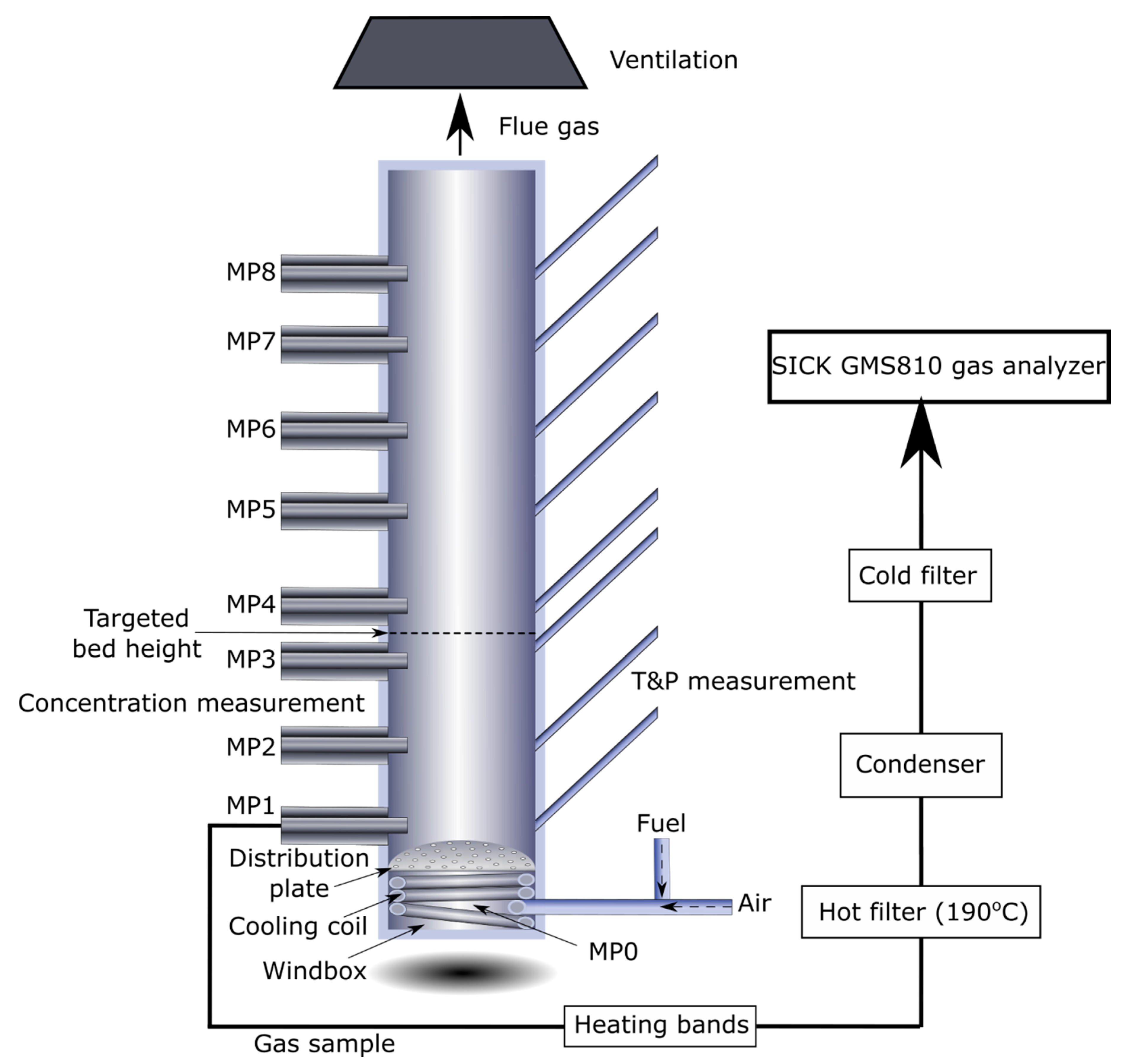
3.1. Experiments with Methane
The three materials (sand, ilmenite and C28) were examined at five different bed temperatures (600, 750, 800, 850, 900 °C). The results from these experiments using methane as fuel are also presented in
Tables S1–S3 in the
supplementary material. The vertical temperature profiles measured for the systems operated with sand, ilmenite and C28 at different bed temperatures can be seen in
Figure 4. The bed heights were estimated for the three bed materials at all bed temperatures. The average bed height was 15.6 cm for sand, 15.3 cm for ilmenite and 14.6 cm for C28, based on the pressure drop measurements for experiments with methane as fuel. The estimated bed height is, therefore, between measurement points 3 and 4 for all three materials. In all gas concentration measurements with methane as fuel, the methane concentration at the inlet was 9.07%, and the measured concentrations in the reactor are dry since steam is condensed prior to the gas analyser.
