Quantitative Ultrasound Texture Analysis to Assess the Spastic Muscles in Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Testing Position
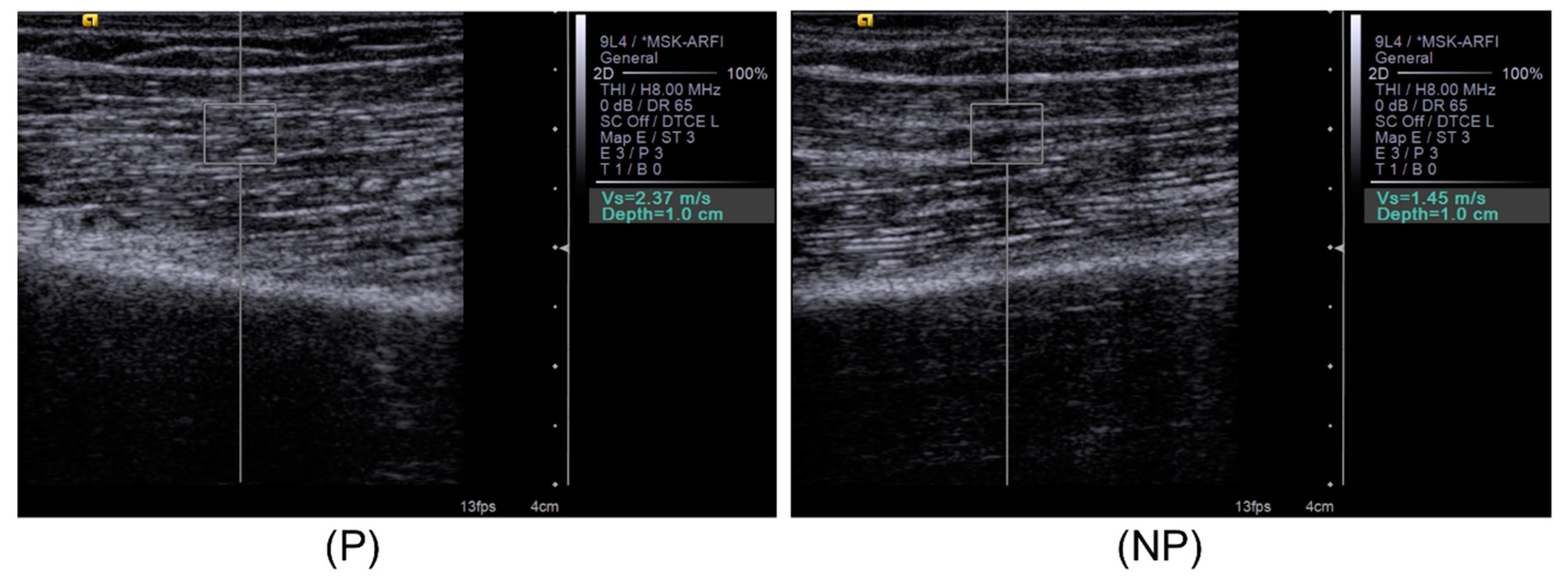
2.3. Bicep Brachii Muscle Shear Wave Velocity and Gray Scale Image Capture
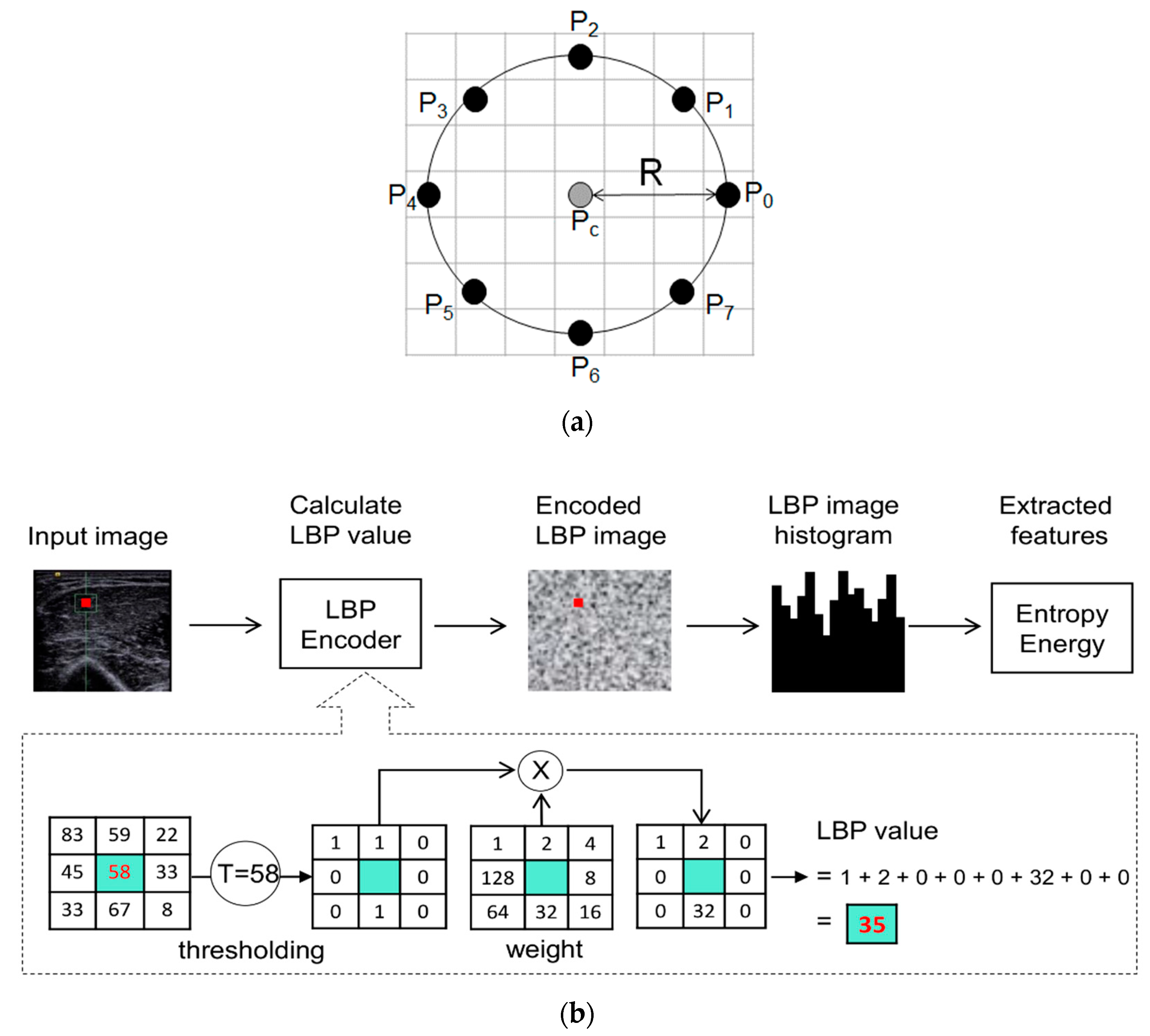
2.4. Image Processing
2.5. Assessment of Functional Performance
2.6. Statistical Analysis
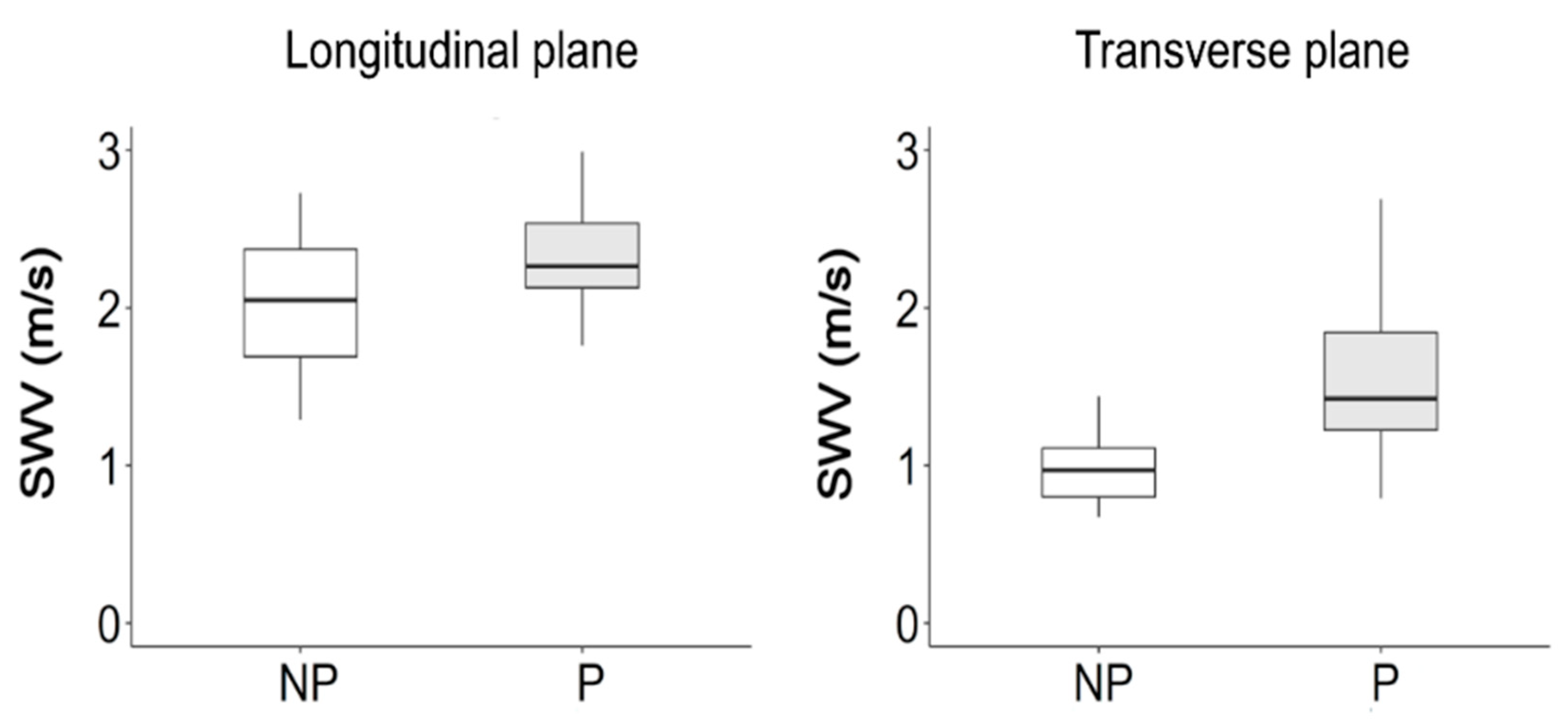
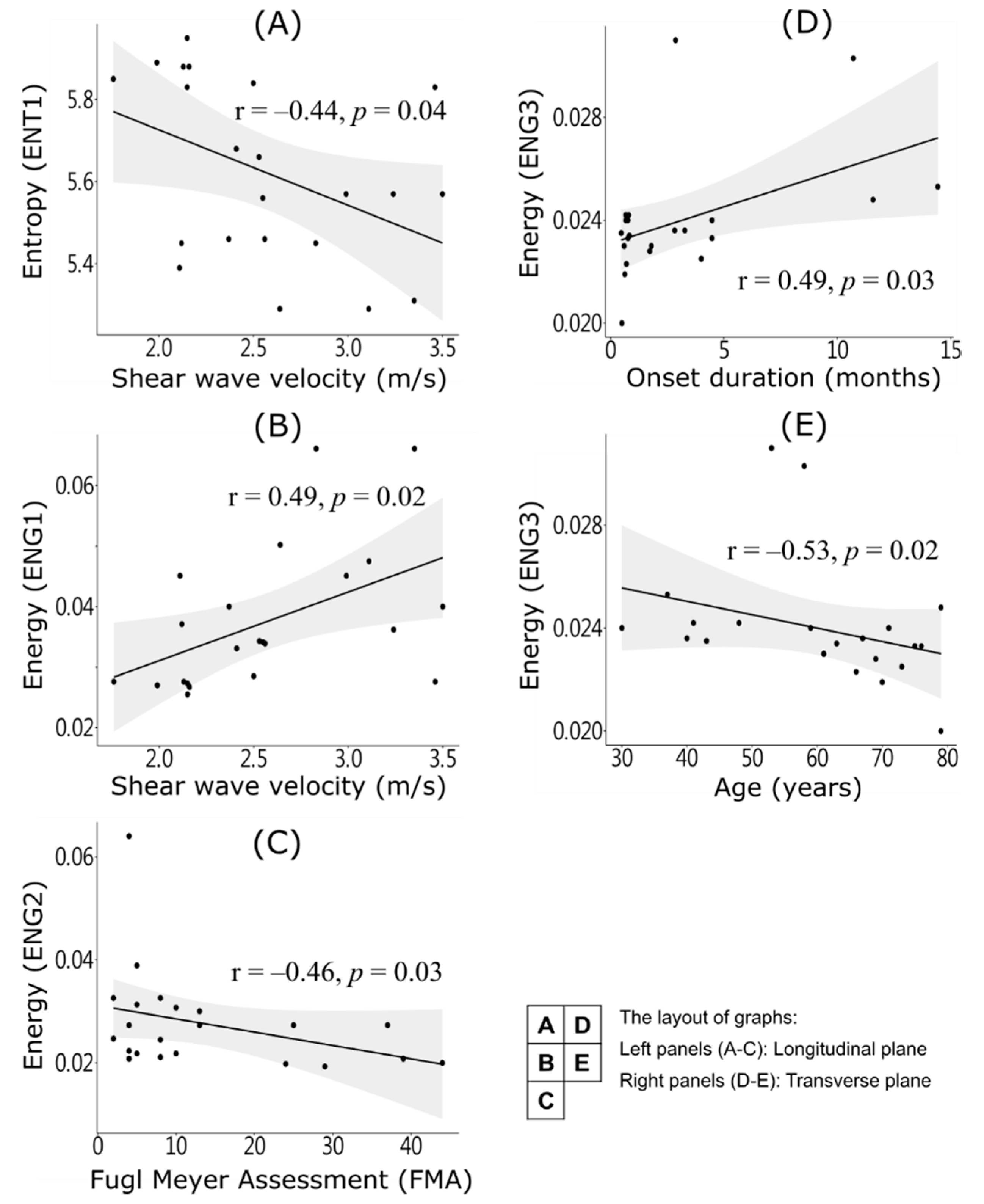
3. Results
4. Discussion
4.1. In-Homogenous Muscle Texture
4.2. Fiber Orientation and Shear Wave Velocity
4.3. Region of Interest (ROI) Size
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maurits, N.M.; Bollen, A.E.; Windhausen, A.; De Jager, A.E.J.; Van Der Hoeven, J.H. Muscle ultrasound analysis: Normal values and differentiation between myopathies and neuropathies. Ultrasound Med. Biol. 2003, 29, 215–225. [Google Scholar] [CrossRef]
- Lee, S.S.; Spear, S.; Rymer, W.Z. Quantifying changes in material properties of stroke-impaired muscle. Clin. Biomech. 2015, 30, 269–275. [Google Scholar] [CrossRef]
- Fridén, J.; Lieber, R.L. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve 2003, 27, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tong, K.Y.; Hu, X. The Effect of Poststroke Impairments on Brachialis Muscle Architecture as Measured by Ultrasound. Arch. Phys. Med. Rehabil. 