1. Introduction
Boba milk tea originated in Taiwan in the 1980s, became very popular in Asia in the 1990s, and has been increasingly popular around the world since 2000 [
1,
2]. The “boba” balls, which are also called “pearl” balls, are the central ingredient of the milk tea. It is usually made of tapioca, or tapioca mixed with other ingredients, such as konjac powder, sweet potato, chamomile, and so on [
3,
4,
5]. There have been some studies on the calory content and obesity risk of milk tea [
6], but there have been few reports related to the improvement of milk tea technology, including that related to the “boba” balls.
Orange is rich in nutrition. It not only contains nutrient elements needed by the human body, it also contains plant phenols and flavonoids, containing an especially high content of Vc [
7,
8]. It is one of the most popular fruits in the world. Neves, Trombin, and Marques et al. analysed the global consumption of frozen concentrate orange juice from 2013 to 2018. They summarized the consumer behaviour of three kinds of orange juice in the main market worldwide [
9]. A large number of studies have shown that eating a large amount of citrus fruits is beneficial to anti-cancer functions and prevention of cardiovascular diseases [
10,
11,
12,
13].
In this project, orange juice was wrapped in calcium alginate film to obtain fruit balls, which were used in milk tea, forming a new type of fruit ball drink. This technique was reported in cell immobilization during the last century. For example, Rickert et al. used calcium alginate gel to embed bacteria for the fermentation of organic acids [
14]. By dropping Calcium ions (Ca
2+) into sodium alginate solution, Ca
2+ diffused to the periphery and reacted with sodium alginate to form a gel film to wrap the liquid core, and hollow micro-capsules were prepared. Some scholars have performed studies on the influence of processing conditions of calcium alginate gel (CAG). The physical properties of CAG beads change with heat treatment, including rupture strength, size, sphericity and porous structures [
15,
16].
In general, calcium alginate gel is a solid gel; the CAG studied in this paper has an orange-flavoured drink core. When you bite it, the juice flows out of the inner core, like jam, giving people a sweet and sour, delicious, unprecedented eating experience. It is also a calcium alginate immobilized embedding technology, which has the potential to maintain the stability of sensitive food ingredients, especially some natural health ingredients, such as flavones, active proteins, sterols, enzymes, probiotics and vitamins, etc. By applying calcium alginate ball embedding methods, important breakthroughs could result in some key food industry technologies, thus achieving ideal sensory, physical and chemical indicators, and shelf life, while also achieving functional food design. For example, Lin et al. wrapped astaxanthin in the CAG. The results showed that after storage at 25 °C for 21 days, the contents of astaxanthin in the CAG both remained above 90% of their original amount [
17]. This provides an effective way of developing the stability of sensitive compounds. Jiang et al. fixed bifidobacteria in carboxymethyl chitin–calcium alginate gel, the bifidobacteria could be delivered to the gut without being dissolved in the gastric juice [
18]. Here, “boba” balls made of calcium alginate for use in milk tea were studied. Operational conditions such as drop height, flow velocity, and sodium alginate and calcium chloride solution concentration were investigated. The diameter, mechanical strength, loading ratio and encapsulation rate of the “boba” balls are discussed. The sensory and microorganisms changes of the “boba” balls at different temperatures were studied, and their shelf life is predicted by using the Arrhenius equation.
2. Materials and Methods
2.1. Materials
Orange juice (sugar: 66°Bx, acidity: 4.16%), modified starch (hydroxypropyl distarch phosphate from potato, stable to temperature, acidity and shear force), calcium chloride anhydrous and sodium alginate were provided by Hangzhou Bodo Industry and Trade Co., LTD., Hangzhou, China. Sucrose and table salt were bought from a market; citric acid anhydrous was purchased from Shandong Zhongchuang Lemon Biochemical Co., LTD., Anqiu, Shandong, China. Sunset yellow, lemon yellow, and orange essence were purchased from Hangzhou Lvjing Flavor Co., LTD., Shandong, China, Xanthan gum and Guar bean gum were purchased from Hebei Jinfeng Chemical Co., LTD., Hengshui, Hebei, China. The companies above were located in China. All of the above materials were of food grade.
2.2. The “boba” Ball Preparation
The composition of the orange-flavoured drink, which was the core of the “boba” ball, was carried out with reference to Wu et al. [
19] and was improved for processing. It included sucrose (22.80%), citric acid anhydrous (0.50%), salt (0.04%), sunset yellow (0.0012%), citric yellow (0.006%), orange essence (0.07%), Xanthan (0.1%), and guar gum (0.25%). All the ingredients were put into pure boiling water (100 °C) to be fully dissolved, after which the mixture was cooled to 50–60 °C, and concentrated orange juice (5%), modified starch (3.75%), calcium chloride anhydrous (1%) were added and blended together. Homogenizer (Shanghai Zollo Instrument Co., Ltd., Shanghai, China) was used to homogenize the orange flavoured drink at the pressure of 18–22 mpa for 30 min.
