Vermiremediation of Biomixtures from Biobed Systems Contaminated with Pesticides
Abstract
1. Introduction
2. Material and Methods
2.1. Uncontaminated and Contaminated Biomixtures
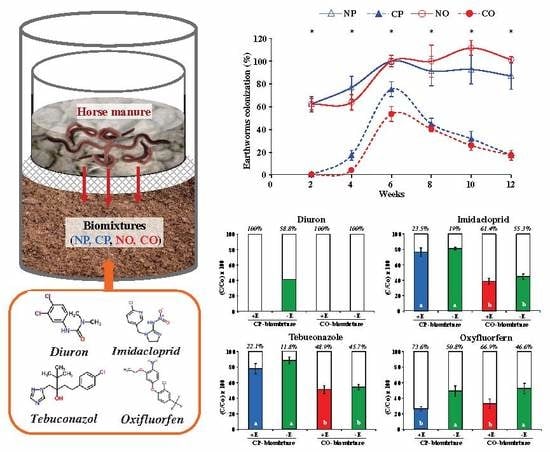
2.2. Experimental Layout
2.3. Pesticides Analysis in the Biomixtures
2.4. Chemical and Dehydrogenase Activity in the Biomixtures
2.5. Statistical Analysis
3. Results and Discussion
3.1. Development of Earthworms in the Different Biomixtures
3.2. Evolution of Dehydrogenase Activity during the Vermiremediation Process
3.3. Chemical Changes in the Different Biomixtures
3.4. Dissipation of Pesticide Residues in the Biomixtures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castillo, M.D.P.; Torstensson, L.; Stenström, J. Biobeds for environmental protection from pesticide use—A review. J. Agric. Food Chem. 2008, 56, 6206–6219. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Moreno, L.; Nogales, R.; Romero, E. Biodegradation of high doses of commercial pesticide products in pilot-scale biobeds using olive-oil agroindustry wastes. J. Environ. Manag. 2017, 204, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.A.; Gebler, L.; Niemeyer, J.; Itako, A.T. Destination of pesticide residues on biobeds: State of the art and future perspectives in Latin America. Chemosphere 2020, 248, 126038. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.; Delgado-Moreno, L.; Nogales, R. Pesticide Dissipation and Enzyme Activities in Ungrassed and Grassed Biomixtures, Composed of Winery Wastes, Used in Biobed Bioremediation Systems. Water Air. Soil Poll. 2019, 230, 33. [Google Scholar] [CrossRef]
- Romero, I.A.; van Dillewijn, P.; Nesme, J.; Sørensen, S.J.; Romero, E. Improvement of pesticide removal in contaminated media using aqueous extracts from contaminated biopurification systems. Sci. Total Environ. 2019, 691, 749–759. [Google Scholar] [CrossRef]
- De Wilde, T.; Debaer, C.; Ryckeboer, J.; Springael, D.; Spanoghe, P. The influence of small-and large-scale composting on the dissipation of pesticide residues in a biopurification matrix. J. Sci. Food Agric. 2010, 90, 1113–1120. [Google Scholar] [CrossRef]
- Karas, P.A.; Perruchon, C.; Karanasios, E.; Papadopoulou, E.S.; Manthou, E.; Sitra, S.; Ehaliotis, C.; Karpouzas, D.G. Integrated biodepuration of pesticide contaminated wastewaters from the fruit-packaging industry: Bioaugmentation, risk assessment and optimized management. J. Hazard. Mater. 2016, 320, 635–644. [Google Scholar] [CrossRef]
- Nogales, R.; Romero, E.; Fernández-Gómez, M. Vermicompostaje: Procesos, Productos y Aplicaciones; Mundi Prensa: Madrid, Spain, 2014; p. 172. [Google Scholar]
- Nogales, R.; Fernández-Gomez, M.J.; Delgado-Moreno, L.; Castillo-Díaz, J.M.; Romero, E. Eco-friendly vermitechnological winery waste management: A pilot-scale study. SN Appl. Sci. 2020, 2, 653. [Google Scholar] [CrossRef]
- Singh, A.; Singh, G.S. Vermicomposting: A Sustainable tool for environmental equilibria. Environ. Qual. Manage 2017, 27, 23–40. [Google Scholar] [CrossRef]
- Dominguez, J.; Edwards, C.A. Biology and ecology of earthworm species used for vermicomposting. In Vermiculture Technology: Earthworms, Organic Waste and Environmental Management; Edwards, C.A., Arancon, N.Q., Sherman, R., Eds.; CRC Press: Boca Raton, CA, USA, 2011; pp. 27–38. [Google Scholar]