2007, 88, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Khoury, V.; Cardinal, É.; Brassard, P. Atrophy and Fatty Infiltration of the Supraspinatus Muscle: Sonography Versus MRI. Am. J. Roentgenol. 2008, 190, 1105–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.; Verrijp, K.N.; Arts, I.M.; Van Der Laak, J.A.; Hoogerbrugge, P.M.; Van Engelen, B.G.; Verrips, A. Skeletal Muscle Ultrasound: Correlation Between Fibrous Tissue and Echo Intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Dubois, G.J.; Bachasson, D.; Lacourpaille, L.; Benveniste, O.; Hogrel, J. Local Texture Anisotropy as an Estimate of Muscle Quality in Ultrasound Imaging. Ultrasound Med. Biol. 2018, 44, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- König, T.; Steffen, J.; Rak, M.; Neumann, G.; Von Rohden, L.; Tönnies, K.D. Ultrasound texture-based CAD system for detecting neuromuscular diseases. Int. J. Comput. Assist. Radiol. Surg. 2014, 10, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Payá, J.J.; Ríos-Díaz, J.; Tembl-Ferrairó, J.I.; Vázquez-Costa, J.F.; Del Baño-Aledo, M.E.; Medina-Mirapeix, F. Quantitative Muscle Ultrasonography Using Textural Analysis in Amyotrophic Lateral Sclerosis. Ultrason. Imaging 2017, 39, 357–368. [Google Scholar] [CrossRef]
