Structural, Morphological, and Electrochemical Performance of CeO2/NiO Nanocomposite for Supercapacitor Applications
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of CeO2/NiO Nanocomposite
2.2. Characterization Techniques
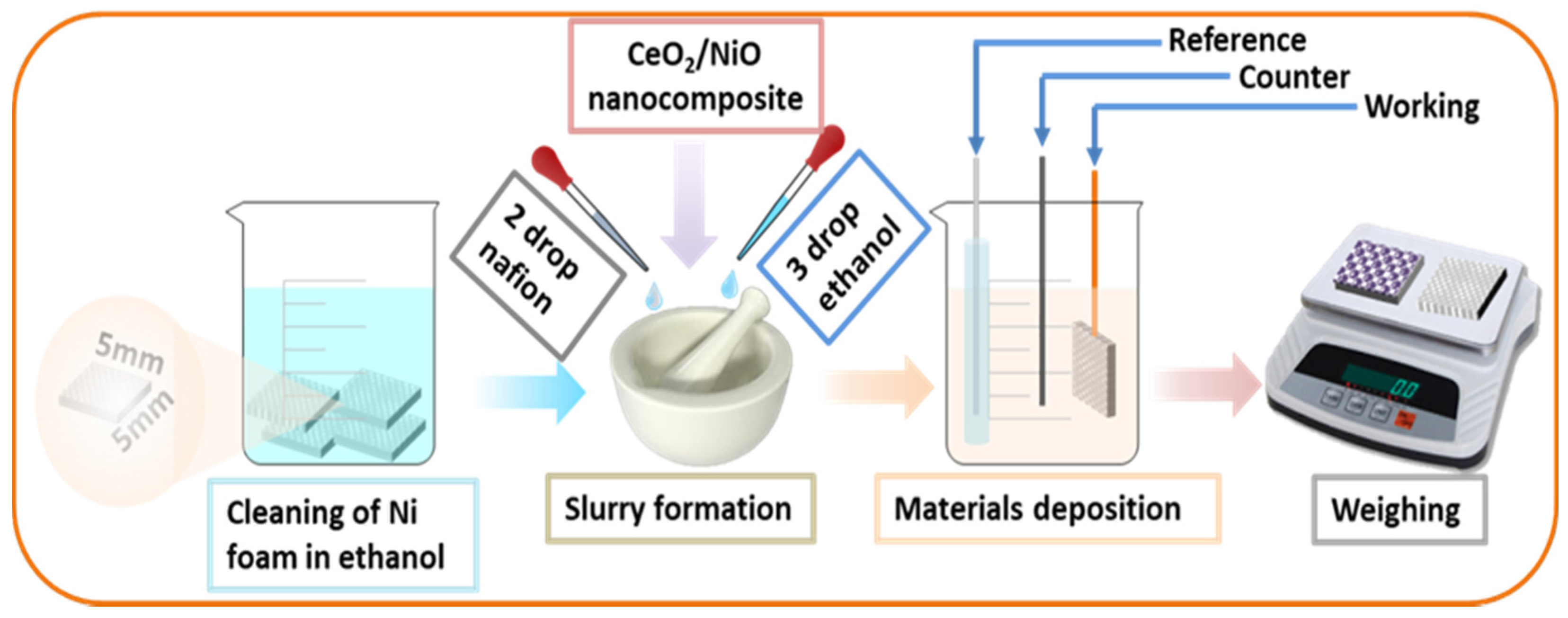
2.3. Fabrication of Supercapacitor Electrode and Electrochemical Tests
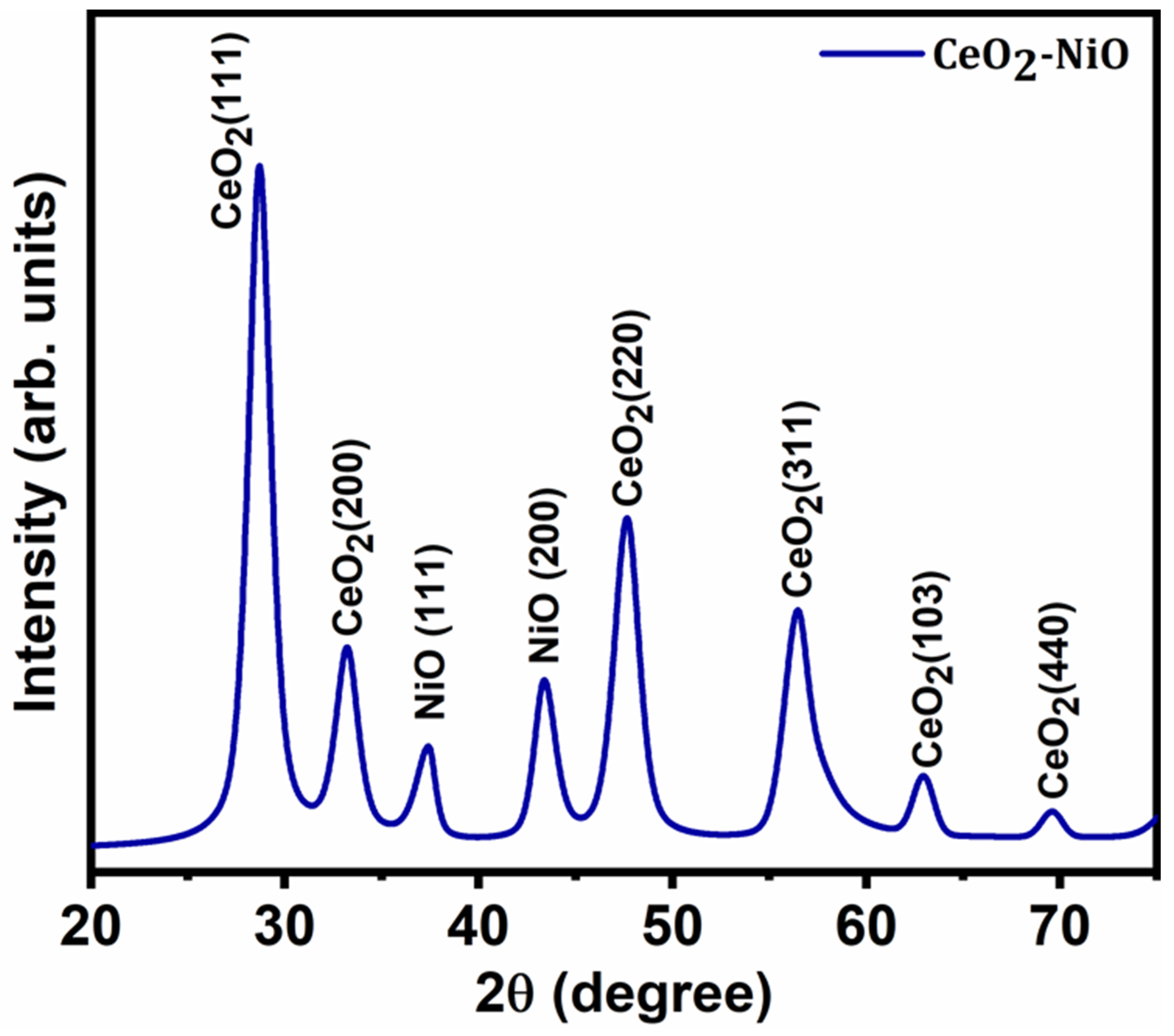
