.J.; Wilson, C.; Shah, N.N.; Hewitt, N.J. Tariff-based load shifting for domestic cascade heat pump with enhanced system energy efficiency and reduced wind power curtailment. Appl. Energy 2020, 257, 113976. [Google Scholar] [CrossRef]
- Angenendt, G.; Zurmühlen, S.; Rücker, F.; Axelsen, H.; Sauer, D.U. Optimization and operation of integrated homes with photovoltaic battery energy storage systems and power-to-heat coupling. Energy Convers. Manag. X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Lamaison, N.; Collette, S.; Vallée, M.; Bavière, R. Storage influence in a combined biomass and power-to-heat district heating production plant. Energy 2019, 186, 115714. [Google Scholar] [CrossRef]
- Olsthoorn, D.; Haghighat, F.; Mirzaei, P.A. Integration of storage and renewable energy into district heating systems: A review of modelling and optimization. Sol. Energy 2016, 136, 49–64. [Google Scholar] [CrossRef]
- Lund, H.; Werner, S.; Wiltshire, R.; Svendsen, S.; Thorsen, J.E.; Hvelplund, F.; Mathiesen, B.V. 4th Generation District Heating (4GDH). Integrating smart thermal grids into future sustainable energy systems. Energy 2014, 68, 1–11. [Google Scholar] [CrossRef]
- Münster, M.; Morthorst, P.E.; Larsen, H.V.; Bregnbæk, L.; Werling, J.; Lindboe, H.H.; Ravn, H. The role of district heating in the future Danish energy system. Energy 2012, 48, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Mikkola, J.; Lund, P.D. Modeling flexibility and optimal use of existing power plants with large-scale variable renewable power schemes. Energy 2016, 112, 364–375. [Google Scholar] [CrossRef]
- Li, Z.; Wu, W.; Shahidehpour, M.; Wang, J.; Zhang, B. Combined heat and power dispatch considering pipeline energy storage of district heating network. IEEE Trans. Sustain. Energy 2016, 7, 12–22. [Google Scholar] [CrossRef]
- Cellura, M.; Campanella, L.; Ciulla, G.; Guarino, F.; Lo Brano, V.; Cesarini, D.N.; Orioli, A. The redesign of an Italian building to reach net zero energy performances: A case study of the SHC Task 40—ECBCS Annex 52. ASHRAE Trans. 2011, 117, 331–339. [Google Scholar]
- Cellura, M.; Ciulla, G.; Guarino, F.; Longo, S. The redesign of a Rural Building in a Heritage Site in Italy: Towards the Net Zero Energy Target. Buildings 2017, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Werner, S. International review of district heating and cooling. Energy 2017, 137, 617–631. [Google Scholar] [CrossRef]
- Schmidt, D. Low Temperature District Heating for Future Energy Systems. Energy Procedia 2018, 149, 595–604. [Google Scholar] [CrossRef]
- del Hoyo Arce, I.; Herrero López, S.; López Perez, S.; Rämä, M.; Klobut, K.; Febres, J.A. Models for fast modelling of district heating and cooling networks. Renew. Sustain. Energy Rev. 2018, 82, 1863–1873. [Google Scholar] [CrossRef]
- Andersen, A.N.; Østergaard, P.A. Support schemes adapting district energy combined heat and power for the role as a flexibility provider in renewable energy systems. Energy 2020, 192, 116639. [Google Scholar] [CrossRef]
- Ortiz, J.; Guarino, F.; Salom, J.; Corchero, C.; Cellura, M. Stochastic model for electrical loads in Mediterranean residential buildings: Validation and applications. Energy Build. 2014, 80, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Guarino, F.; Cassarà, P.; Longo, S.; Cellura, M.; Ferro, E. Load match optimisation of a residential building case study: A cross-entropy based electricity storage sizing algorithm. Appl. Energy 2015, 154, 380–391. [Google Scholar] [CrossRef]
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar] [CrossRef]
- Tronchin, L.; Manfren, M.; Nastasi, B. Energy efficiency, demand side management and energy storage technologies—A critical analysis of possible paths of integration in the built environment. Renew. Sustain. Energy Rev. 2018, 95, 341–353. [Google Scholar] [CrossRef]
- Singh Gaur, A.; Fitiwi, D.Z.; Curtis, J. Heat Pumps and Their Role in Decarbonising Heating Sector: A Comprehensive Review. Available online: http://aei.pitt.edu/102238/ (accessed on 23 April 2020).
- Vanhoudt, D.; Geysen, D.; Claessens, B.; Leemans, F.; Jespers, L.; Van Bael, J. An actively controlled residential heat pump: Potential on peak shaving and maximization of self-consumption of renewable energy. Renew. Energy 2014, 63, 531–543. [Google Scholar] [CrossRef]
- Sweetnam, T.; Fell, M.; Oikonomou, E.; Oreszczyn, T. Domestic demand-side response with heat pumps: Controls and tariffs. Build. Res. Inf. 2019, 47, 344–361. [Google Scholar] [CrossRef] [Green Version]
- Patteeuw, D.; Henze, G.P.; Helsen, L. Comparison of load shifting incentives for low-energy buildings with heat pumps to attain grid flexibility benefits. Appl. Energy 2016, 167, 80–92. [Google Scholar] [CrossRef]
- Hedegaard, K.; Mathiesen, B.V.; Lund, H.; Heiselberg, P. Wind power integration using individual heat pumps—Analysis of different heat storage options. Energy 2012, 47, 284–293. [Google Scholar] [CrossRef]
- Fischer, D.; Madani, H. On heat pumps in smart grids: A review. Renew. Sustain. Energy Rev. 2017, 70, 342–357. [Google Scholar] [CrossRef] [Green Version]
- Bach, B.; Werling, J.; Ommen, T.; Münster, M.; Morales, J.M.; Elmegaard, B. Integration of large-scale heat pumps in the district heating systems of Greater Copenhagen. Energy 2016, 107, 321–334. [Google Scholar] [CrossRef] [Green Version]
- Lund, R.; Persson, U. Mapping potential heat sources for heat pumps in district heating in Denmark. Energy 2016, 110, 129–138. [Google Scholar] [CrossRef]
- Arat, H.; Arslan, O. Optimization of district heating system aided by geothermal heat pump: A novel multistage with multilevel ANN modelling. Appl. Therm. Eng. 2017, 111, 608–623. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K.; Yang, W.M. Advances in heat pump systems: A review. Appl. Energy 2010, 87, 3611–3624. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, X. Handbook of Energy Systems in Green Buildings; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783662491201. [Google Scholar]
- Chwieduk, D. Analysis of Utilisation of Renewable Energies As Heat Sources for Heat. Renew. Energy 1996, 9, 720–723. [Google Scholar] [CrossRef]
- Chwieduk, B.; Chwieduk, D. Performance analysis of a PV driven heat pump system during a heating season in high latitude countries. In Proceedings of the 12 th IEA Heat Pump Conference, Warsaw, Poland, 29 May 2017; pp. 1–10. [Google Scholar]
- Kim, J.H.; Shcherbakova, A. Common failures of demand response. Energy 2011, 36, 873–880. [Google Scholar] [CrossRef]
- Hu, J.; Chen, W.; Yang, D.; Zhao, B.; Song, H.; Ge, B. Energy performance of ETFE cushion roof integrated photovoltaic/thermal system on hot and cold days. Appl. Energy 2016, 173, 40–51. [Google Scholar] [CrossRef]
- Bogdan, Ž.; Kopjar, D. Improvement of the cogeneration plant economy by using heat accumulator. Energy 2006, 31, 2285–2292. [Google Scholar] [CrossRef]
- Beck, T.; Kondziella, H.; Huard, G.; Bruckner, T. Optimal operation, configuration and sizing of generation and storage technologies for residential heat pump systems in the spotlight of self-consumption of photovoltaic electricity. Appl. Energy 2017, 188, 604–619. [Google Scholar] [CrossRef]
- Franco, A.; Fantozzi, F. Experimental analysis of a self consumption strategy for residential building: The integration of PV system and geothermal heat pump. Renew. Energy 2016, 86, 1075–1085. [Google Scholar] [CrossRef]