- Molinari, F.; Caresio, C.; Acharya, U.R.; Mookiah, M.R.K.; Minetto, M.A. Advances in Quantitative Muscle Ultrasonography Using Texture Analysis of Ultrasound Images. Ultrasound Med. Biol. 2015, 41, 2520–2532. [Google Scholar] [CrossRef]
- Sogawa, K.; Nodera, H.; Takamatsu, N.; Mori, A.; Yamazaki, H.; Shimatani, Y.; Izumi, Y.; Kaji, R. Neurogenic and Myogenic Diseases: Quantitative Texture Analysis of Muscle US Data for Differentiation. Radiology 2017, 283, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Faust, O.; Molinari, F.; Sree, S.V.; Junnarkar, S.P.; Sudarshan, V. Ultrasound-based tissue characterization and classification of fatty liver disease: A screening and diagnostic paradigm. Knowl. Based Syst. 2015, 75, 66–77. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Krishnan, M.M.R.; Molinari, F.; Saba, L.; Ho, S.Y.S.; Ahuja, A.T.; Ho, S.C.; Nicolaides, A.; Suri, J.S. Atherosclerotic Risk Stratification Strategy for Carotid Arteries Using Texture-Based Features. Ultrasound Med. Biol. 2012, 38, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nasser, M.; Melendez, J.; Moreno, A.; Omer, O.A.; Puig, D. Breast tumor classification in ultrasound images using texture analysis and super-resolution methods. Eng. Appl. Artif. Intell. 2017, 59, 84–92. [Google Scholar] [CrossRef]