- Rodriguez-Campos, J.; Dendooven, L.; Alvarez-Bernal, D.; Contreras-Ramos, S.M. Potential of earthworms to accelerate removal of organic contaminants from soil: A review. Appl. Soil Ecol. 2014, 79, 10–25. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Olle, M. Review: Vermicompost, its importance and benefit in agriculture. J. Agric. Sci. 2019, 2, 93–98. [Google Scholar]
- Shi, Z.; Liua, J.; Tanga, Z.; Zhaob, Y.; Wanga, C. Vermiremediation of organically contaminated soils: Concepts, current status, and future perspectives. Appl. Soil Ecol. 2020, 147, 103377. [Google Scholar] [CrossRef]
- Rorat, A.; Wloka, D.; Grobelak, A.; Grosser, A.; Sosnecka, A.; Milczarek, M.; Jelonek, P.; Vandenbulcke, F.; Kacprzak, M. Vermiremediation of polycyclic aromatic hydrocarbons and heavy metals in sewage sludge composting process. J. Environ. Manag. 2017, 187, 347–353. [Google Scholar] [CrossRef]
- Suthar, S.; Sajwan, P.; Kumar, K. Vermiremediation of heavy metals in wastewater sludge from paper and pulp industry using earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2014, 109, 177–184. [Google Scholar] [CrossRef]
- Usmani, Z.; Kumar, V.; Mritunjay, S.K. Vermicomposting of coal fly ash using epigeic and epi-endogeic earthworm species: Nutrient dynamics and metal remediation. RSC Adv. 2017, 7, 4876–4890. [Google Scholar] [CrossRef]
- Jordão, C.P.; Pereira, W.L.; Carari, D.M.; Fernandes, R.B.A.; De Almeida, R.M.; Fontes, M.P.F. Adsorption from Brazilian soils of Cu(II) and Cd(II) using cattle manure vermicompost. Int. J. Environ. Studies 2011, 68, 719–736. [Google Scholar] [CrossRef]
- Liu, B.; Wua, C.H.; Pana, P.; Fua, Y.; Heb, Z.; Wua, L.; Li, Q. Remediation effectiveness of vermicompost for a potentially toxic metal contaminated tropical acidic soil in China. Ecotoxicol. Environ. Saf. 2019, 182, 109394. [Google Scholar] [CrossRef]
- Fernández-Bayo, J.D.; Nogales, R.; Romero, E. Winery vermicomposts to control the leaching of diuron, imidacloprid and their metabolites: Role of dissolved organic carbon content. J. Environ. Sci. Health Part B 2015, 50, 190–200. [Google Scholar] [CrossRef]
- Castillo-Diaz, J.M.; Beguet, J.; Martin-Laurent, F.; Nogales, R.; Romero, E. Multidisciplinary assessment of pesticide mitigation in soil amended with vermicomposted agroindustrial wastes. J. Hazard. Mat. 2016, 304, 379–387. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Peña, A. Compost and vermicompostof olive cake to bioremediate triazines-contaminated soil. Sci. Tot. Environ. 2009, 407, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, X.; Li, Y.; Huang, D.; Donga, J.; Li, F. Enhancement effect of two ecological earthworm species (Eisenia foetida and Amynthas robustus E. Perrier) on removal and degradation processes of soil DDT. J. Environ. Monit. 2012, 14, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Diaz, J.M.; Delgado-Moreno, L.; Núñez, R.; Nogales, R.; Romero, E. Enhancing pesticide degradation using indigenous microorganisms isolated under high pesticide load in bioremediation systems with vermicomposts. Bioresour. Technol. 2016, 214, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Haby, V.A. Simplified colorimetric determination of soil organic matter. Soil Sci. 1971, 112, 137–141. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Pramanik, P.; Safique, S.; Jahan, A.; Bhagat, R.M. Effect of vermicomposting on treated hard stem leftover wastes from pruning of tea plantation: A novel approach. Ecol. Eng. 2016, 97, 410–415. [Google Scholar] [CrossRef]