- Jarre, M.; Noussan, M.; Simonetti, M. Primary energy consumption of heat pumps in high renewable share electricity mixes. Energy Convers. Manag. 2018, 171, 1339–1351. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, L.; Wang, X.; Sun, T.; Wang, J.; Zhang, S. Flue gas recovery system for natural gas combined heat and power plant with distributed peak-shaving heat pumps. Appl. Therm. Eng. 2017, 111, 599–607. [Google Scholar] [CrossRef]
- Tjaden, T.; Schnorr, F.; Weniger, J.; Bergner, J.; Quaschning, V. Einsatz von PV-Systemen mit Wärmepumpen und Batteriespeichern zur Erhöhung des Autarkiegrades in Einfamilienhaushalten. In Proceedings of the 30. Symp. Photovoltaische Solarenergi, Berlin, Germany, 4–6 March 2015; p. 20. [Google Scholar]
- Fischer, D.; Rautenberg, F.; Wirtz, T.; Wille-Haussmann, B.; Madani, H. Smart Meter Enabled Control for Variable Speed Heat Pumps to Increase PV Self-Consumption. In Proceedings of the 24th IIR International Congress of Refrigeration, Yokohama, Japan, 16–22 August 2015; pp. 4049–4056. [Google Scholar]
- Binder, J.; Williams, C.O.O.; Kelm, T. Increasing pv self-consumption, domestic energy autonomy and grid compatibility of pv systems using heat pumps, thermal storage and battery storage. 27 Eur. Photovolt. Sol. Energy Conf. Exhib. 2012, 4030–4034. [Google Scholar] [CrossRef]
- David, A.; Mathiesen, B.V.; Averfalk, H.; Werner, S.; Lund, H. Heat Roadmap Europe: Large-scale electric heat pumps in district heating systems. Energies 2017, 10, 578. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Kelly, N.J.; Richardson, I.; Thomson, M. Assessing heat pumps as flexible load. Proc. Inst. Mech. Eng. Part A J. Power Energy 2013, 227, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, E.A.M.; Asare-Bediako, B.; De Koning, C.P.; Frunt, J.; Slootweg, J.G. Assessment of an algorithm to utilize heat pump flexibility-theory and practice. In Proceedings of the 2015 IEEE Eindhoven PowerTech, Eindhoven, The Netherlands, 29 June–2 July 2015. [Google Scholar]
- Arteconi, A.; Polonara, F. Assessing the demand side management potential and the energy flexibility of heat pumps in buildings. Energies 2018, 11, 1846. [Google Scholar] [CrossRef] [Green Version]
- Lizana, J.; Chacartegui, R.; Barrios-Padura, A.; Valverde, J.M. Advances in thermal energy storage materials and their applications towards zero energy buildings: A critical review. Appl. Energy 2017, 203, 219–239. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Ciulla, G.; Lo Brano, V.; Cellura, M.; Franzitta, V.; Milone, D. A finite difference model of a PV-PCM system. Energy Procedia 2012, 30, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Bastien, D.; Athienitis, A.K. Passive thermal energy storage, part 2: Design methodology for solaria and greenhouses. Renew. Energy 2017, 103, 537–560. [Google Scholar] [CrossRef]
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef] [PubMed]
- Del Pero, C.; Aste, N.; Paksoy, H.; Haghighat, F.; Grillo, S.; Leonforte, F. Energy storage key performance indicators for building application. Sustain. Cities Soc. 2018, 40, 54–65. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, L.F.; Solé, A.; Barreneche, C. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef] [Green Version]
- Abedin, A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- A Review on High Temperature Thermochemical Heat Energy Storage. Renew. Sustain. Energy Rev. Available online: https://oatao.univ-toulouse.fr/15979/ (accessed on 23 April 2020).
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Thermochemical Energy Storage Systems: Modelling, Analysis and Design. Available online: https://ir.library.dc-uoit.ca/handle/10155/119 (accessed on 23 April 2020).
- Kuznik, F.; Johannes, K. Thermodynamic efficiency of water vapor/solid chemical sorption heat storage for buildings: Theoretical limits and integration considerations. Appl. Sci. 2020, 10, 489. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Riffat, S.B. Thermochemical energy storage technologies for building applications: A state-of-the-art review. Int. J. Low-Carbon Technol. 2013, 8, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Wang, R.Z.; Wang, L.W. Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Han, Y.; Yu, R.; Cheng, Z. Thermochemical storage analysis of the dry reforming of methane in foam solar reactor. Energy Convers. Manag. 2018, 158, 489–498. [Google Scholar] [CrossRef]
- Sorption Thermal Energy Storage. Available online: https://link.springer.com/chapter/10.1007/978-3-319-96640-3_4 (accessed on 23 April 2020).
- Krönauer, A.; Lävemann, E.; Brückner, S.; Hauer, A. Mobile Sorption Heat Storage in Industrial Waste Heat Recovery. Energy Procedia 2015, 73, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Reiser, A.; Bogdanović, B.; Schlichte, K. The application of Mg-based metal-hydrides as heat energy storage systems. Int. J. Hydrogen Energy 2000, 25, 425–430. [Google Scholar] [CrossRef]
- Stengler, J.; Linder, M. Thermal energy storage combined with a temperature boost: An underestimated feature of thermochemical systems. Appl. Energy 2020, 262, 114530. [Google Scholar] [CrossRef]
- Tesio, U.; Guelpa, E.; Verda, V. Integration of thermochemical energy storage in concentrated solar power. Part 2: Comprehensive optimization of supercritical CO2 power block. Energy Convers. Manag. X 2020, 6, 100038. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Moghtaderi, B. Techno-economic analysis of an integrated liquid air and thermochemical energy storage system. Energy Convers. Manag. 2020, 205, 112341. [Google Scholar] [CrossRef]
- Lizana, J.; Bordin, C.; Rajabloo, T. Integration of solar latent heat storage towards optimal small-scale combined heat and power generation by Organic Rankine Cycle. J. Energy Storage 2020, 29, 101367. [Google Scholar] [CrossRef]
- Lass-Seyoum, A.; Blicker, M.; Borozdenko, D.; Friedrich, T.; Langhof, T. Transfer of laboratory results on closed sorption thermo- chemical energy storage to a large-scale technical system. Energy Procedia 2012, 30, 310–320. [Google Scholar] [CrossRef]
- van Helden, W.; Thür, A.; Weber, R.; Furbo, S.; Gantenbein, P.; Heinz, A.; Salg, F.; Kerskes, H.; Williamson, T.; Sörensen, H.; et al. Parallel development of three com- pact systems for seasonal solar thermal storage: Introduction. In Proceedings of the Innostock 2012, 12th International Conference on Energy Storage, Llleida, Spain, 16–18 May 2012. [Google Scholar]
- van Essen, V.M.; Zondag, H.A.; Schuitema, R.; van Helden, W.G.J.; Rindt, C.C.M.; Van Essen, V.M.; Zondag, H.A.; Schuitema, R.; Van Helden, W.G.J.; Rindt, C.C.M. Materials for thermochemical storage: Characterization of magnesium sulfate. In Proceedings of the Proceedings Eurosun, Lisbon, Portugal, 7–10 October 2008; pp. 4–9. [Google Scholar]
- Cammarata, A.; Verda, V.; Sciacovelli, A.; Ding, Y. Hybrid strontium bromide-natural graphite composites for low to medium temperature thermochemical energy storage: Formulation, fabrication and performance investigation. Energy Convers. Manag. 2018, 166, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Poppi, S.; Sommerfeldt, N.; Bales, C.; Madani, H.; Lundqvist, P. Techno-economic review of solar heat pump systems for residential heating applications. Renew. Sustain. Energy Rev. 2018, 81, 22–32. [Google Scholar] [CrossRef]
- IUPAC Compendium of Chemical Terminology. Available online: http://publications.iupac.org/compendium/index.html (accessed on 24 March 2020).