- Hirvasniemi, J.; Thevenot, J.; Immonen, V.; Liikavainio, T.; Pulkkinen, P.; Jämsä, T.; Arokoski, J.; Saarakkala, S. Quantification of differences in bone texture from plain radiographs in knees with and without osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1724–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; He, W.; Du, L.-J.; Chen, J.; Park, D.; Wells, M.; Fowlkes, J.B.; O’Dell, M. Quantitative Ultrasound Imaging to Assess the Biceps Brachii Muscle in Chronic Post-Stroke Spasticity: Preliminary Observation. Ultrasound Med. Biol. 2018, 44, 1931–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-H.; Ho, Y.-C.; Hsiao, M.-Y.; Chen, W.-S.; Wang, T.-G. Evaluation of Post-Stroke Spastic Muscle Stiffness Using Shear Wave Ultrasound Elastography. Ultrasound Med. Biol. 2017, 43, 1105–1111. [Google Scholar] [CrossRef]
- Abouelkaram, S.; Suchorski, K.; Buquet, B.; Berge, P.; Culioli, J.; Delachartre, P.; Basset, O. Effects of muscle texture on ultrasonic measurements. Food Chem. 2000, 69, 447–455. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, W.-S.; Park, G.-Y.; Wang, T.-G.; Lew, H.L. Musculoskeletal Sonoelastography: A Focused Review of its Diagnostic Applications for Evaluating Tendons and Fascia. J. Med. Ultrasound 2012, 20, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Ojala, T.; Pietikäinen, M.; Harwood, D. A comparative study of texture measures with classification based on featured distributions. Pattern Recognit. 1996, 29, 51–59. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Pillen, S.; Van Alfen, N. Skeletal muscle ultrasound. Neurol. Res. 2011, 33, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Tsui, P.-H. Ultrasound Detection of Scatterer Concentration by Weighted Entropy. Entropy 2015, 17, 6598–6616. [Google Scholar] [CrossRef] [Green Version]
- Turo, D.; Otto, P.; Shah, J.P.; Heimur, J.; Gebreab, T.; Zaazhoa, M.; Armstrong, K.; Gerber, L.H.; Sikdar, S. Ultrasonic Characterization of the Upper Trapezius Muscle in Patients with Chronic Neck Pain. Ultrason. Imaging 2013, 35, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Gennisson, J.; Deffieux, T.; Mace, E.; Montaldo, G.; Fink, M.; Tanter, M. Viscoelastic and Anisotropic Mechanical Properties of in vivo Muscle Tissue Assessed by Supersonic Shear Imaging. Ultrasound Med. Biol. 2010, 36, 789–801. [Google Scholar] [CrossRef]
- Bird, M.; Le, D.; Shah, J.; Gerber, L.; Tandon, H.; DeStefano, S.; Sikdar, S. Characterization of local muscle fiber anisotropy using shear wave elastography in patients with chronic myofascial pain. In Proceedings of the 2017 IEEE International Ultrasonics Symposium IUS, Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar]
- Liu, L.; Fieguth, P.; Guo, Y.; Wang, X.; Pietikäinen, M. Local binary features for texture classification: Taxonomy and experimental study. Pattern Recognit. 2017, 62, 135–160. [Google Scholar] [CrossRef] [Green Version]