In
Figure 4, it can firstly be observed that the temperature profile is rather similar for all bed materials for the respective bed temperatures. The gases are rapidly heated upon entering the bed from the wind box below the distribution plate. The temperature is then rather constant in the bed (MP1–MP3) and for the most part also between MP3 and MP4, where the splash zone is considered to be located. The highest temperature is usually found just above the bed (MP4). In the freeboard (MP4–MP8), the temperature level is slowly decreased towards a value which is closer to the setpoint of the electrically heated furnace, which surrounds the reactor. The measured temperatures are overall very similar for the three bed materials although some differences can be observed in the freeboard at 700, 750 and 900 °C.
Since it is expected that increased temperature results from heat release from fuel combustion, it is interesting to observe if such temperature gains can be seen for the three bed materials. It is observed that there are differences in the ΔT between MP4 and MP5 for the three bed materials, which therefore could indicate that there are also differences in fuel conversion in this region for these materials. The temperature in the freeboard drops overall for all tested materials and bed temperatures but the drop is more significant at higher temperatures. The reason for this temperature drop is that the furnace temperature is below the temperature in the reactor where the exothermic reaction heats the inside of the reactor resulting in a heat loss to the surrounding furnace. It should also be mentioned that the operation of the ventilation system above the furnace is expected to cool the top of the freeboard to some extent. A more pronounced temperature drop, therefore, indicates that little or no fuel combustion takes place in this zone. A less pronounced temperature drop could indicate that more methane combustion takes place in the freeboard. Except for the measurements at 900 °C, where sand appears have a smaller temperature drop than the other two bed materials, it is difficult to observe any significant differences between the bed materials. The difficulty to see temperature differences, in general, can be traced to the fact that the convective heat transfer from the gas is lower compared to radiative heat transfer from bed materials and walls. It is also possible that conductive heat transfer from walls to the thermocouples could influence the measurements. This could, therefore, limit the possibility to observe changes in measured temperature based on temperature differences in the gas, especially in the freeboard where the presence of particles is scarce. In order to get a better idea about the differences between the three bed materials, gas concentrations measurements should, therefore, be consulted.
In relation to the gas concentration measurements, it is somewhat uncertain how representative the measured value for the average gas concentration in the bed is. There are two main reasons for this. The first reason for this is related to the nature of fluidized beds, where the gas concentration can be assumed to be different in the emulsion phase and in the bubble phase, at least according to the two-phase theory for fluidization. As a result of the presence of these two theoretically distinguishable phases, it could be expected that the probe manages to sample more gas from the emulsion phase where the gas is flowing upwards with a lower velocity. It is, in this experiment, unknown from which phase the extracted gas originates from (emulsion phase, bubble phase or both) which could have an impact on the measured concentration. It should also be mentioned that the gas velocity is the same for the different bed materials and the particle size is similar. A quick estimation of bubble diameters, bubble velocities and bubble fractions based on equations provided by Kunii and Levenspiel [
31] show that only minor differences between the three bed materials can be expected. The same conclusion is drawn when comparing the apparent overall coefficients of gas interchange between bubble and emulsion phases based on the equations presented by Sit and Grace [
32]. This indicates that the three cases should have a similar share of extracted gas from bubble and emulsion phase and that the mass transfer between bubble and emulsion phase should be similar. In summary, the results for different bed materials should be useful for comparison.