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Sobri, S.; Koohi-Kamali, S.; Rahim, N.A. Solar photovoltaic generation forecasting methods: A review. Energy Convers. Manag. 2018, 156, 459–497. [Google Scholar] [CrossRef]
- Stutz, B.; Le Pierres, N.; Kuznik, F.; Johannes, K.; Palomo Del Barrio, E.; Bédécarrats, J.P.; Gibout, S.; Marty, P.; Zalewski, L.; Soto, J.; et al. Stockage thermique de l’énergie solaire. Comptes Rendus Phys. 2017, 18, 401–414. [Google Scholar] [CrossRef]
- Le Pierrès, N.; Huaylla, F.; Stutz, B.; Perraud, J. Long-term solar heat storage process by absorption with the KCOOH/H2O couple: Experimental investigation. Energy 2017, 141, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- Bush, H.E.; Loutzenhiser, P.G. Solar electricity via an Air Brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Fe2O3/Fe3O4 redox reactions: Thermodynamic and kinetic analyses. Sol. Energy 2018, 174, 617–627. [Google Scholar] [CrossRef]
- Hutchings, K.N.; Wilson, M.; Larsen, P.A.; Cutler, R.A. Kinetic and thermodynamic considerations for oxygen absorption/desorption using cobalt oxide. Solid State Ionics 2006, 177, 45–51. [Google Scholar] [CrossRef]
- Singh, A.; Tescari, S.; Lantin, G.; Agrafiotis, C.; Roeb, M.; Sattler, C. Solar thermochemical heat storage via the Co3O4/CoO looping cycle: Storage reactor modelling and experimental validation. Sol. Energy 2017, 144, 453–465. [Google Scholar] [CrossRef]
- Balasubramanian, G.; Ghommem, M.; Hajj, M.R.; Wong, W.P.; Tomlin, J.A.; Puri, I.K. Modeling of thermochemical energy storage by salt hydrates. Int. J. Heat Mass Transf. 2010, 53, 5700–5706. [Google Scholar] [CrossRef]
- Visscher, K.; Veldhuis, J.B.J. Comparison of candidate materials for seasonal storage of solar heat through dynamic simulation of building and renewable energy system. In Proceedings of the Ninth International IBPSA Conference, Montreal, QC, Canada, 15–18 August 2005; pp. 1285–1292. [Google Scholar]
- Lucio, B.; Romero, M.; González-Aguilar, J. Analysis of solid-state reaction in the performance of doped calcium manganites for thermal storage. Solid State Ionics 2019, 338, 47–57. [Google Scholar] [CrossRef]
- Imponenti, L.; Albrecht, K.J.; Wands, J.W.; Sanders, M.D.; Jackson, G.S. Thermochemical energy storage in strontium-doped calcium manganites for concentrating solar power applications. Sol. Energy 2017, 151, 1–13. [Google Scholar] [CrossRef]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as Adsorbents for Low Temperature Heating and Cooling Applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef] [PubMed]
- N’Tsoukpoe, K.E.; Perier-Muzet, M.; Le Pierrès, N.; Luo, L.; Mangin, D. Thermodynamic study of a LiBr–H2O absorption process for solar heat storage with crystallisation of the solution. Sol. Energy 2014, 104, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Leonzio, G. Solar systems integrated with absorption heat pumps and thermal energy storages: State of art. Renew. Sustain. Energy Rev. 2017, 70, 492–505. [Google Scholar] [CrossRef]
- Hui, L.; N’Tsoukpoe, K.E.; Lingai, L. Evaluation of a seasonal storage system of solar energy for house heating using different absorption couples. Energy Convers Manag. 2011, 52, 2427–2436. [Google Scholar] [CrossRef]
- Li, G.; Hwang, Y.; Radermacher, R. Radermacher Review of cold storage materials for air condition application. Int. J. Refrig 2012, 35, 2053–2077. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Wang, L.W. Theoretical and experimental investigation of a closed sorption thermal storage prototype using LiCl/water. Energy 2015, 93, 1523–1534. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Li, T.X. Experimental investigation on an open sorption thermal storage system for space heating. Energy 2017, 141, 2421–2433. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, R.Z.; Li, T.X.; Nomura, Y. Investigation of a 10 kWh sorption heat storage device for effective utilization of low-grade thermal energy. Energy 2016, 113, 739–747. [Google Scholar] [CrossRef]
- Advanced Storage Concepts for Solar and Low Energy Buildings of the Solar Heating and Cooling Programme of the International Energy Agency. 23 Pages Final Report of Subtask B—Chemical and Sorption Storage. Available online: https://www.iea-shc.org/publications-category?CategoryID=63 (accessed on 23 April 2020).
- Rammelberg, H.U.; Schmidt, T.; Ruck, W. Hydration and dehydration of salt hydrates and hydroxides for thermal energy storage—kinetics and energy release. Energy Procedia 2012, 30, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Fumey, B.; Weber, R.; Gantenbein, P.; Daguenet-Frick, X.; Stoller, S.; Fricker, R.; Dorer, V. Operation Results of a Closed Sorption Heat Storage Prototype. Energy Procedia 2015, 73, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Daguenet-Frick, X.; Dudita, M.; Omlin, L.; Paul, G. Seasonal Thermal Energy Storage with Aqueous Sodium Hydroxide—Development and Measurements on the Heat and Mass Exchangers. Energy Procedia 2018, 155, 286–294. [Google Scholar] [CrossRef]
- Lepinasse, E.; Spinner, B. Production de froid par couplage de re´ acteurs solide-gaz II: Performance d’un pilote de 1 à 2 kW. Rev. Int. Froid. 1994, 17, 323–328. [Google Scholar] [CrossRef]
- Bao, H.S.; Oliveira, R.Z.; Wang, R.Z.; Wang, L.W. Choice of low temperature salt for a resorption refrigerator. Ind. Eng. Chem. Res. 2010, 49, 4897–4903. [Google Scholar] [CrossRef]
- Bao, H.S.; Wang, R.Z.; Wang, L.W. A resorption refrigerator driven by low grade thermal energy. Energy Convers. Manag. 2011, 52, 2339–2344. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Wang, R.Z.; Kiplagat, J.K.; Wang, C.Y. Novel composite sorbent for resorption systems and for chemisorption air conditioners driven by low generation temperature. Renew. Energy 2009, 34, 2757–2764. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Wang, R.Z. Performance of solid–gas reaction heat transformer system with gas valve control. Chem. Eng. Sci. 2010, 65, 2910–2920. [Google Scholar] [CrossRef]
- Ma, Z.; Bao, H.; Roskilly, A.P. Seasonal solar thermal energy storage using thermochemical sorption in domestic dwellings in the UK. Energy 2019, 166, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Li, T.X.; Yan, T.; Wang, R.Z. Advanced thermochemical resorption heat transformer for high-efficiency energy storage and heat transformation. Energy 2019, 175, 1222–1233. [Google Scholar] [CrossRef]
- Nevau, P.; Mazet, N.; Michel, B. Experimental investigation of an innovative thermochemical process operating with a hydrate salt and moist air for thermal storage of solar energy. Appl. Energy 2014, 129, 177–186. [Google Scholar]
- Ferrucci, F.; Stitou, D.; Ortega, P.; Lucas, F. Mechanical compressor-driven thermochemical storage for cooling applications in tropical insular regions. Concept and efficiency analysis. Appl. Energy 2018, 219, 240–255. [Google Scholar] [CrossRef]
- Lehmann, C.; Beckert, S.; Gläser, R.; Kolditz, O.; Nagel, T. Assessment of adsorbate density models for numerical simulations of zeolite-based heat storage applications. Appl. Energy 2017, 185, 1965–1970. [Google Scholar] [CrossRef]
- Finck, C.; Li, R.; Kramer, R.; Zeiler, W. Quantifying demand flexibility of power-to-heat and thermal energy storage in the control of building heating systems. Appl. Energy 2018, 209, 409–425. [Google Scholar] [CrossRef]
- Zettl, B.; Englmair, G.; Steinmaurer, G. Development of a revolving drum reactor for open-sorption heat storage processes. Appl. Therm. Eng. 2014, 70, 42–49. [Google Scholar] [CrossRef]
- Kerskes, H.; Mette, B.; Bertsch, F.; Asenbeck, S.; Drück, H. Development of a thermo-chemical energy storage for solar thermal applications. In Proceedings of the ISES, Solar World Congress, Kassel, Germany, 28 August–2 September 2011. [Google Scholar]
- Energy-Hub for Residential and Commercial Districts and Transport.D3.2 Report on a Combination of Thermal Storage Techniques and Components. Available online: https://www.e-hub.org/pdf/D3.2_Thermal_storage_techniques_components.pdf (accessed on 23 April 2020).