- Wang, Y.; Cang, T.; Zhao, X.; Yu, R.; Chen, L.; Wu, C.H.; Wang, Q. Comparative acute toxicity of twenty-four insecticides to earthworm, Eisenia fétida. Ecotoxicol. Environ. Saf. 2012, 79, 122–128. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual, A World Compendium, 14th ed.; British Crop Protection Council: Surry, UK, 2006; pp. 598–599. [Google Scholar]
- Wang, X.; Zhu, X.; Peng, Q.; Wang, Y.; Ge, J.; Yang, G.; Wang, X.; Cai, L.; Shen, W. Multi-level ecotoxicological effects of imidacloprid on earthworm (Eisenia fetida). Chemosphere 2019, 219, 923–932. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Romero, E.; Nogales, R. Impact of imidacloprid residues on the development of Eisenia fetida during vermicomposting of greenhouse plant waste. J. Hazard. Mater. 2011, 192, 1886–1889. [Google Scholar] [CrossRef]
- Zang, Y.; Zhong, Y.; Luo, Y.; Kong, Z.M. Genotoxicity of two novel pesticides for the earthworm, Eisenia fetida. Environ. Pollut. 2000, 108, 271–278. [Google Scholar] [CrossRef]
- Chen, J.; Saleem, M.; Wang, C.; Liang, W.; Zhang, Q. Individual and combined effects of herbicide tribenuron-methyl and fungicide tebuconazole on soil earthworm Eisenia fetida. Sci. Rep. 2018, 8, 2967. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance oxyfluorfen. EFSA J. 2010, 8, 1906. [Google Scholar] [CrossRef]
- Barrena, R.; Vazquez, F.; Sanchez, A. Dehydrogenase activity as a method for monitoring the composting process. Bioresour. Technol. 2008, 99, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.M.; Romero, E.; Nogales, R. Dynamics of microbial communities related to biochemical parameters during vermicomposting and maturation of agroindustrial lignocellulose wastes. Bioresour. Technol. 2013, 146, 345–354. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Díaz-Raviña, M.; Romero, E.; Nogales, R. Recycling of environmentally problematic plant wastes generated from greenhouse tomato crops through vermicomposting. Int. J. Environ. Sci. Technol. 2013, 10, 697–708. [Google Scholar] [CrossRef]
- Muñoz-Leo, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole Application Decreases Soil Microbial Biomass and Activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Chen, H.; Yi, Z.; Choi, M.M. Influence of short-time imidacloprid and acetamiprid application on soil microbial metabolic activity and enzymatic activity. Environ. Sci. Pollut. Res. Int. 2014, 21, 10129–10138. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Romero, E.; Nogales, R. Feasibility of vermicomposting for vegetable greenhouse waste recycling. Bioresour. Technol. 2010, 101, 9654–9660. [Google Scholar] [CrossRef]
- Beffa, T.; Blanc, M.; Marilley, L.; Lott Fischer, J.; Lyon, P.-F.; Aragno, M. Taxonomic and metabolic microbial diversity during composting. In The Science of Composting; de Bertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Blackies Academic and Professional: Glasgow, Scotland, 1995; Volume 1, pp. 149–161. [Google Scholar]