- Júnior, N.D.S.P.; Matta, T.T.; Alvarenga, A.V.; Pereira, W.C.D.A.; De Oliveira, L.F. Reliability of ultrasound texture measures of Biceps Brachialis and Gastrocnemius Lateralis muscles’ images. Clin. Physiol. Funct. Imaging 2015, 37, 84–88. [Google Scholar] [CrossRef]

| Variables | Value | Range |
|---|---|---|
| Hemiplegic type (hemorrhage/infraction) | 5/17 | |
| Hemiplegic side (L/R) | 12/10 | |
| MAS | ||
| 0 | 3 | |
| 1 | 8 | |
| 2 | 10 | |
| 3 | 1 | |
| Age (years) | 59.1 ± 14.6 | (30–79) |
| Body height (cm) | 163.9 ± 7.2 | (149–179) |
| Body weight (kg) | 66.6 ± 8.8 | (50–83) |
| Duration from stroke onset (months) | 3.2 ± 4.0 | (0.47–14.43) |
| FMA | 18.1 ± 14.8 | (2–44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.-T.; Wei, T.-S.; Ching, C.T.-S. Quantitative Ultrasound Texture Analysis to Assess the Spastic Muscles in Stroke Patients. Appl. Sci. 2021, 11, 11. https://doi.org/10.3390/app11010011
Liu P-T, Wei T-S, Ching CT-S. Quantitative Ultrasound Texture Analysis to Assess the Spastic Muscles in Stroke Patients. Applied Sciences. 2021; 11(1):11. https://doi.org/10.3390/app11010011
Chicago/Turabian StyleLiu, Peng-Ta, Ta-Sen Wei, and Congo Tak-Shing Ching. 2021. "Quantitative Ultrasound Texture Analysis to Assess the Spastic Muscles in Stroke Patients" Applied Sciences 11, no. 1: 11. https://doi.org/10.3390/app11010011