The second reason is that the measured gas concentration is affected by the depth at which the gas sampling takes place. This was evaluated in separate experiment with sand as bed material where the gas sampling point was varied between one side of the reactor wall to the other. In this experiment it could be observed that less fuel conversion took place in the middle of the tube where a higher CH4 concentration and lower CO2 concentrations was observed, compared to close to the reactor walls where more fuel was converted. This could, for example, be explained by the fact that bubbles are more easily formed in the middle of the bed and gas present in the bubbles has lower residence time in the bed. At 900 °C, the difference in measured CO2 concentration was quite significant at MP3, 6.9% in the middle compared to around 9.6% by the reactor walls. These measurements supported the choice to place the gas sampling location at a point approximately halfway into the middle of the tube, which should provide an estimated gas concentration, which is the most representative overall. It should be said that, at 600 °C, the measured concentrations were already very similar at MP1 and throughout the whole bed. This indicates that the gases were well mixed when entering the bed and a larger concentration difference over the cross section when the in-bed fuel conversion is higher.
The measured concentration should, nevertheless, provide for a good comparison between different cases and it is also observed overall that the measured concentrations align well with the measured values in the freeboard which supports the conclusion that the measured values are reliable.
A first observation, which can be seen in
Figure 5, is that the methane concentration in the bed decreases with increasing bed temperature, thus displaying that more combustion of methane takes place in the lower part of the reactor at higher bed temperatures. This is expected since methane combustion is promoted by a higher temperature in general and, therefore, there is less unreacted methane in the freeboard. This results in a temperature drop in the freeboard when little or no unreacted methane is left in the flue gas (see
Figure 4) and heat is instead transferred from the reactor towards the inside of the furnace, which was approximately 50–100 °C cooler than the targeted bed temperature.
At a bed temperature of 600 °C, there is almost no methane conversion taking place in any of the systems. When the bed temperature was increased further, ignition of the fuel took place in the lower part of the freeboard with a visible flame and audible sound, which occurred around 700 °C for all bed materials. At a bed temperature of 750 °C, a significant part of the methane appears to be burnt between MP4 and MP5, which is the lower part of the freeboard, which is in line with the observations in the temperature profile in
Figure 4, where the temperature was more constant at 750 °C between MP4 and MP5 compared to higher bed temperatures. At higher bed temperatures, a clearer decrease in temperature was observed between these two measurement points. This could be due to almost no remaining unreacted methane being detected in the freeboard at bed temperatures > 750 °C, resulting in a flue gas with mostly inert compounds at MP6 or higher up in the freeboard. It can be noted that it was difficult to keep the wind box temperature below 500 °C in some cases and especially with ilmenite as a bed material. Based on the measured gas concentrations, it is, however, clear that ignition did not take place in the wind box.
In the experiments with C28 as bed material, it can be observed that the measured methane concentration is lower in the lower part of the bed (MP1-MP3) compared to just above the bed at MP4 and the measured concentration is almost the same at MP5 at a bed temperature of 800–850 °C. Some indications of similar character can be observed with ilmenite at a bed temperature of 800–850 °C. This does not occur with sand as the bed material, where the methane concentration is gradually reduced along the height of the reactor. This might seem surprising at first, but this is most likely explained by the gas–solid reactions with the oxygen carrier. As described earlier, it is probable that gas from the emulsion phase is sampled compared to gas in the bubble phase. In the systems with oxygen carrier materials, gas–solid reactions are likely to occur in the emulsion phase, where gas–solid contact is better. It could, therefore, be possible to obtain a higher methane conversion in the emulsion phase if the oxygen carrier has a high reactivity with the fuel gas (such as, for example, C28). At the top of the bed the gas from emulsion phase and bubble phase is mixed and the sampled gas in the freeboard can be expected to contain more equal quantities of gas from bubble and emulsion phase. This could, therefore, explain the increased methane concentration with the oxygen carriers at MP4-MP5, where gas from the bubble phase is expected to have a higher methane concentration. The increase in methane concentration is a result of the amount of gas in the bubble/emulsion phase and the gas conversion in each phase. Nevertheless, the results support the hypothesis that the gas–solid reaction takes place in the emulsion phase, which takes place to an especially high extent with C28 as the bed material.