- de Boer, R.; Smeding, S.; Zondag, H.A.; Krol, G. Development of a prototype system for seasonal solar heat storage using an open sorption process. In Proceedings of the Eurotherm Semin, #99—Adv Therm Energy Storage, Lleida, Spain, 28–30 May 2014; pp. 1–9. [Google Scholar]
- Johannes, K.; Kuznik, F.; Hubert, J.-L.; Durier, F.; Obrecht, C. Design and characterisation of a high powered energy dense zeolite thermal energy storage system for buildings. Appl. Energy 2015, 159, 80–86. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Heintz, J.; Lagre, D.; Luo, L.; Durier, F. Experimental and numerical investigations of a zeolite 13X/water reactor for solar heat storage in buildings. Energy Convers. Manag. 2016, 108, 488–500. [Google Scholar] [CrossRef]
- Mehlhorn, D.; Valiullin, R.; Kärger, J.; Schumann, K.; Brandt, A.; Unger, B. Transport enhancement in binderless zeolite X- and A-type molecular sieves revealed by PFG NMR diffusometry. Elesvier 2014, 188, 126–132. [Google Scholar] [CrossRef]
- Chan, K.C.; Chao, C.Y.H.; Sze-To, G.N.; Hui, K.S. Performance predictions for a new zeolite 13X/CaCl2 composite adsorbent for adsorption cooling systems. Int. J. Heat Mass Transf. 2012, 55, 3214–3224. [Google Scholar] [CrossRef]
- Hongois, S.; Kuznik, F.; Stevens, P.; Roux, J.J. Development and characterisation of a new MgSO4-zeolite composite for long-term thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1831–1837. [Google Scholar] [CrossRef]
- Whiting, G.T.; Grondin, D.; Stosic, D.; Bennici, S.; Auroux, A. Zeolite-MgCl2 composites as potential long-term heat storage materials: Influence of zeolite properties on heats of water sorption. Sol. Energy Mater. Sol. Cells 2014, 128, 289–295. [Google Scholar] [CrossRef]
- Dawoud, B.; Aristov, Y. Experimental study on the kinetics of water vapour sorption on selective water sorbents, silica gel and alumina under typical operating conditions of sorption heat pumps. Int. J. Heat Mass Transf. 2003, 46, 273–281. [Google Scholar] [CrossRef]
- Jänchen, J.; Schumann, K.; Thrun, E.; Brandt, A.; Unger, B.; Hellwig, U. Preparation, hydrothermal stability and thermal adsorption storage properties of binderless zeolite beads. Int. J. Low-Carbon Technol. 2012, 7, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Englmair, G.; Zettl, B.; Lager, D. Characterisation of a Rotating Adsorber Designed for Thermochemical Heat Storage Processes. In Proceedings of the EUROSUN2014, International Conference on Solar Energy and Buildings, EuroSun Aix-les-Bains, France, 16–19 September 2015; pp. 1–8. [Google Scholar]
- Finck, C.; Henquet, E.; van Soest, C.; Oversloot, H.; de Jong, A.-J.; Cuypers, R.; van‘t Spijker, H. Experimental Results of a 3 kWh Thermochemical Heat Storage Module for Space Heating Application. Energy Procedia 2014, 48, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Cuypers, R.; Maraz, N.; Eversdijk, J.; Finck, C.; Henquet, E.; Oversloot, H.; van’t Spijker, H.; de Geus, A. Development of a Seasonal Thermochemical Storage System. Energy Procedia 2012, 30, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Jänchen, J.; Ackermann, D.; Weiler, E.; Stach, H.; Brösicke, W. Calorimetric investigation on zeolites, AlPO4’s and CaCl2 impregnated attapulgite for thermochemical storage of heat. Thermochim. Acta 2005, 434, 37–41. [Google Scholar] [CrossRef]
- Engel, G. Sorption thermal energy storage: Hybrid coating/granules adsorber design and hybrid TCM/PCM operation. Energy Convers. Manag. 2019, 184, 466–474. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Magnesium sulphate-silicone foam composites for thermochemical energy storage: Assessment of dehydration behaviour and mechanical stability. Sol. Energy Mater. Sol. Cells 2019, 200, 109992. [Google Scholar] [CrossRef]
- Fasano, M.; Falciani, G.; Brancato, V.; Palomba, V.; Asinari, P.; Chiavazzo, E.; Frazzica, A. Atomistic modelling of water transport and adsorption mechanisms in silicoaluminophosphate for thermal energy storage. Appl. Therm. Eng. 2019, 160, 114075. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Wang, R. A consolidated calcium chloride-expanded graphite compound for use in sorption refrigeration systems. Carbon N. Y. 2007, 45, 390–396. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Wang, R.Z.; Wang, C. Evaluation of the cooling performance of a consolidated expanded graphite-calcium chloride reactive bed for chemi- sorption icemaker. Int. J. Refrig. 2007, 30, 103–112. [Google Scholar] [CrossRef]
- Van Pal, M.D.; Smeding, S. Thermally driven ammonia-salt type ii heat pump: Development and test of a prototype licl. Energy 2007, 2–7. [Google Scholar] [CrossRef]
- Aidoun, Z.; Ternan, M. Salt impregnated carbon fibres as the reactive medium in a chemical heat pump: The NH3–CoCl2 system. Appl. Therm. Eng. 2002, 22, 1163–1174. [Google Scholar] [CrossRef]
- Ristic´, A.; Logar, N.Z.; Henninger, S.K. The performance of small-pore- microporous aluminophosphates in low-temperature solar energy storage: The structure-property relationship. Adv. Funct. Mater. 2012, 22, 1952–1957. [Google Scholar] [CrossRef]
- Kuznik, F.; Johannes, K.; Obrecht, C.; David, D. A review on recent developments in physisorption thermal energy storage for building applications. Renew. Sustain. Energy Rev. 2018, 94, 576–586. [Google Scholar] [CrossRef]
- Jänchen, J.; Ackermann, D.; Stach, H.; Brösicke, W. Studies of the water adsorption on Zeolites and modified mesoporous materials for seasonal storage of solar heat. Sol. Energy 2004, 76, 339–344. [Google Scholar] [CrossRef]
- Developed Materials for Thermal Energy Storage: Synthesis and Characterization. Available online: https://www.sciencedirect.com/science/article/pii/S1876610214027453 (accessed on 23 April 2020).
- Ponomarenko, I.V.; Glaznev, I.S.; Gubar, A.V.; Aristov, Y.I.; Kirik, S.D. Synthesis and water sorption properties of a new composite “CaCl2 confined into SBA-15 pores”. Microporous Mesoporous Mater. 2010, 129, 243–250. [Google Scholar] [CrossRef]
- Tanashev, Y.Y.; Krainov, A.V.; Aristov, Y. Thermal conductivity of composite sorbents “salt in porous matrix” for heat storage and transformation. Appl. Therm. Eng. 2014, 61, 96–99. [Google Scholar] [CrossRef]
- Mahon, D.; Henshall, P.; Claudio, G.; Eames, P.C. Feasibility study of MgSO4 + zeolite based composite thermochemical energy stores charged by vacuum flat plate solar thermal collectors for seasonal thermal energy storage. Renew. Energy 2020, 145, 1799–1807. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Y.; Ding, B.; Yu, G.; Ye, F.; Xu, C. Structure and hydration state characterizations of MgSO4-zeolite 13x composite materials for long-term thermochemical heat storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110047. [Google Scholar] [CrossRef]
- Posern, K.; Kaps, C. Calorimetric studies of thermochemical heat storage materials based on mixtures of MgSO4 and MgCl2. Thermochim. Acta 2010, 129, 243–250. [Google Scholar] [CrossRef]
- Casey, S.P.; Elvins, J.; Riffat, S.; Robinson, A. Salt impregnated desiccant matrices for “open” thermochemical energy storage—Selection, synthesis and characterisation of candidate materials. Energy Build. 2014, 84, 412–425. [Google Scholar] [CrossRef]
- Chen, C.; Lovegrove, K.M.; Sepulveda, A.; Lavine, A.S. Design and optimization of an ammonia synthesis system for ammonia-based solar thermochemical energy storage. Sol. Energy 2018, 159, 992–1002. [Google Scholar] [CrossRef]
- Liu, T.; Bai, Z.; Zheng, Z.; Liu, Q.; Lei, J.; Sui, J.; Jin, H. 100 kWe power generation pilot plant with a solar thermochemical process: Design, modeling, construction, and testing. Appl. Energy 2019, 251, 113217. [Google Scholar] [CrossRef]
- Zondag, H.; Kikkert, B.; Smeding, S.; de Boer, R.; Bakker, M. Prototype thermo- chemical heat storage with open reactor system. Appl. Energy 2013, 109, 360–365. [Google Scholar] [CrossRef]
- Study on Medium-Temperature Chemical Heat Storage using Mixed Hydroxides. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0140700709000449 (accessed on 23 April 2020).