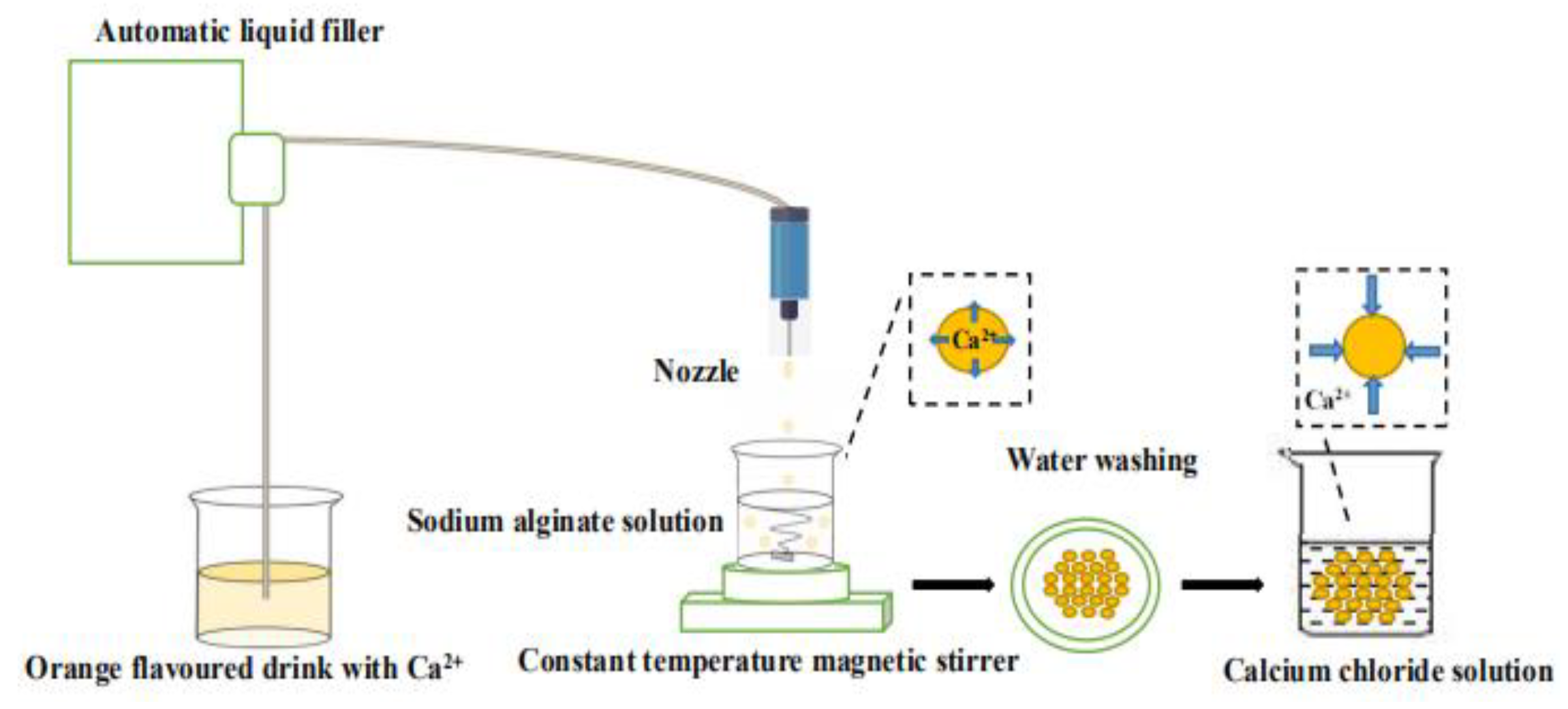
The preparation process of the “boba” ball is shown in
Figure 1. The orange fruit-flavoured drink was added to the sodium alginate solution drop by drop using the Automatic liquid filler (Newking Pump Co., Ltd., Shangdong, China). After 1 min of reaction, calcium alginate balls were filtered out with a stainless-steel filter spoon, and were washed with distilled water and then transferred into 5% calcium chloride solution to react for 15 min. Finally, the residual calcium chloride outside of the “boba” ball was washed off with distilled water again, and the preparation was completed. All the above operations were carried out at room temperature. The prepared “boba” balls were stored in 0.9% sodium chloride solution for property detection.