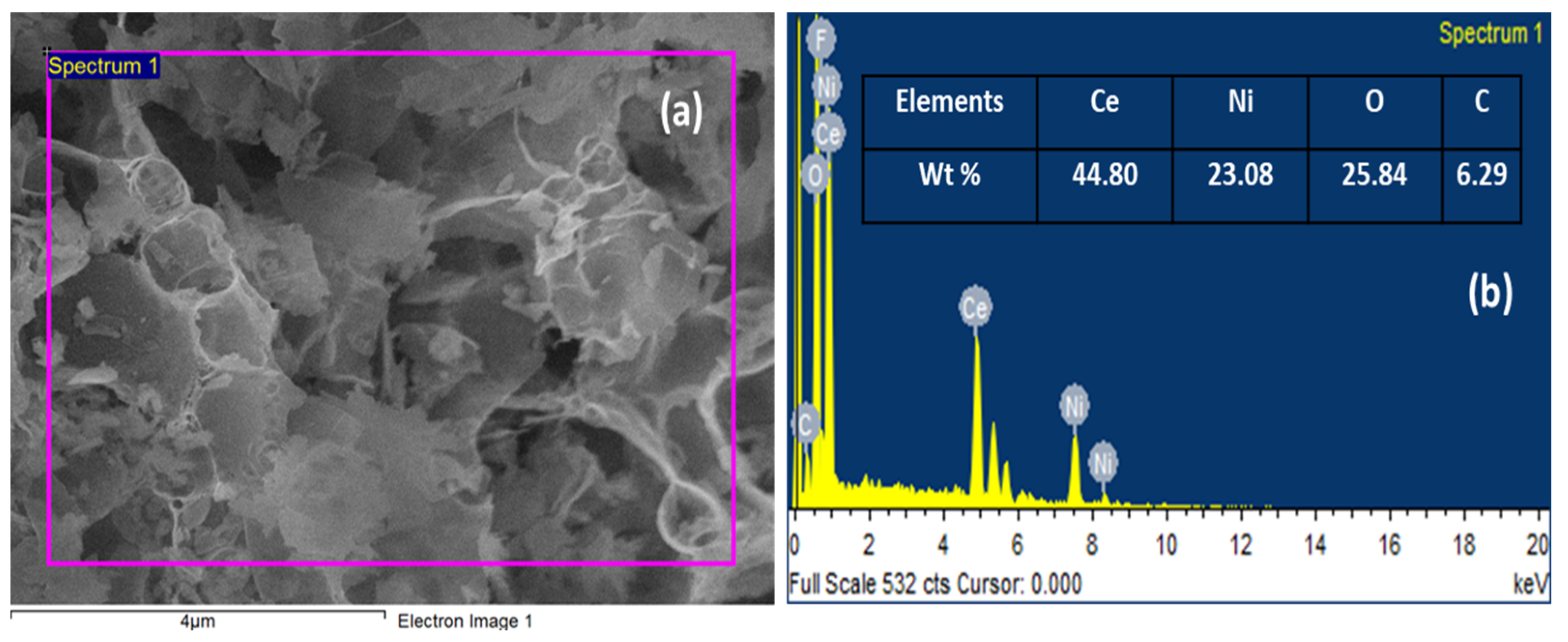
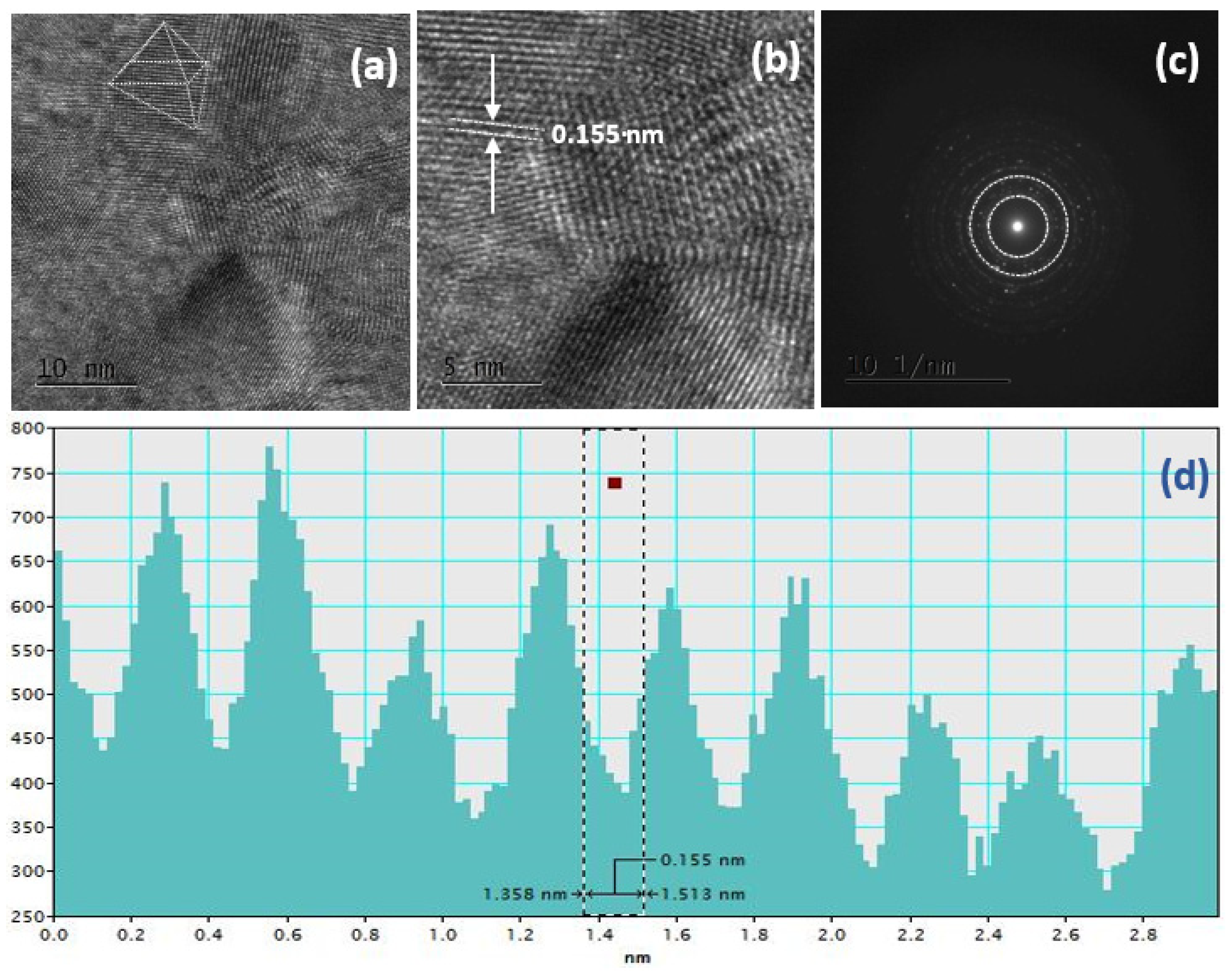
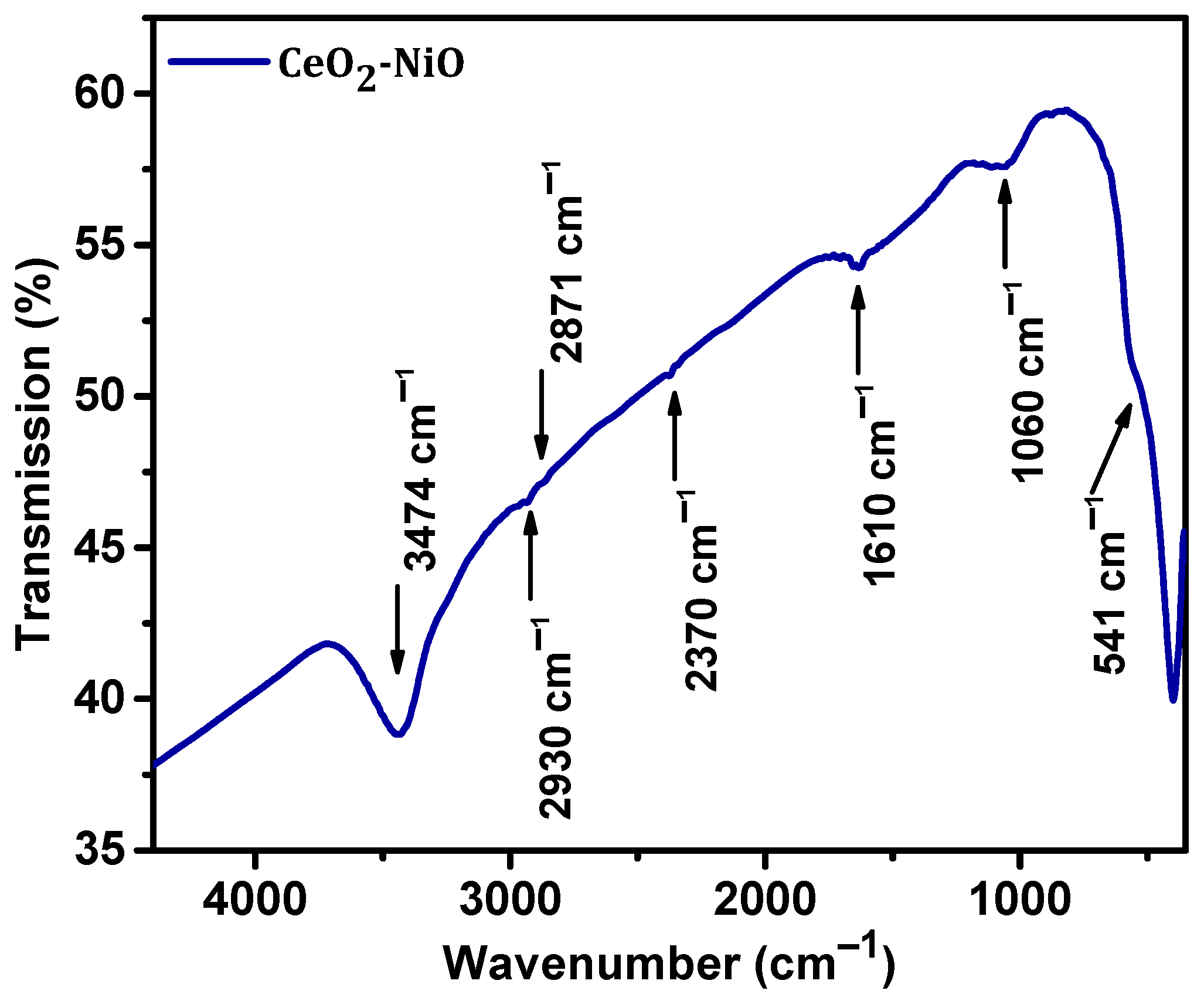
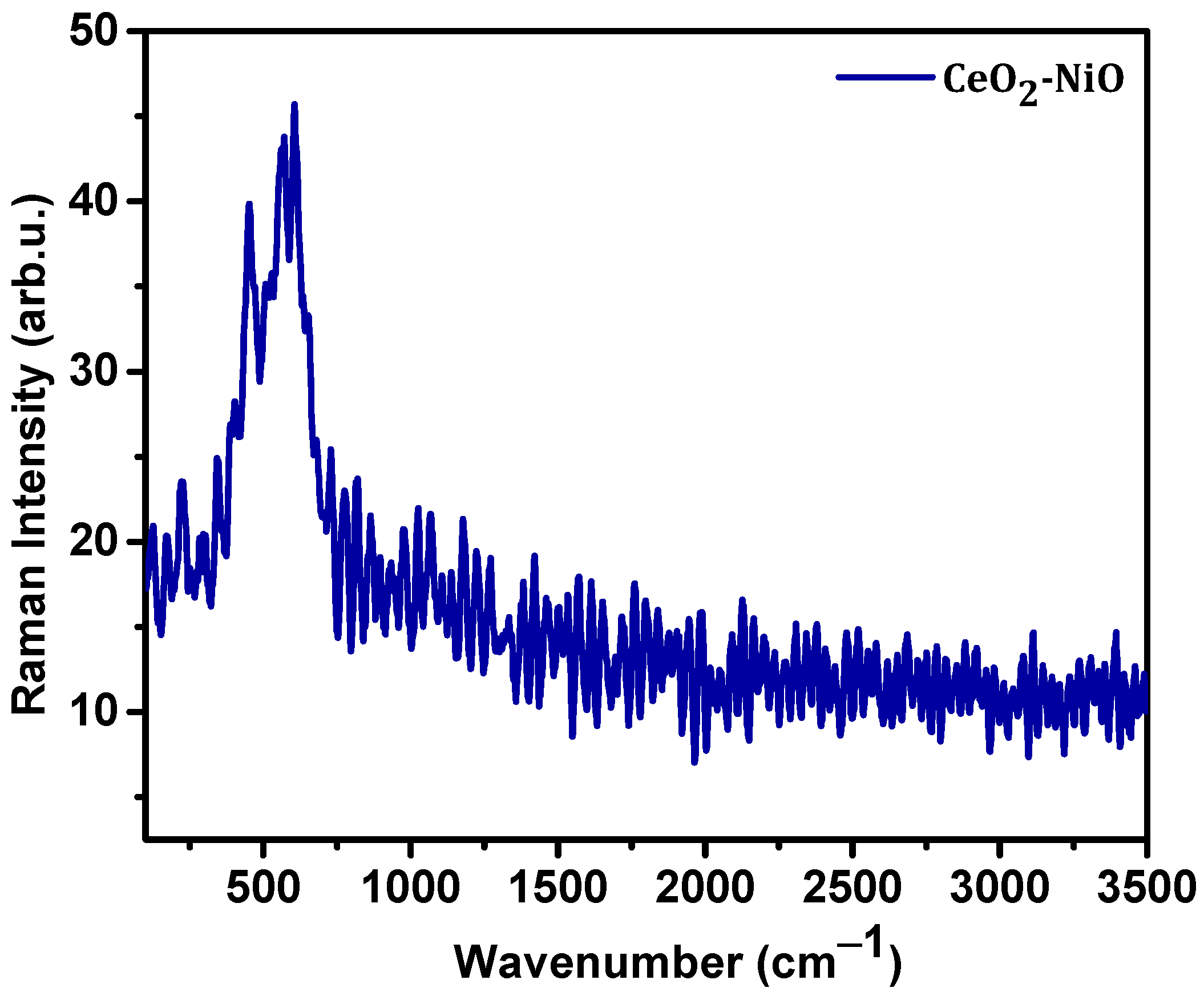
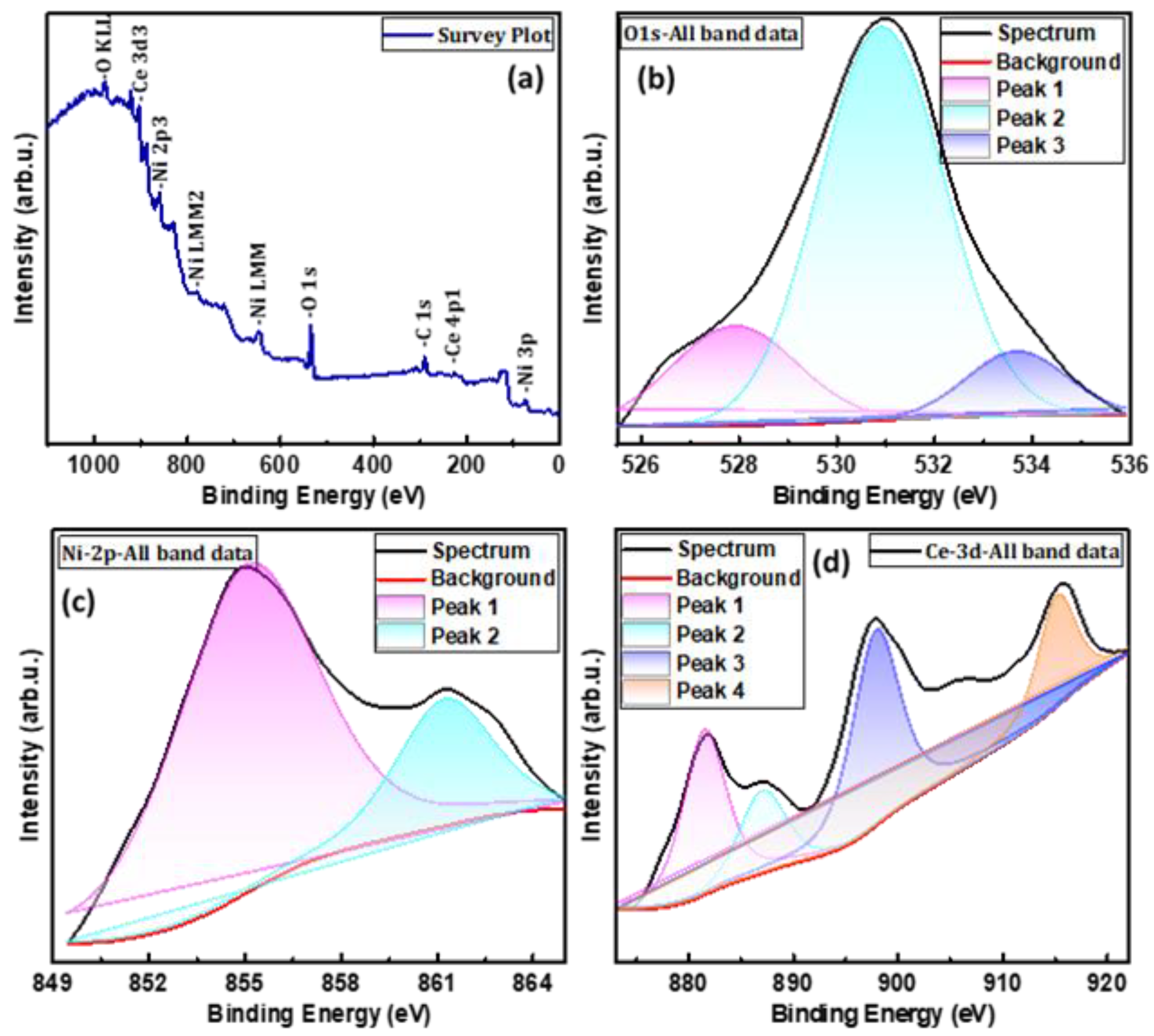