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid–gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Milone, C. Efficiency improvement of heat storage materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 162, 31–39. [Google Scholar] [CrossRef]
- Shkatulov, A.; Krieger, T.; Zaikovskii, V.; Chesalov, Y.; Aristov, Y. Doping magnesium hydroxide with sodium nitrate: A new approach to tune the dehydration reactivity of heat-storage materials. ACS Appl. Mater. Interfaces 2014, 6, 19966–19977. [Google Scholar] [CrossRef]
- Sunku Prasad, J.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Huang, C.; Xu, M.; Huai, X. Experimental investigation on thermodynamic and kinetic of calcium hydroxide dehydration with hexagonal boron nitride doping for thermochemical energy storage. Chem. Eng. Sci. 2019, 206, 518–526. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Sodium-based hydrides for thermal energy applications. Appl. Phys. A Mater. Sci. Process. 2016, 122, 406. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, C.Y. Thermal analysis of exothermic process in a magnesium hydride reactor with porous metals. Chem. Eng. Sci. 2013, 98, 273–281. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.; Fang, Z.Z. Metal hydrides for high-temperature power generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Zhao, C.Y. Dehydration/hydration of MgO/H2O chemical thermal storage system. Energy 2015, 82, 611–618. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Kim, S.T.; Miura, H.; Kato, Y.; Aristov, Y.I. Adapting the MgO-CO2 working pair for thermochemical energy storage by doping with salts. Energy Convers. Manag. 2019, 185, 473–481. [Google Scholar] [CrossRef]
- Gigantino, M.; Kiwic, D.; Steinfeld, A. Thermochemical energy storage via isothermal carbonation-calcination cycles of MgO-stabilized SrO in the range of 1000–1100 °C. Sol. Energy 2019, 188, 720–729. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Pel, L.; Adana, O.C.G. Experimental studies for the cyclability of salt hydrates for thermochemical heat storage. J. Energy Storage 2016, 5, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas-solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Zondag, H.A.; van Essen, V.M.; Bleijendaal, L.P.J.; Kikkert, B.; Bakker, M. Application of MgCl2 6H2O for thermochemical seasonal solar heat storage. In Proceedings of the 5th Int Renew Energy Storage Conference IRES 2010, Berlin, Germany, 22–24 November 2010. [Google Scholar]
- Mehrabadi, A.; Farid, M. New salt hydrate composite for low-grade thermal energy storage. Energy 2018, 164, 194–203. [Google Scholar] [CrossRef]
- De Boer, R.; Haije, W.G.; Veldhuis, J.B.J.; Smeding, S.F. Solid-sorption cooling with integrated thermal storage the SWEAT prototype. In Proceedings of the International Conference Heat Powered Cycles, Larnaca, Cyprus, 10–13 October 2004. [Google Scholar]
- Sakamoto, Y.; Yamamoto, H. Performance of Thermal Energy Storage Unit Using Solid Ammoniated Salt (CaCl2-NH3 System). Nat. Resour. 2014, 5, 337–342. [Google Scholar]
- Review on Advanced of Solar Assisted Chemical Heat Pump Dryer for Agriculture Produce. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1364032110003473 (accessed on 23 April 2020).
- Stitou, D.; Mazet, N.; Mauran, S. Experimental investigation of a solid/gas thermochemical storage process for solar air conditioning. Energy 2012, 41, 261–270. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Wang, Y.; Ling, X.; Jin, X. The role of sensible heat in a concentrated solar power plant with thermochemical energy storage. Energy Convers. Manag. 2019, 190, 42–53. [Google Scholar] [CrossRef]
- Khosa, A.A.; Zhao, C.Y. Heat storage and release performance analysis of CaCO3/CaO thermal energy storage system after doping nano silica. Sol. Energy 2019, 188, 619–630. [Google Scholar] [CrossRef]
- Fernández, R.; Ortiz, C.; Chacartegui, R.; Valverde, J.M.; Becerra, J.A. Dispatchability of solar photovoltaics from thermochemical energy storage. Energy Convers. Manag. 2019, 191, 237–246. [Google Scholar] [CrossRef]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Perez-Maqueda, L.A. Carbonation of Limestone Derived CaO for Thermochemical Energy Storage: From Kinetics to Process Integration in Concentrating Solar Plants. ACS Sustain. Chem. Eng. 2018, 6, 6404–6417. [Google Scholar] [CrossRef] [Green Version]
- Meroueh, L.; Yenduru, K.; Dasgupta, A.; Jiang, D.; AuYeung, N. Energy storage based on SrCO3 and Sorbents—A probabilistic analysis towards realizing solar thermochemical power plants. Renew. Energy 2019, 133, 770–786. [Google Scholar] [CrossRef]
- Takasu, H.; Hoshino, H.; Tamura, Y.; Kato, Y. Performance evaluation of thermochemical energy storage system based on lithium orthosilicate and zeolite. Appl. Energy 2019, 240, 1–5. [Google Scholar] [CrossRef]
- Kerskes, H.; Mette, B.; Bertsch, F.; Asenbeck, S.; Drück, H. Chemical energy storage using reversible solid/gas-reactions (CWS)—Results of the research project. Energy Procedia 2012, 30, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Pebernet, L.; Ferrieres, X.; Pernet, S.; Michielsen, B.L.; Rogier, F.; Degond, P. Discontinuous Galerkin method applied to electromagnetic compatibility problems: Introduction of thin wire and thin resistive material models. IET Sci. Meas. Technol. 2008, 2, 395–401. [Google Scholar] [CrossRef]
- Bales, C.; Gantenbein, P.; Jaenig, D.; Kerskes, H.; Summer, K.; Van Essen, M.; Weber, R. Laboratory Tests of Chemical Reactions and Prototype Sorption Storage Units, A Report of IEA Solar Heating and Cooling programme—Task 32. Sol. Heat. Cool. Program. IET Sci. Meas. Technol. 2008, 2, 395–401. [Google Scholar]
- Iammak, K.; Wongsuwan, W.; Kiatsiriroj, T. Investigation of modular chemical energy storage performance. In Proceedings of the Proc jt int conf energy environ, Hua Hin, Thailand, 1–3 December 2004. [Google Scholar]
- Koepf, E.; Villasmil, W.; Meiera, A. Pilot-scale solar reactor operation and characterization for fuel production via the Zn/ZnO thermochemical cycle. J. Appl. Energy 2015, 165, 1004–1023. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Thomey, D.; de Oliveira, L.; Happich, C.; Roeb, M.; Sattler, C.; Tsongidis, N.I.; Sakellariou, K.G.; Pagkoura, C.; Karagiannakis, G.; et al. Oxide particles as combined heat storage medium and sulphur trioxide decomposition catalysts for solar hydrogen production through sulphur cycles. Int. J. Hydrogen Energy 2019, 44, 9830–9840. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Moghtaderi, B. Thermodynamic analysis of a novel hybrid thermochemical-compressed air energy storage system powered by wind, solar and/or off-peak electricity. Energy Convers. Manag. 2019, 180, 1268–1280. [Google Scholar] [CrossRef]
- Tescari, S.; Singh, A.; Agrafiotis, C.; de Oliveira, L.; Breuer, S.; Schlögl-Knothe, B.; Roeb, M.; Sattler, C. Experimental evaluation of a pilot-scale thermochemical storage system for a concentrated solar power plant. Appl. Energy 2017, 189, 66–75. [Google Scholar] [CrossRef]
- Zhou, X.; Mahmood, M.; Chen, J.; Yang, T.; Xiao, G.; Ferrari, M.L. Validated model of thermochemical energy storage based on cobalt oxides. Appl. Therm. Eng. 2019, 159, 113965. [Google Scholar] [CrossRef]
- Yilmaz, D.; Darwish, E.; Leion, H. Investigation of the combined Mn-Si oxide system for thermochemical energy storage applications. J. Energy Storage 2020, 28, 101180. [Google Scholar] [CrossRef]