2.3. Preparation of “boba” Ball Storage Solution
CAG showed a three-dimensional network structure, known as an “egg box” structure, that enables small molecules of water-soluble substances to pass through freely [
20]. The swelling property of CAG was affected by pH value and stabilised at solution pH < 6. This indicates that calcium alginate would gradually decompose in salt solution with pH greater than 5.5 [
21]. Therefore, “boba” balls must be stored in environments with pH values less than 5.5. There were some sugar, acid, pigment, essence and other small molecule water-soluble substances in the “boba” balls. To prevent the loss of solute and maintain the pH of the environment, the formulation of the “boba” ball storage solution was prepared as follows: sucrose 22.80%, citric acid 0.50%, salt 0.04%, sunset yellow 0.0012%, citric yellow 0.006%, orange essence 0.07%. The pH value of the storage solution was measured to be about 2.05 ± 0.05 using a pH meter (INESA Scientific Instrument Co., Ltd., Shanghai, China).
2.4. Determination of “boba” Ball Properties
Measurement of diameter: 20 capsules were randomly selected, the diameters were measured one by one using Vernier calipers (Shanghai Meinite Industrial Co., Ltd., Shanghai, China), and the average value was taken [
22].
Determination of mechanical strength: The single-axis pressing method was used for measurement. A “boba” ball was placed on the tray of the analytical balance (Mettler-Toledo, LLC, Shanghai, China), and positive pressure was applied to the “boba” ball until it burst. The balance showed a sudden change of pressure from low to high, and then to low; the maximum positive pressure was recorded, namely the mechanical strength of the “boba” ball. Twenty “boba” balls were randomly selected for each detection, and the mechanical strength was represented by the average value [
20].
The orange fruit-flavoured drink loading ratio and encapsulation rate:
The encapsulation rate is an important parameter in the preparation of “boba” balls. The higher, the better.
2.5. Shelf Life Determination and Prediction
The “boba” balls were placed in a sealed glass jar by adding the storage solution, pasteurized at 70–80 °C for 30 min, and were then stored at 20 °C, 25 °C, 30 °C, 35 °C and 40 °C, respectively.
Total bacterial colony determination was conducted with reference to GB 4789.2—National Standard Food Microbiology Examination for Food Safety [
23].
The sensory evaluation indexes included colour, aroma, taste and appearance, and the standards are shown in