- Liaquat, R.; Jamal, A.; Tauseef, I.; Qureshi, Z.; Farooq, U.; Imran, M.; Ali, M.I. Characterizing Bacterial Consortia from an Anaerobic Digester Treating Organic Waste for Biogas Production. Pol. J. Environ. Stud. 2017, 26, 709–716. [Google Scholar] [CrossRef]
- Suthar, S. Vermicomposting potential of Perionyx sansibaricus (Perrier) in different waste materials. Bioresour. Technol. 2007, 98, 1231–1237. [Google Scholar] [CrossRef]
- Dutra, E.S.; Pascon, R.C.; Vallim, M.A. São Paulo Zoo composting as a source of bacteria with bioremediation potential. Afr. J. Microbiol. Res. 2013, 7, 5200–5206. [Google Scholar]
- Semple, K.T.; Reid, B.J. Impact of composting strategies on the treatment of soils contaminated with organic pollutants. Environ. Pollut. 2001, 112, 269–283. [Google Scholar] [CrossRef]
- Pelosi, C.; Barot, S.; Capowiez, Y.; Hedde, M.; Vandenbulcke, F. Pesticides and earthworms. A review. Agron. Sustain. Dev. 2014, 34, 199–228. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Mazzia, C.; Capowiez, Y.; Rault, M. Carboxylesterase activity in earthworm gut contents: Potential (eco)toxicological implications. Comp. Biochem. Physiol. 2009, 150C, 503–551. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Vivas, C.S.; Martinez, M.J.; Guerrero, J.A. 14C Tebuconazole degradation in Colombian soils. Commun. Abric. Appl. Biol. Sci. 2010, 75, 173–181. [Google Scholar]




| Diuron | Imidacloprid | Tebuconazol | Oxifluorfen | Dimethoate | |
|---|---|---|---|---|---|
| CP | 0.89 ± 0.14 | 23.2 ± 0.5 | 24.0 ± 1.6 | 42.9 ± 2.4 | nd |
| CO | 0.55 ± 0.02 | 28.2 ± 0.5 | 42.3 ± 3.0 | 66.8 ± 5.4 | nd |
| Biomixture a | pH | EC dS m−1 | TOC g kg−1 | WSC g kg−1 | N g kg−1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | F | t-test | I | F | t-test | I | F | t-test | I | F | t-test | I | F | t-test | ||
| NP | +E | 7.0 | 7.1 | 0.42 | 3.0 | 2.7 | 0.07 | 139 | 131 | 0.15 | 2.2 | 2.0 | 0.15 | 7.1 | 8.9 | 0.01 * |
| CP | +E | 7.1 | 7.2 | 0.43 | 3.5 | 3.1 | 0.02 * | 114 | 108 | 0.39 | 1.3 | 1.1 | 0.10 | 6.4 | 7.3 | 0.04 * |
| -E | 7.2 | 0.42 | 3.5 | 0.99 | 112 | 0.72 | 1.2 | 0.18 | 6.2 | 0.44 | ||||||
| NO | +E | 8.1 | 8.0 | 0.18 | 1.5 | 1.3 | 0.13 | 151 | 104 | 0.00 * | 9.6 | 7.2 | 0.01 * | 8.4 | 13.3 | 0.00 * |
| CO | +E | 8.1 | 7.8 | 0.09 | 2.3 | 1.6 | 0.01 * | 143 | 125 | 0.01 * | 6.5 | 4.1 | 0.00 * | 12.5 | 13.6 | 0.07 |
| -E | 7.9 | 0.04 | 2.3 | 0.99 | 138 | 0.18 | 5.8 | 0.01 * | 12.9 | 0.61 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Moreno, L.; Nogales, R.; Romero, E. Vermiremediation of Biomixtures from Biobed Systems Contaminated with Pesticides. Appl. Sci. 2020, 10, 3173. https://doi.org/10.3390/app10093173
Delgado-Moreno L, Nogales R, Romero E. Vermiremediation of Biomixtures from Biobed Systems Contaminated with Pesticides. Applied Sciences. 2020; 10(9):3173. https://doi.org/10.3390/app10093173
Chicago/Turabian StyleDelgado-Moreno, Laura, Rogelio Nogales, and Esperanza Romero. 2020. "Vermiremediation of Biomixtures from Biobed Systems Contaminated with Pesticides" Applied Sciences 10, no. 9: 3173. https://doi.org/10.3390/app10093173
APA StyleDelgado-Moreno, L., Nogales, R., & Romero, E. (2020). Vermiremediation of Biomixtures from Biobed Systems Contaminated with Pesticides. Applied Sciences, 10(9), 3173. https://doi.org/10.3390/app10093173