In order to simplify a comparison between the different bed materials,
Figure 6 can be consulted, where the results for all bed materials are added together at the respective bed temperatures. At 600 °C, it can be observed in
Figure 6 that the difference between the three materials is small, where little/no methane conversion is observed. At all other bed temperatures (750–900 °C), however, it can be observed that the methane concentration is overall lower in the bed, using ilmenite and C28 as bed materials, compared to the results with sand. The reason for this difference in gas conversion can be explained by the gas–solid reaction with the oxygen carrier, which only occurs in the system with ilmenite and C28. This reaction can, therefore, be considered as especially important to obtain a high methane conversion in the bed. It should be mentioned that the reaction with the bed material itself is endothermic but the reoxidation is exothermic, so the net heat release corresponds to the heat of combustion for methane. It should be mentioned that limitations in mass transport between the bubble and emulsion phase can have a significant impact on the comparison between sand and the oxygen carriers. In the extreme case that the mass transfer from the bubbles to the emulsion phase would be very poor, and if there are no bed particles present in the bubbles, the contact between fuel and bed material would be negligible. This would have resulted in identical fuel conversion for different kinds of bed material. It can be observed that this is not the case here. It is likely that gas–solid reactions occur in the emulsion phase, between fuel and oxygen carrier. Gas–solid reactions could, however, also take place as a result of particles present in the bubbles. It should also be noted that the degree of mixing in the fluidized bed, where the experiments take place, can affect the experimental results. Since the mixing of the fluidized bed can vary between different reactors, it is important to keep this in mind when evaluating the difference between different bed materials.
It is possible to estimate the degree of methane conversion based on the CH4 inlet concentration, the measured concentration of CH4 and the fact that 2 mol of steam is produced per mol CH4 according to Equation (8). At the highest tested bed temperature (900 °C), for example, the methane conversion is almost complete in the bed with C28 and ilmenite (corresponding to 99.7% and 99.3% methane conversion at MP2, respectively), whereas the measured methane conversion (based on the measured CH4 concentration) with sand was equal to 86.7%.
The trend is the same at 850 °C at the same measurement point, where the methane conversion was 98.7% for C28, 90.4% ilmenite and 61.7% for sand. This displays that almost 100% oxidation of the methane can be achieved in the bed in the presence of an oxygen carrier, which cannot be said when using sand, where a significant part of the combustion of methane takes place above the bed at these bed temperatures.
In the comparison between the bed materials, it should be mentioned that different bed masses are used which could have an influence on the result, where a larger specific bed inventory (see
Table 3) could potentially result in a higher degree of fuel conversion. It is not possible to evaluate the effect of this in this experiment, but it should be mentioned that operation with C28 results in higher fuel conversion than ilmenite, but with a smaller specific bed inventory compared to ilmenite. Regarding the bed inventory, it should also be added that the pressure is overall higher in the bed and in the wind box in the system with ilmenite since the bed density is higher. This could cause more significant fluctuations in pressure where bursts of larger bubbles could pass through the bed.
In order to confirm that the converted methane is fully oxidized to CO2, and not converted to CO, the measured concentrations of CO2 and CO can be consulted. The measured CO2 concentrations confirm that changes in the CH4 concentration results in the opposite direction for the CO2 concentration. In cases where methane is completely converted, the concentration of CO2 is equal to the inlet concentration of CH4 at the inlet when taking into account the condensed steam, which does not reach the gas analyser. In the comparison between C28 and sand, it can, for example, be observed that the CO2 concentration is higher in the bed for all the measurement points in the bed temperature interval 750–900 °C. To summarize, it can be clearly be seen that more fuel conversion takes place in the bed with the two oxygen carrier materials compared to sand.