Table 1. The panel of 12 assessors was aged from 20 to 36 years and possessed expertise in the sensory evaluation of food. Approximately 20 “boba” balls stored at a constant temperature were taken out, kept in a plate at room temperature for 30 min, and served to each panellist along with the questionnaire. The samples were presented one at a time, with about a 5 min wait between samples. The intervals for sensory evaluation of the “boba” balls varied at different storage temperatures: balls stored at 20–30 °C were evaluated every 5–9 days, and balls stored at 35 °C and 40 °C were evaluated every 1–2 days. The intensities of the sensory attributes are presented as the mean of the scores provided by the 12 panel assessors. If the sensory score was below 80, the product was considered to be unable to meet consumers’ expectations and reach the shelf-life limit.
According to [
24,
25], the sensory evaluation response function F(X) at a certain temperature can be expressed as follows:
where k is the sensory evaluation change reaction rate, and t is the period for which the food was stored. Here, the k value at a certain temperature can be derived from the slope of the regression equation between the response value F(X) and time. The effect of temperature on the change reaction of sensory evaluation can described by the Arrhenius relationship.
Taking the logarithm on both sides of the Arrhenius function:
where Ea is the activation energy in J/mol, R is the gas constant in J/(K mol) and is equal to 8.314, k
0 is a pre-exponential factor, and T is absolute temperature in K (273 °C).
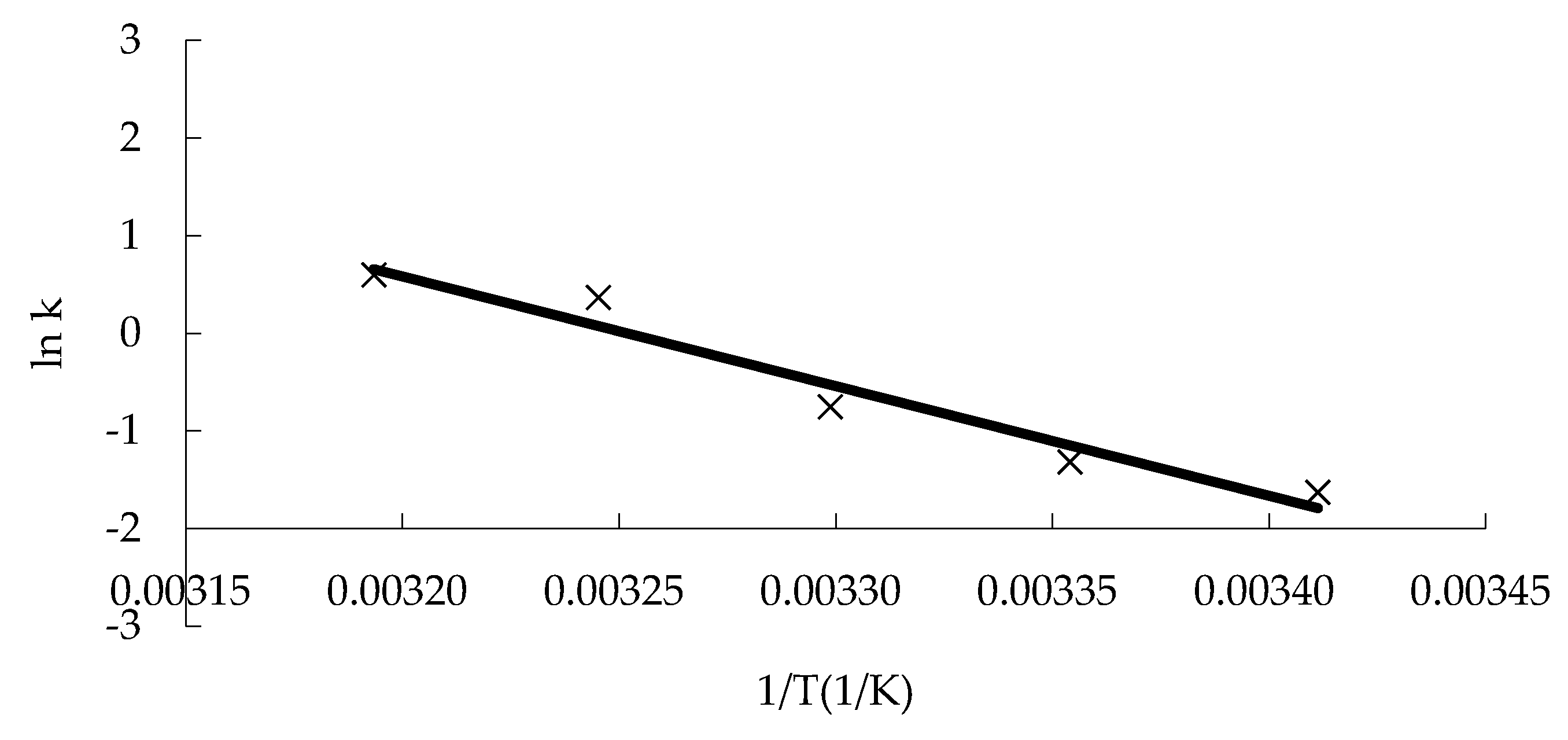
Here, the Ea value and k0 can be derived from the slope of the regression equation between ln k and 1/T.
2.6. Statistical Analysis
The Data Processing System (DPS) software v13.5 was applied to fix the experiment data and establish the mathematical model [
26]. The Origin software (OriginLab Corporation, Northampton, MA, USA) was applied to paint the response surface.
3. Results and Discussion
3.1. Selection of Hose Diameter
The diameter of the straws used for “boba” milk tea is generally about 10.00–12.00 mm. To allow the “boba” ball pass through the straw smoothly, the diameter of the prepared balls generally needs to be 1.00–2.00 mm lower than this. The objective of this project was to prepare “boba” balls with a diameter of about 8–10 mm; therefore, it was advisable to use a hose with a diameter of 8 mm.
3.2. Influence of Drop Height
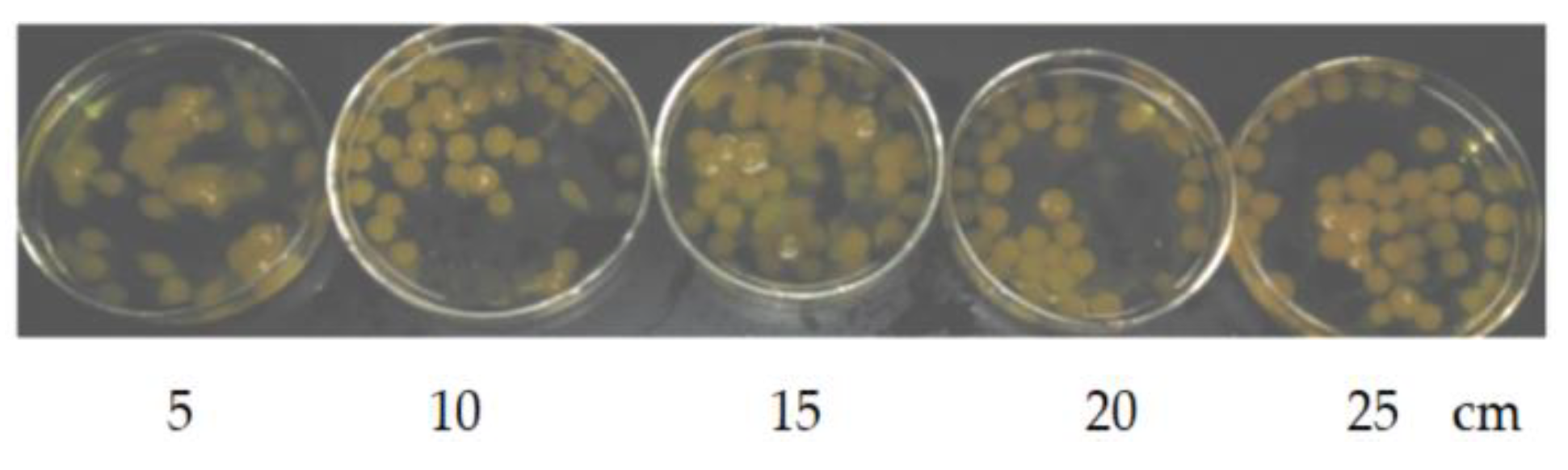
Figure 2 shows the influence of drop height on the “boba” balls. The velocity of the automatic liquid filler was 60 mL/min, the sodium alginate solution was 0.8%. It was found that if the drop height wasn’t high enough, calcium alginate bal would easily float on the surface, and the final ball was often elliptical (see the drop height of 5 cm in
Figure 2); with increasing drop height, the probability of elliptical balls gradually decreased (see the drop height 5–20 cm in
Figure 2). When the drop height was greater than 20 cm, the “boba” balls were basically spherical. Therefore, it is advisable to choose a drop height greater than 20 cm. The drop height selected here was 25 cm.