- Smithson, G.L.; Bakhshi, N.N. The kinetics and mechanism of the hydation of magnesium oxide in a batch reactor. Can. J. Chem. Eng. 1969, 47, 508–513. [Google Scholar] [CrossRef]
- Feitknecht, W.; Braun, H. Der Mechanismus der Hydratation von Magnesiumoxid mit Wasserdampf. Helv. Chim. Acta 1967, 50, 2040–2053. [Google Scholar] [CrossRef]
- Dai, L.; Long, X.F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Schaube, F.; Kohzer, A.; Schutz, J.; Worner, A.; Müller-Steinhagen, H. De- and rehy- dration of Ca(OH)2 in a reactor with direct heat transfer for thermochemical heat storage. Part A: Experimental results. Chem. Eng. Res. Des. 2013, 91, 856–864. [Google Scholar] [CrossRef]
- Fujii, I.; Tsuchiya, K.; Higano, M.; Yamada, J. Studies of an energy storage system by use of the reversible chemical reaction: CaO + H2O ⇌ Ca(OH)2. Sol. Energy 1985, 34, 367–377. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Appl. Energy 2016, 175, 277–284. [Google Scholar] [CrossRef]
- Cosquillo Mejia, A.; Afflerbach, S.; Linder, M.; Schmidt, M. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor. Appl. Therm. Eng. 2020, 169, 114961. [Google Scholar] [CrossRef]
- Long, X.F.; Dai, L.; Lou, B.; Wu, J. The kinetics research of thermochemical energy storage system Ca(OH)(2)/CaO. Int. J. Energy Res. 2017, 41, 1004–1013. [Google Scholar] [CrossRef]
- Andre, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 707–715. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Zhao, J. Development on thermochemical energy storage based on CaO-based materials: A review. Sustainability 2018, 10, 2660. [Google Scholar] [CrossRef] [Green Version]
- Composite Material for High-Temperature Thermochemical Energy Storage Using Calcium Hydroxide and Ceramic Foam. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/est2.53 (accessed on 29 March 2020).
- Ogura, H.; Yamamoto, T.; Kage, H. Efficiencies of CaO/H2O/Ca(OH)2 Chemical Heat Pump for Heat Storing and Heating/Cooling. Energy 2003, 28, 1479–1493. [Google Scholar] [CrossRef]
- Arjmand, M.; Liu, L.; Neretnieks, I. Exergetic Efficiency of High-Temperature-Lift Chemical Heat Pump (CHP) Based on CaO/CO2 and CaO/H2O Working Pairs. Int. J. Energy Res. 2013, 37, 1122–1131. [Google Scholar] [CrossRef]
- Mette, B.; Kerskes, H.; Drück, H.; Müller-Steinhagen, H. New highly efficient regeneration process for thermochemical energy storage. Appl. Energy 2013, 109, 352–359. [Google Scholar] [CrossRef]
- Shelyapina, M.G. Metal hydrides for energy storage. Handb. Ecomater. 2019, 2, 775–810. [Google Scholar]
- Humphries, T.D.; Sheppard, D.A.; Li, G.; Rowles, M.R.; Paskevicius, M.; Matsuo, M.; Aguey-Zinsou, K.F.; Sofianos, M.V.; Orimo, S.I.; Buckley, C.E. Complex hydrides as thermal energy storage materials: Characterisation and thermal decomposition of Na2Mg2NiH6. J. Mater. Chem. A 2018, 6, 9099–9108. [Google Scholar] [CrossRef] [Green Version]
- Ward, P.A.; Corgnale, C.; Teprovich, J.A.; Motyka, T.; Hardy, B.; Sheppard, D.; Buckley, C.; Zidan, R. Technical challenges and future direction for high-efficiency metal hydride thermal energy storage systems. Appl. Phys. A Mater. Sci. Process. 2016, 122, 426. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Teprovich, J.A.; Zidan, R.; Ward, P.A. High temperature thermal energy storage in the CaAl2 system. J. Alloys Compd. 2017, 735, 2611–2615. [Google Scholar]
- Javadian, P.; Gharibdoust, S.H.P.; Li, H.W.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Reversibility of LiBH4 Facilitated by the LiBH4-Ca(BH4)2 Eutectic. J. Phys. Chem. C 2017, 121, 18439–18449. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Sheppard, D.A.; Buckley, C.E. Lithium imide systems for high temperature heat storage in concentrated solar thermal systems. J. Alloys Compd. 2017, 716, 291–298. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Buckley, C.E. The potential of metal hydrides paired with compressed hydrogen as thermal energy storage for concentrating solar power plants. Int. J. Hydrogen Energy 2019, 44, 9143–9163. [Google Scholar] [CrossRef]
- Manickam, K.; Mistry, P.; Walker, G.; Grant, D.; Buckley, C.E.; Humphries, T.D.; Paskevicius, M.; Jensen, T.; Albert, R.; Peinecke, K.; et al. Future perspectives of thermal energy storage with metal hydrides. Int. J. Hydrogen Energy 2019, 44, 7738–7745. [Google Scholar] [CrossRef]
- Experimental Study of Salt Hydrates for Thermochemical Seasonal Heat Storage. Available online: https://research.tue.nl/en/publications/experimental-study-of-salt-hydrates-for-thermochemical-seasonal-h (accessed on 23 April 2020).
- Mukherjee, A.; Majumdar, R.; Saha, S.K.; Kumar, L.; Subramaniam, C. Assessment of open thermochemical energy storage system performance for low temperature heating applications. Appl. Therm. Eng. 2019, 156, 453–470. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A systematic multi-step screening of numerous salt hydrates for low temperature ther- mochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Seasonal Storage Coupled to a Solar Combisystem: Dynamic Simulations for Process Dimensioning. Available online: http://proceedings.ises.org/paper/eurosun2010/eurosun2010-0262-Tanguy.pdf (accessed on 29 March 2020).
- Fopah-lele, A.; Gaston, J. Solar Energy Materials & Solar Cells A review on the use of SrBr2 · 6H2O as a potential material for low temperature energy storage systems and building applications. Sol. Energy Mater. Sol. Cells 2017, 164, 175–187. [Google Scholar]
- Lahmidi, H.; Mauran, S.; Goetz, V. Definition, test and simulation of a thermochemical storage process adapted to solar thermal systems. Sol. Energy 2006, 80, 883–893. [Google Scholar] [CrossRef]
- Mauran, S.; Lahmidi, H.; Goetz, V. Solar heating and cooling by a thermochemical process. First experiments of a prototype storing 60 kW h by a solid/gas reaction. Sol. Energy 2008, 82, 623–636. [Google Scholar] [CrossRef]
- Clark, R.J.; Mehrabadi, A.; Farid, M. State of the art on salt hydrate thermochemical energy storage systems for use in building applications. J. Energy Storage 2020, 27, 101145. [Google Scholar] [CrossRef]
- Humphries, T.D.; Møller, K.T.; Rickard, W.D.A.; Sofianos, M.V.; Liu, S.; Buckley, C.E.; Paskevicius, M. Dolomite: A low cost thermochemical energy storage material. J. Mater. Chem. A 2019, 7, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.; Horvat, A.; Sobrino, C.; Sánchez-Delgado, S. Thermochemical conversion of C. cardunculus L. in nitrate molten salts. Appl. Therm. Eng. 2019, 148, 136–146. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Sarrion, B.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Manuel Valverde, J. Large-scale high-temperature solar energy storage using natural minerals. Sol. Energy Mater. Sol. Cells 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Barker, R. The Reversibility of the Reaction CaCO3(s) ⇌ CaO(s) + CO2 (g). J. Appl. Chem. Biotechnol. 1973, 23, 733–742. [Google Scholar] [CrossRef]
- Wu, H.; Salles, F.; Zajac, J. A Critical Review of Solid Materials for Low-Temperature Thermochemical Storage of Solar Energy Based on Solid-Vapour Adsorption in View of Space Heating Uses. Molecules 2019, 24, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belz, K.; Kuznik, F.; Werner, K.F.; Schmidt, T.; Ruck, W.K.L. Thermal energy storage systems for heating and hot water in residential buildings. Elsevier Inc. 2015, 441–465. [Google Scholar] [CrossRef]
- Requirements to Consider when Choosing a Thermochemical Material for Solar Energy Storage. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0038092X13003484 (accessed on 23 April 2020).