Some of the differences in methane conversion and CO
2 formation can be explained by the CO concentration measurements (see
Figure 7). It can be observed that the CO concentration in the bed is overall highest in the system with ilmenite as a bed material. The concentration of CO is overall low in the freeboard, especially with sand and ilmenite. In the bed with C28, the CO concentration is generally the lowest in the bed but the highest above the bed. It is apparent that some of the CH
4 is converted to CO in the bed but that most of it is then converted to CO
2 in the lower part of the freeboard. The CO formation is generally small for all bed materials at high temperature, which aligns well with the previous observations. It should also be mentioned that the measured H
2 concentrations are overall low and have been neglected in this analysis.
In addition, the dependency on the air-to-fuel ratio was investigated where the ratio was varied between 1 to 1.2 for the three bed materials at 750 °C. The results are presented in
Table 5. It can be observed that the air-to-fuel ratio had a limited impact on the conversion of methane in the bed in general for the three bed materials although some improvement in fuel conversion is observed when increasing the AFR. It can also be seen that the same trend with a lower methane concentration with ilmenite and C28 as a bed material compared to sand is observed at each measurement point for other air-to-fuel ratios within the tested interval with the exception of MP1, where the methane concentration was slightly lower with sand as a bed material compared to using ilmenite. It should be noted that the choice of bed temperature for the variation in the AFR could influence the results since the difference in fuel conversion between sand and the two oxygen carriers is observed to be more pronounced at higher bed temperatures.
It should be mentioned that the system with sand has a higher methane conversion in the freeboard than the system with C28. The results for sand and C28 presented in
Table 4 show that the methane concentration is higher with C28 as bed material at MP5 even though more methane conversion takes place in the bed (see MP4). The same trend is observed when looking at the methane concentration at bed temperatures 800–850 °C.
This could potentially be caused by the fact that the concentration of the methane is lower in that region, which results in it being more difficult to have the fuel ignition taking place in the gas phase, compared to the system with sand, where the gas mixture is closer to stoichiometric conditions.
In addition to the results related to fuel conversion, the NO emissions were estimated, and the results are presented in
Table 6. NO emissions from the process are based on the measured concentration of NO in the dry flue gas. Some NO
2 is also likely present albeit in smaller concentration, typically NO
2 is less than 5% of the total NO
x [
33] meaning that the NO concentration should be close to the NO
x concentration in the furnace and at the flue gas stack. The data for MP7 with sand as a bed material at 850 °C are missing.
It can be observed that the measured concentrations of NO are overall low, especially at high temperature. It can also be observed that almost no NO at all is detected in the bed. It may seem contradictory at first that more NO is formed at a lower temperature, since the formation of thermal NOx is expected to be the dominating mechanism for NO formation, since no nitrogen is present in the fuel and no prompt NO can be expected to be formed in this oxygen-rich atmosphere. The tendency for higher NO formation at moderate temperatures could, however, be explained by knowing where in the bed, as well as how, the fuel conversion takes place. The highest NO concentrations are measured above the bed in the cases where a significant part of the methane fuel conversion takes place above the sand bed, i.e., at a bed temperature of 750 °C. This was also the temperature level where flames were most visible and the most audible and visible explosions took place above the bed, as observed from the top of the reactor. Fuel-NOx formation can be excluded as the fuel does not contain any nitrogen compounds, and since the system operates with excess oxygen, any significant prompt NOx formation is unlikely. However, the NO formation can be explained by local hotspots from uncontrolled flame combustion in the freeboard where the thermal inertia is low. Considering the composition of the gas mixture, a very significant increase in gas temperature is expected as the gas ignites. Since thermal NOx formation increases exponentially with temperature, the flame temperature should be controlled. In the bed, where the heat transfer between gas and solids, as well as the thermal inertia, is high, no hotspots are expected and NO formation is not expected, which is confirmed by low NO concentration in that zone for all bed materials regardless of the amount of fuel conversion in the bed. However, high fuel conversion in the bed gives less NO formation in the freeboard and, thus, lower overall emissions, which is also seen by the performed measurements.