3.3. Influence of Flow Velocity
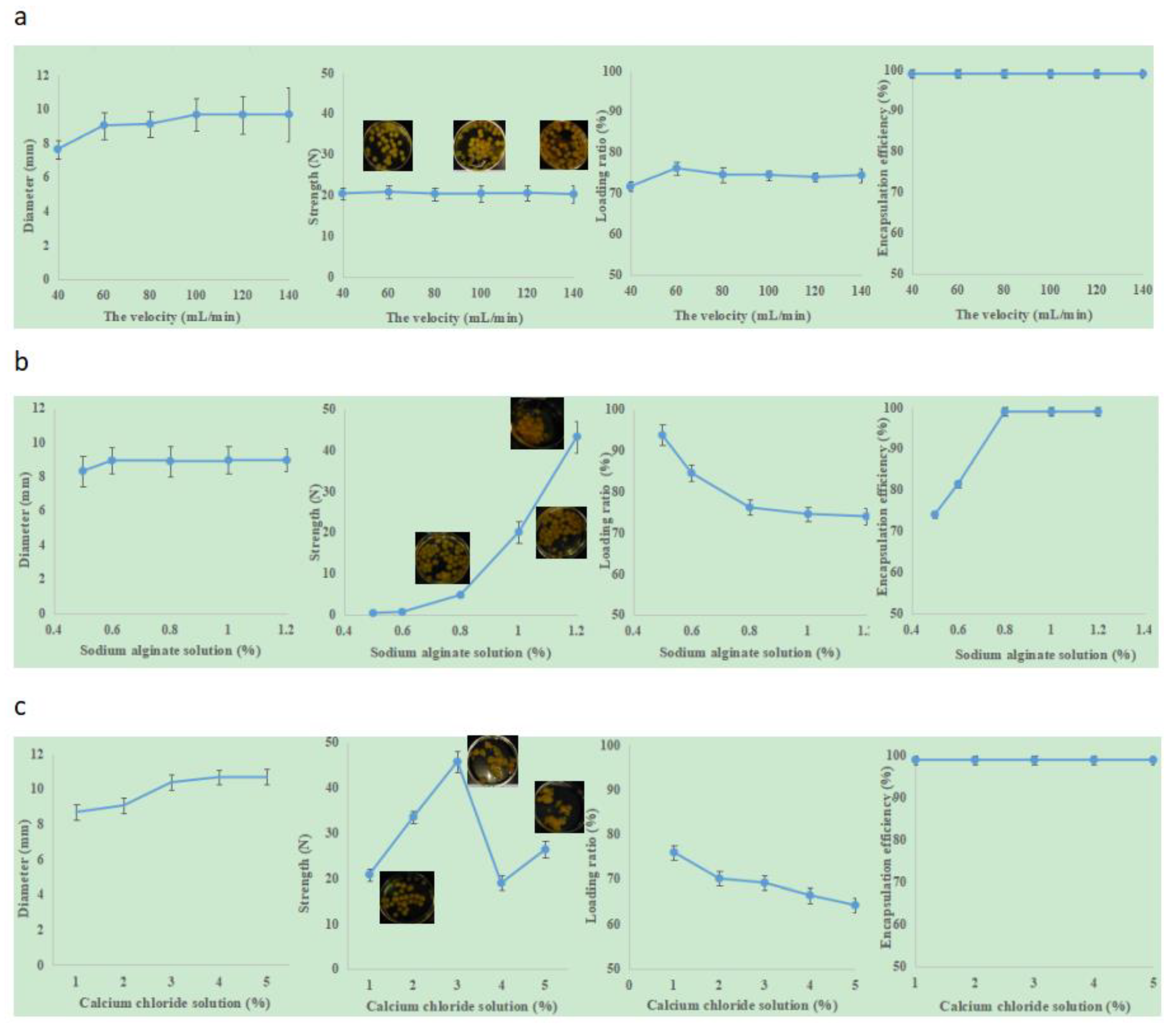
Table 2 and
Figure 3a present the effect of flow velocity on the “boba” ball properties. The diameter of the hose was 8 cm, the drop height was 25 cm, and the concentration of sodium alginate solution was 0.8%. It was found that the flow velocity had a positive effect on the diameter of the “boba” balls (
Table 2). When the flow rate was less than 40 mL/min or greater than 100 mL/min, the produced ball diameters were sometimes smaller than 8 cm or larger than 10 cm. With the increasing flow velocity, there was a directly proportional increase in particle size distribution. This was in good agreement with the report on alginate micro spheres [
27]. These balls wouldn’t be applied to the milk tea, as they would result in a bad consumption experience. Therefore, in order to obtain the required “boba” with suitable diameters of between 8–10 mm, the flow velocity should be controlled at 60–80 mL/min.