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016. Available online: https://www.sciencedirect.com/topics/engineering/thermochemical-energy-storage (accessed on 23 April 2020).
- Schmidt, M.; Linder, M. Power generation based on the Ca(OH)2/CaO thermochemical storage system–Experimental investigation of discharge operation modes in lab scale and corresponding conceptual process design. Appl. Energy 2017, 203, 594–607. [Google Scholar] [CrossRef]
- Fopah-Lele, A.; Rohde, C.; Neumann, K.; Tietjen, T.; Rönnebeck, T.; N’Tsoukpoe, K.E.; Osterland, T.; Opel, O.; Ruck, W.K.L. Lab-scale experiment of a closed thermo- chemical heat storage system including honeycomb heat exchanger. Energy 2016, 114, 225–238. [Google Scholar] [CrossRef]
- Michel, B.; Neveu, P.; Mazet, N. Comparison of closed and open thermochemical processes, for long-term thermal energy storage applications. Energy 2014, 72, 702–716. [Google Scholar] [CrossRef] [Green Version]
- Eigenberger, G.; Ruppel, W. Catalytic fixed-bed reactors. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley–VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Volume 122, pp. 197–205. [Google Scholar]
- Zondag, H.A.; Schuitema, R.; Bleijendaal, L.P.J.; Gores, J.C.; van Essen, M.; van Helden, W.B. R&D of thermochemical reactor concepts to enable heat storage of solar energy in residential houses. In Proceedings of the 3rd International Conference on Energy Sustainability, San Francisco, CA, USA, 19–23 July 2009. [Google Scholar]
- Farcot, L.; Le Pierrès, N.; Michel, B.; Fourmigué, J.-F.; Papillon, P. Numerical investigations of a continuous thermochemical heat storage reactor. J. Energy Storage 2018, 20, 109–119. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. Assessment of a closed thermochemical energy storage using energy and exergy methods. Appl. Energy 2012, 93, 18–23. [Google Scholar] [CrossRef]
- Romanchenko, D.; Kensby, J.; Odenberger, M.; Johnsson, F. Thermal energy storage in district heating: Centralised storage vs. storage in thermal inertia of buildings. Energy Convers. Manag. 2018, 162, 26–38. [Google Scholar] [CrossRef]
- High Energy Density Sorption Heat Storage for Solar Space Heating (Hydes Heat Storage), Final Report of EU Project in the JOULE III Program. Available online: https://op.europa.eu/en/publication-detail/-/publication/91c4b9d0-5775-4bc4-9c2c-184804739041 (accessed on 23 April 2020).
- Nwulu, N.I.; Xia, X. Optimal dispatch for a microgrid incorporating renewables and demand response. Renew. Energy 2017, 101, 16–28. [Google Scholar] [CrossRef]
- Li, T.X.; Wang, R.Z.; Yan, T.; Ishugah, T.F. Integrated energy storage and energy upgrade, combined cooling and heating supply, and waste heat recovery with solid-gas thermochemical sorption heat transformer. Int. J. Heat Mass Transf. 2014, 76, 237–246. [Google Scholar] [CrossRef]
- Yan, T.; Wang, C.Y.; Li, D. Performance analysis of a solid-gas thermochemical composite sorption system for thermal energy storage and energy upgrade. Appl. Therm. Eng. 2019, 150, 512–521. [Google Scholar] [CrossRef]
- Yang, S.; Deng, C.; Liu, Z. Optimal design and analysis of a cascade LiBr/H2O absorption refrigeration/transcritical CO2 process for low-grade waste heat recovery. Energy Convers. Manag. 2019, 192, 232–242. [Google Scholar] [CrossRef]
- Nasri, M.; Burger, I.; Michael, S.; Friedrich, H.E. Waste heat recovery for fuel cell electric vehicle with thermochemical energy storage. In Proceedings of the 1th International Conference on Ecological Vehicles and Renewable Energies, Monte Carlo, Monaco, 6–8 April 2016; pp. 1–6. [Google Scholar]
- Kuwata, K.; Masuda, S.; Kobayashi, N.; Fuse, T.; Okamura, T. Thermochemical Heat Storage Performance in the Gas/Liquid-Solid Reactions of SrCl2 with NH3. Nat. Resour. 2016, 7, 655–665. [Google Scholar]
- Jarimi, H.; Aydin, D.; Yanan, Z.; Ozankaya, G.; Chen, X.; Riffat, S. Review on the recent progress of thermochemical materials and processes for solar thermal energy storage and industrial waste heat recovery. Int. J. Low-Carbon Technol. 2019, 14, 44–69. [Google Scholar] [CrossRef] [Green Version]
- Kerkes, H.; Sommr, K.; Muller, H. Monosorp—ein Integrales Konzept zur Solarthermischen Gebaudeheizung mit Sorptionwarmespeicher. Available online: https://elib.dlr.de/54282/ (accessed on 29 March 2020).
- Development of a Thermo-Chemical Energy Storage for Solar Thermal Applications. Available online: http://www.t.task20.iea-shc.org/data/sites/1/publications/Task42-Development_of_a_Thermo-Chemical_Energy_Storage_for_Solar_Thermal_Applications.pdf (accessed on 29 March 2020).
- Particle Reactors for Solar Thermochemical Processes. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0038092X17304917 (accessed on 23 March 2020).
- Modularer Energiespeicher nach dem Sorptionsprinzip mit hoher Energiedichte (MODESTORE). Available online: https://www.aee-intec.at/modularer-energiespeicher-nach-dem-sorptionsprinzip-mit-hoher-energiedichte-modestore-p45 (accessed on 29 March 2020).
- Mustafa Omer, A. Ground-source heat pumps systems and applications. Renew. Sustain. Energy Rev. 2008, 12, 344–371. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Liu, M.; Steven Tay, N.H.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Hewitt, N.J. Heat pumps and energy storage—The challenges of implementation. Appl. Energy 2012, 89, 37–44. [Google Scholar] [CrossRef]
- Ground Source Heat Pumps. Available online: https://www.sciencedirect.com/topics/engineering/ground-source-heat-pump (accessed on 23 March 2020).
- Molten Silicon Storage of Concentrated Solar Power with Integrated Thermophotovoltaic Energy Conversion. Available online: https://aip.scitation.org/doi/10.1063/1.5067099 (accessed on 24 March 2020).