The measured freeboard gas temperature is close to the temperature of the bed and, thus, is clearly lower than the temperature at which thermal NO formation takes place, according to the Zeldovich mechanism, where local temperatures of at least 1300 °C are required [
34]. The dominating heat transfer mechanism to the thermocouple is radiation from reactor walls and the bed material, as well as direct heat transfer from the walls. With the short path lengths, the emissivity of gas radiation should be less than 0.05, and convection heat transfer from gas is also small. This would mean that local high gas temperatures are poorly visible in the measurement. Thus, the absence of a rise in temperature from MP4 to MP5 in
Figure 4, despite significant fuel conversion taking place—
Figure 5—could be explained by a combination of falling wall temperatures and poor heat transfer from the gas.
It should also be added that there is a difference between flames produced in a fluidized bed and flame combustion with a gas burner for example. There is no sustained flame in the fluidized bed and local measurements cannot be made to characterize the flame and determine local temperatures and gas compositions, but the work must rely on global measurements.
The difference between a system with flame combustion in the freeboard and a system with high in-bed fuel conversion is illustrated in
Figure 7, where a comparison is made between the results in measured concentrations of NO and CH
4 for sand at 750 °C and for C28 at 900 °C.
In
Figure 8, it can be observed that, with sand at 750 °C, fuel conversion takes place mainly above the bed. This is obvious since the concentration of CH
4 is reduced significantly between MP4 and MP5. Between the same measurement points, the NO concentration increases significantly. This could be compared with the experiment with C28 at 900 °C, where CH
4 conversion takes place in the bed and almost no NO emissions are detected at any height in the reactor. The difference in the results between temperatures 750 and 900 °C can be further emphasized by observing
Table 5 where the measured NO emissions are at least 79 ppm for all bed materials at MP8 at 750 °C, whereas no more than 11 ppm is detected at MP8 at 900 °C. This represents, therefore, a reduction in NO emissions by more than 93% compared to a case with flame combustion.
It can be observed that the measured concentration of NO is higher with ilmenite as a bed material than with sand at 750 °C and 800 °C. A similar amount of methane conversion appears to take place above the bed in those cases, so the reason could instead be connected to the availability of oxygen in the bed. In the bed with an oxygen carrier, oxygen is available both in gaseous and solid phase in the form of the oxygen carrier material, which could contribute to a higher risk of NO formation. When C28 is used as a bed material, the measured NO concentration is lower, however, and this could be explained by the fact that more fuel conversion takes place in the bed with this bed material, which means that less fuel conversion can take place above the bed and cause local hotspots where NO may be formed. It should also be mentioned that NO may be reduced by reacting with the oxygen carrier to form N2 (while the oxygen carrier is oxidized) but the reaction rate for this reaction may vary for different conditions.
3.2. Experiments with Off-Gas
The same bed materials (sand, ilmenite and C28) were also evaluated with PSA off-gas as a fuel at five different bed temperatures (600, 650, 700, 750, 800 °C). The data from the experiments using PSA off-gas as fuel are also presented in
Tables S4–S7 in the
supplementary material. The vertical temperature profile of the systems for the experiments can be observed in
Figure 9. In the experiments with off-gas as fuel, the average bed height was 15.4 cm for sand, 15.6 cm for ilmenite and 14.6 cm for C28.
At high temperatures, it was difficult to keep the temperature in the wind box below the limit at which the gas mixture ignites in the wind box. This occurred for all bed materials when operating at a bed temperature of 800 °C and with C28 at 750 °C. In those cases, the wind box temperature was significantly higher and complete conversion of CH4, CO and H2 was observed at all measurement points, which is why these results were excluded from this article. The apparent reason for this taking place is that the cooling of the wind box was not sufficiently efficient to hinder ignition at these temperature levels. For these cases, it is observed that the fuel is almost completely converted already at MP1, which makes these cases irrelevant in the analysis. The temperature profiles overall look quite similar for the three bed materials, although some differences are seen in the freeboard.