Figure 3a shows that the loading ratio reached its optimal value (76.15 ± 1.51) when the flow velocity was 60 mL/min. Under this preparation condition, the flow velocity had little effect on encapsulation efficiency and mechanical strength. The encapsulation efficiencies of the “boba” balls were all 100%, and the mechanical strength maintained stability.
3.4. Influence of Sodium Alginate Solution Concentration
Figure 3b shows the influence of different concentrations of sodium alginate on the “boba” ball properties. The diameter of the hose was 8 cm, the drop height was 25 cm, the flow rate was 60 mL/min, and the concentration of calcium chloride solution was 1.0%. It was found that the sodium alginate solution concentration had positive effects on the diameter, mechanical strength, loading ratio and encapsulation efficiency of the “boba” balls. when the sodium alginate solution concentration was greater than 0.6%, the diameter maintained stability. However, if its concentration was below 0.5%, there were some “boba” balls with diameters <8.00 mm. The hinge structure formed by sodium alginate and calcium ions changed with the concentration of sodium alginate, and affected the diameter, mechanical strength, loading ratio and encapsulation efficiency of the “boba” ball [
27,
28,
29]. The encapsulation efficiency of the “boba” ball was 74.07 ± 0.87% (0.5% sodium alginate) and 81.48 ± 0.75% (0.6% sodium alginate). When the concentration of sodium alginate was higher than 0.8%, the encapsulation reached 100%. However, when the concentration of sodium alginate reached’ 1.2%, the “boba” balls tended to stick together and were not conducive to being cleaned separately; this situation would increase their preparation time. The mechanical strength increased exponentially with increasing concentration of sodium alginate. The relationship between the concentration of sodium alginate and the strength of the “boba” balls was as follows, and correlation coefficient was 0.9786:
When the concentration of sodium alginate increased from 0.8% to 1.0%, the strength increased from 4.83 ± 0.50 to 20.14 ± 2.60, and the crushing resistance increased by more than four times. To give the “boba” balls a certain crushing resistance and 100% encapsulation efficiency in production, 1% sodium alginate was deemed to be the best.
3.5. Influence of Calcium Chloride Solution Concentration
Figure 3c shows the influence of different concentrations of calcium chloride on the “boba” ball properties. The diameter of the hose was 8 cm, the drop height was 25 cm, the flow rate was 60 mL/min, and the concentration of sodium alginate solution concentration was 1.0%. It was found that the calcium chloride concentration had positive effects on the diameter, mechanical strength, and the loading ratio of the “boba” balls. The encapsulation efficiency was 100% under the condition of 1–5%. With the increase of calcium chloride concentration, the loading ratio decreased, and the mechanical strength first went up, then went down, and then went up again. When the calcium chloride solution was 3%, the strength reached its maximum. The calcium ion/alginate ratio here was found to present an optimized ratio. The increase of calcium content could increase the crosslinking density and shrinkage of matrix [
30], but also produced more carbon dioxide, leading to a more porous and weak matrix [
31]. The “boba” balls’ average diameter gradually increased from 8.70 ± 0.70 to 10.73 ± 1.30, and was then maintained at a certain level. When the calcium chloride concentration reached or exceeded 2%, the “boba” balls stuck together easily, showed the astringency of calcium chloride and presented a poor sensory evaluation experience. Taking the above factors into consideration, the 1% calcium chloride concentration was determined to be the best.
3.6. Microbial Colonies/Sensory Changes and Prediction of Shelf Life for the “boba” Ball
The “boba” balls were prepared according to the optimized experimental conditions described above, and stored at different temperatures. During the 90 days of storage, no microorganisms were detected. This was due to pasteurization and sealed filling. Therefore, the microbiological safety of the product could be guaranteed. The product’s shelf life is dependent on changes in sensory properties. Colour, aroma, stratification and capsule breakage rate were the main factors affecting sensory indicators.