- Datas, A.; Ramos, A.; Martí, A.; del Cañizo, C.; Luque, A. Ultra high temperature latent heat energy storage and thermophotovoltaic energy conversion. Energy 2016, 107, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Fitó, J.; Coronas, A.; Mauran, S.; Mazet, N.; Perier-Muzet, M.; Stitou, D. Hybrid system combining mechanical compression and thermochemical storage of ammonia vapor for cold production. Energy Convers. Manag. 2019, 180, 709–723. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Moghtaderi, B. A unique phase change redox cycle using CuO/Cu2O for utility-scale energy storage. Energy Convers. Manag. 2019, 188, 366–380. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, D.; Lu, C.; He, Q.; Liu, W. A review of thermal energy storage in compressed air energy storage system. Energy 2019, 188, 115993. [Google Scholar] [CrossRef]
- Rodriguez-Hidalgo, M.C.; Rodriguez-Aumente, P.A.; Lecuona-Neumann, A.; Legrand, M. Thermo-chemical storage for renewable energies based on absorption: Getting a uniform injection into the grid. Appl. Therm. Eng. 2019, 146, 338–345. [Google Scholar] [CrossRef]

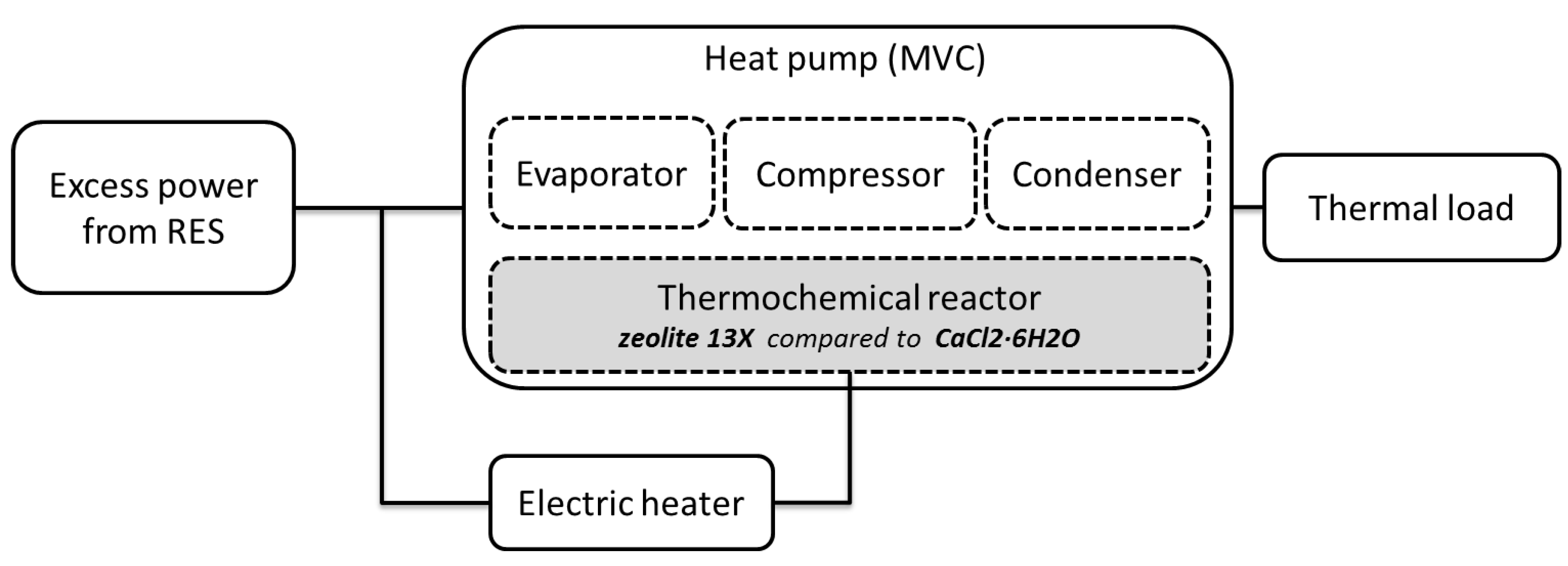




| TES System | Capacity (kWh/t) | Power (MW) | Efficiency (%) | Storage Period | Cost (€/kWh) |
|---|---|---|---|---|---|
| Sensible | 10–50 | 0.001–10.0 | 50–90 | days/months | 0.1–10 |
| Latent | 50–100 | 0.001–1.0 | 75–90 | hours/months | 10–50 |
| Thermochemical | 120–250 | 0.01–1.0 | 75–100 | hours/days | 8–100 |
| Project Name/Institution | Description | Storage System |
|---|---|---|
| MONOSORP [305] (2006) |
| Zeolite 4A |
| Institute for Solar Technology SPF [241] (2006) |
| Zeolite 13X |
| ECN 1 [227] (2010) |
| MgCl2 ⋅6H2O |
| CWS 2 [306] (2011) |
| LiCl with Zeolite 13X used as additive |
| ECN [211] (2013) |
| MgCl2 · H2O |
| Energy hub-ECN [178,179] (2013–2014) |
| Zeolite 13X |
| ASIC 3 [176] (2014) |
| Zeolite 4A (Zeolite 13X) |
| STAID 4 [180] (2015) |
| Zeolite 13X |
| ESSI 5 [276] (2016) |
| SrBr2 ⋅6H2O |
| STAID [181] (2016) |
| Zeolite 13X |
| NSFC 6 [159] (2017–2018) |
| Activated alumina/LiCl |
| Project Name/Institution | Description | Storage System |
|---|---|---|
| SWEAT 1/ECN [229] (2004) |
| Na2S/H2O |
| MCES 2 [242] (2004) |
| Na2S⋅9H2O and graphite used as additive |
| MODESTORE [141,307] (2006) |
| Silica gel |
| SOLAR-STORE [308] (2006) |
| SrBr2 with expanded natural graphite |
| SOLAR-STORE [278] (2008) |
| SrBr2 |
| Fraunhofher [136] (2012) |
| Zeolite/CaCl2 |
| E-hub/Project [190] (2012) |
| Zeolite |
| E-hub/Project [189] (2014) |
| Zeolite 5A |
| COMTES 3 [309] (2015) |
| Zeolite 13XBF |
| COMTES [163] (2015) |
| NaOH/H2O |
| SJTU 4 [160] (2016) |
| LiCl with expanded graphite |
| HSR-SPF 5 [164] (2018) |
| NaOH/H2O |
| Heat STRESS [170] (2019) |
| CaCl2/NH3 |
| University of Newcastle [245] (2019) |
| Co3O4/CoO |
| RESTRUCTURE [247] (2019) |
| Co3O4/CoO |
| References | Application | Storage Material | Performance Indicators |
|---|---|---|---|
| Cammarata et al. [139] | Power-to-heat (household application) | SrBr2/H2O | Energy density: 500 kJ/kg |
| Ferrucci et al. [173] | Power-to-heat (integrated into electric driven cooling system) | BaCl2/NH3 | Energy density: 200 kJ/kg COP = 4.8 |
| Finck et al. [175] | Power-to-heat (integrated into electric driven cooling system) | Zeolite 13X/H2O | Capacity: 5.6 kWh Efficiency: 0.96 |
| Wu et al. [245] | Power-to-heat (to power) | Co3O4/CoO | Energy density: 3.9 kWh/m3 Efficiency: 56.4% |
| Fernandez et al. [235] | Power-to-heat (to power) | CaCO3/CaO | Overall plant Efficiency: 39% |
| Wu et al. [316] | Power-to-heat (to power) | CuO/Cu2O | Energy density: 1600 kJ/kg Efficiency: 50% |
| Rodriguez et al. [318] | Power-to-heat (to power) | NH3/LiNO3 | Capacity: 0.36 MWh Efficiency: 44.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Airò Farulla, G.; Cellura, M.; Guarino, F.; Ferraro, M. A Review of Thermochemical Energy Storage Systems for Power Grid Support. Appl. Sci. 2020, 10, 3142. https://doi.org/10.3390/app10093142
Airò Farulla G, Cellura M, Guarino F, Ferraro M. A Review of Thermochemical Energy Storage Systems for Power Grid Support. Applied Sciences. 2020; 10(9):3142. https://doi.org/10.3390/app10093142
Chicago/Turabian StyleAirò Farulla, Girolama, Maurizio Cellura, Francesco Guarino, and Marco Ferraro. 2020. "A Review of Thermochemical Energy Storage Systems for Power Grid Support" Applied Sciences 10, no. 9: 3142. https://doi.org/10.3390/app10093142
APA StyleAirò Farulla, G., Cellura, M., Guarino, F., & Ferraro, M. (2020). A Review of Thermochemical Energy Storage Systems for Power Grid Support. Applied Sciences, 10(9), 3142. https://doi.org/10.3390/app10093142