In order to get a better overview of the fuel conversion in the reactor, the gas concentration measurements of fuel gas components are evaluated. A first observation, which could be made from this data, is that very little/no hydrogen is measured during any of the experiments, which indicates that hydrogen reacts quickly in presence of oxygen, as could be expected. The focus was therefore to evaluate the concentrations of CO, CH
4 and CO
2 to monitor the fuel conversion in the system. The ratio of the measured concentration of unburnt carbon in the form of CO and CH
4 in relation to the total measured carbon (CO, CH
4 and CO
2) as a measure of the gas conversion. The results are presented in
Figure 10, where ratio at the inlet of 40% is also included.
It can be observed that part of the PSA off-gas is already burnt in the bed at a bed temperature of 600 °C with all bed materials. It is interesting to see that some fuel conversion takes place at these moderate temperatures, which are low in relation to optimal temperatures for OCAC operation. It can also be seen that a significant part of the fuel conversion takes place above the bed and almost all fuel is converted at MP5 ≥ 650 °C with all bed materials. Close to complete carbon conversion is observed at 700–750 °C at the top of the reactor with all three bed materials. The carbon conversion in the bed is also similar for the bed materials. The results in
Figure 10 indicate overall small differences between the different bed materials. This suggests that the choice of bed material may not be so important to achieve high fuel conversion with PSA off-gas as fuel, as opposed to methane where there was a visible difference. At a bed temperature of 750 °C, for example, it is observed that the system with sand as a bed material has a lower content of unburnt carbon in the bed than the system with ilmenite at MP1-MP4. For sand at MP1 at bed temperatures of 700 and 750 °C, the measured concentration of CO
2 indicates that almost complete fuel gas conversion takes place at the very bottom of the bed below MP1. This is, however, contradictory to the results from the results above this point, which are more in line with expectations and the rest of the results, which indicate that the result at MP1 for these two temperatures could be outliers. This is also the reason for the fact that these points are not connected with a line to the rest of the data at the respective bed temperatures.
The difference in concentrations of CO and CH
4 is presented in
Table 7. The ratio is 1 at the inlet.
In
Table 6, it can be observed that the ratio is generally below 1 in the bed, thus indicating higher gas conversion of CO compared to CH
4 in the bed, whereas the ratio is above 1 above the bed, where the ratio is especially high with sand. The ratio is higher above the bed because of significant methane conversion taking place above the bed, whereas the CO concentration is more constant over the height of the reactor. This highlights, once again, the difficulty to convert methane in the bed and motivates the focus on this compound in these experiments.
Based on overall results from this study, it should be possible to use a bubbling fluidized bed, operated in OCAC configuration, to convert the targeted fuel mix of PSA off-gas and light hydrocarbons, such as methane, in the dense bed. This could be important for the implementation of the proposed process application to use an OCAC-reactor as a heat source for steam reforming, where this kind of fuel mix is intended. The NO emissions were also monitored with PSA off-gas as fuel. The results are presented in
Figure 11.
It can be observed that the NO emissions are overall low with all bed materials. The NO concentration is especially low in the bed and, in general at bed temperatures above 600°C. The system with C28 as a bed material results in slightly higher NO emissions overall than the other systems, which could be connected to the previously mentioned effect that the bed material could present more oxidizing conditions than sand, for example.
Combustion with low NO emissions is one of the key advantages considered when evaluating the possibility to integrate OCAC with SMR, instead of using the conventional SMR furnace with gas-fired burners. The conventional furnace operates with flame combustion, causing the significant formation of thermal NO
x. With standard industrial gas burners and combustion in the presence of a flame, emissions corresponding to 100 ppm NO
x can be expected [
35]. The experimental results in this work indicate that operation with oxygen carriers as bed materials at high bed temperatures in OCAC configuration should enable fuel conversion in the bed without the presence of a flame and, thus, provide very low NO
x formation.