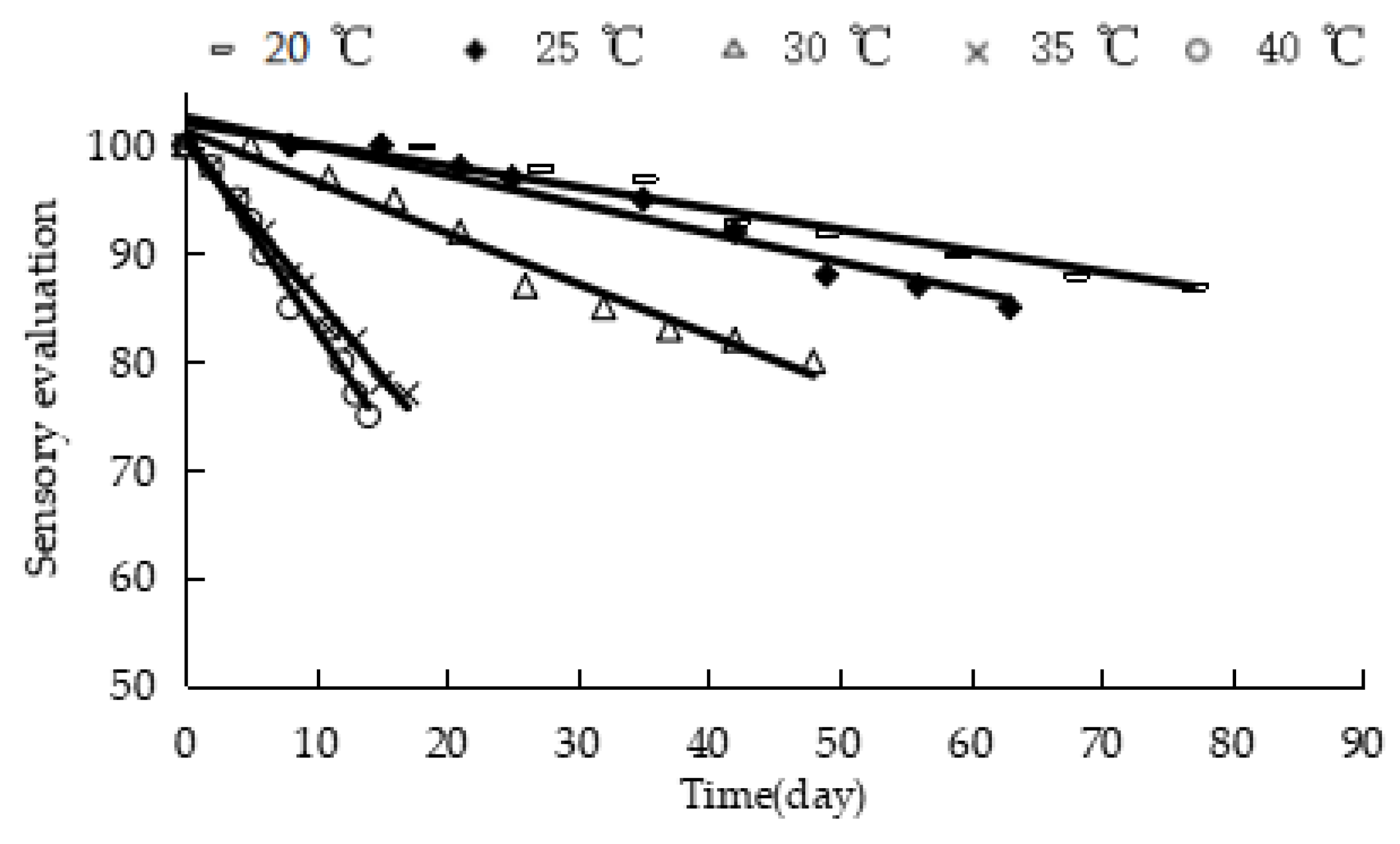
Figure 4 shows the response of the sensory evaluation of “boba” balls stored at different temperatures. The sensory evaluation values of the product were linearly correlated with storage time, the correlation coefficient R
2 of the fitting regression curve was above 0.94 (
Table 3), and the k value was derived, which exhibited a constant value at a constant temperature. The linear equation of ln k against 1/T was obtained according to Equation (4) (
Figure 5). The activation energy Ea (93.45 kJ/mol) and the pre-finger factor k
0 (7.46 × 10
15) were calculated. Then, the mathematical model equation reflecting the change of the quality of the “boba” balls was established, as follows:
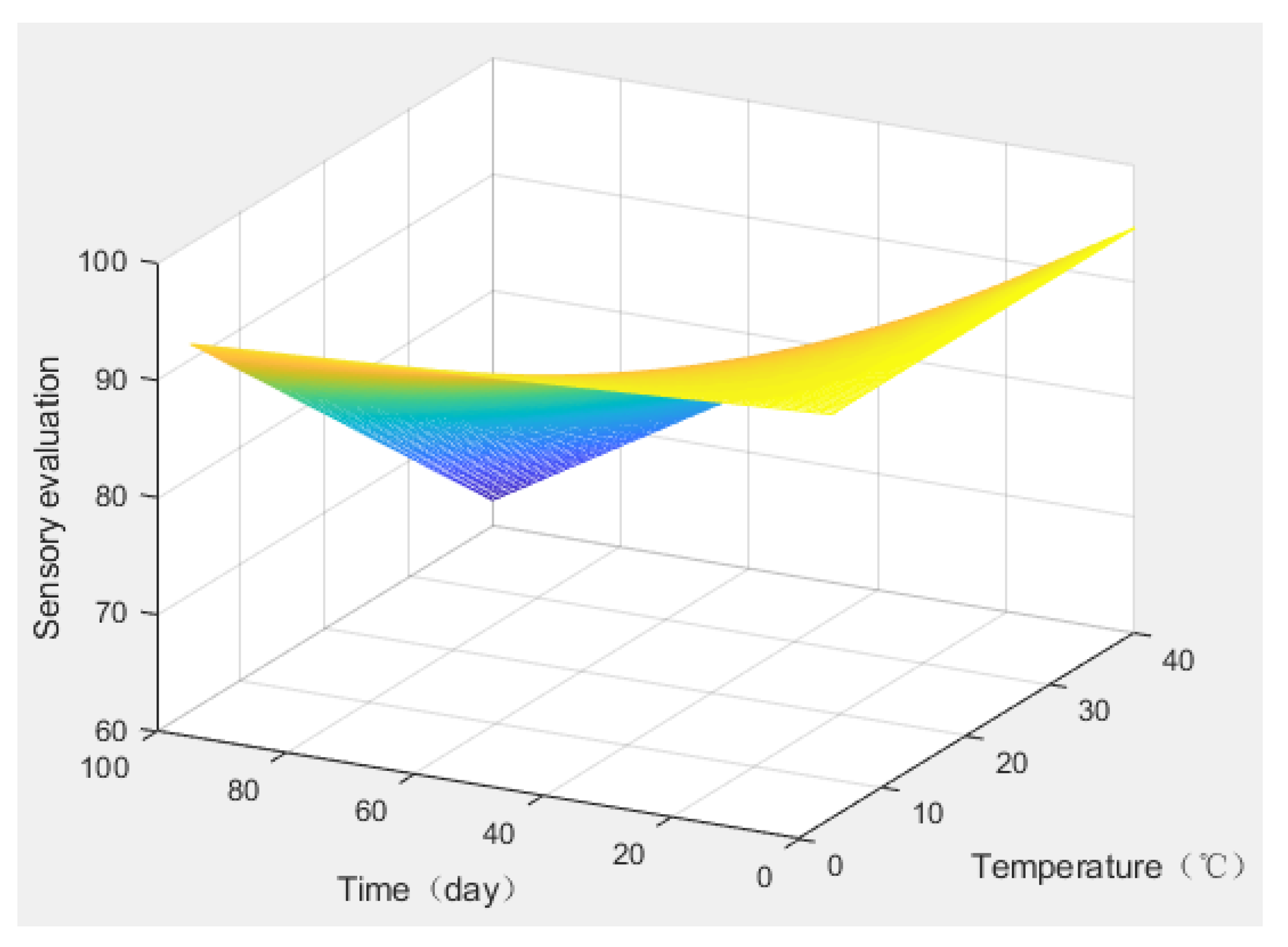
The response surface of the sensory evaluation changed with time and temperature was determined (
Figure 6). When the sensory score was below 80, the product was considered to be unable to meet consumers’ exp ectations and reach the shelf-life limit. Therefore, product shelf life could be predicted for different storage temperatures (
Table 3). When the storage temperature was lower than 10 °C, the shelf life of the “boba” balls was 465 days, exceeding one year.
4. Conclusions
The “boba” balls used in milk tea, which are made of calcium alginate, were studied here. The optimal technological parameters for the preparation of “boba” balls were discussed. The diameter of the liquid adding tube was 8 mm, the drop height was 25 cm, the sodium alginate solution concentration was 1.0%, the flow velocity was 60 mL/min, and the concentration of calcium chloride concentration was 1%. The prepared “boba” balls were stored under different temperature conditions. No microbes were detected after storage for 90 days. The sensory evaluation value of the “boba” ball decreased gradually with storage time at different temperatures. The mathematical model for predicting shelf life was as follows Equation (6).
According to the above mathematical model, the shelf life of the “boba” ball developed could reach more than 1 year in a refrigerated environment at temperatures lower than 10 °C.