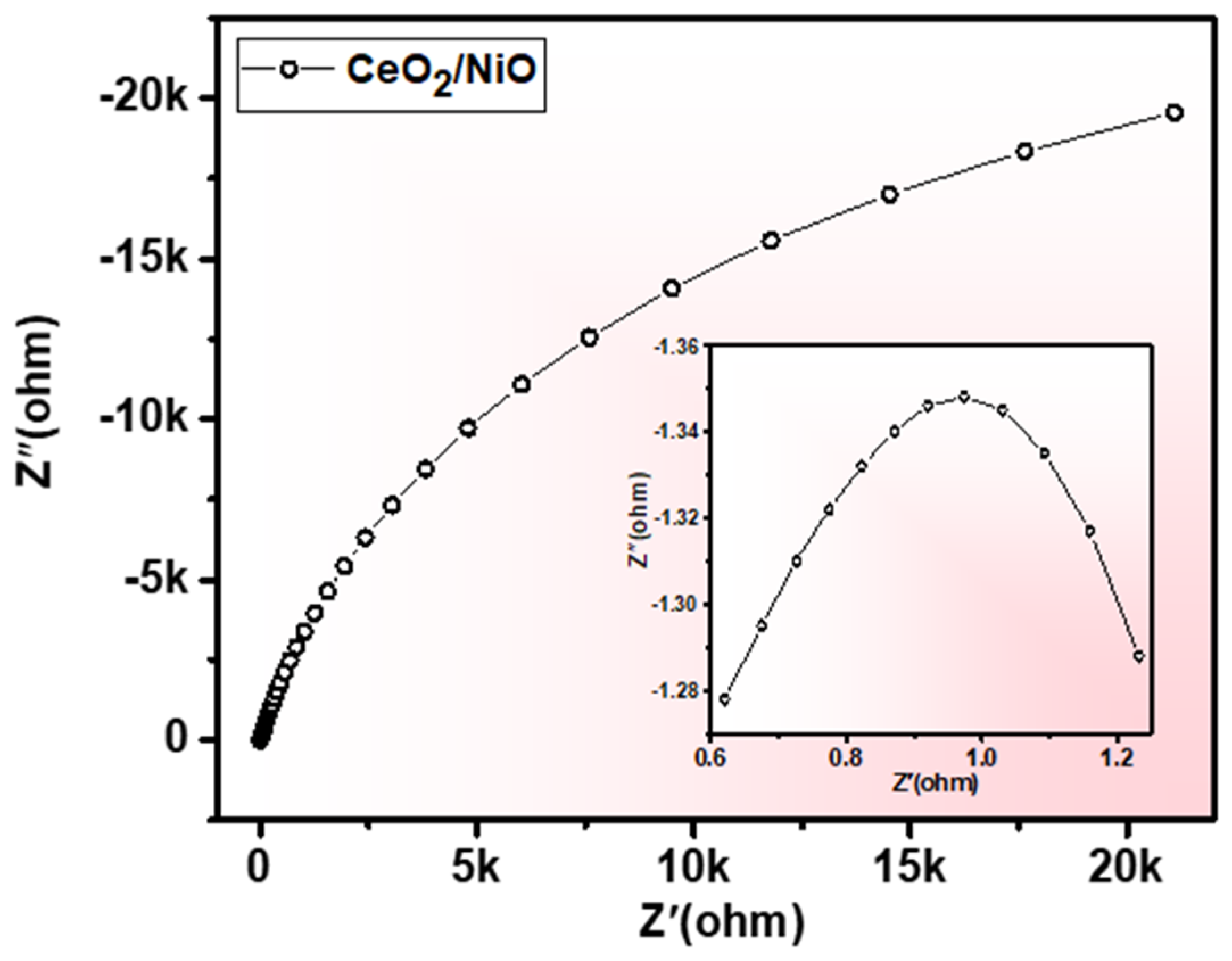
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Mao, L.; Cheng, Q.; Li, J.; Liao, F.; Yang, G.; Xie, L.; Zhao, C.; Chen, L. Two-dimensional Spinel Structured Co-based Materials for High Performance Supercapacitors: A Critical Review. Chem. Eng. J. 2020, 387, 124081. [Google Scholar] [CrossRef]
- Yi, T.F.; Wei, T.T.; Mei, J.; Zhang, W.; Zhu, Y.; Liu, Y.G.; Luo, S.; Liu, H.; Lu, Y.; Guo, Z. Approaching High-Performance Supercapacitors via Enhancing Pseudocapacitive Nickel Oxide-Based Materials. Adv. Sustain. Syst. 2020, 4, 1900137. [Google Scholar] [CrossRef]
- Huang, C.; Hao, C.; Zheng, W.; Zhou, S.; Yang, L.; Wang, X.; Zhu, L. Synthesis of polyaniline/nickel ox-ide/sulfonated graphene ternary composite for all-solid-state asymmetric supercapacitor. Appl. Surf. Sci. 2020, 505, 144589. [Google Scholar] [CrossRef]
- Raghavendra, K.V.G.; Vinoth, R.; Zeb, K.; Gopi, C.V.M.; Sambasivam, S.; Kummara, M.R.; Kim, H.J. An in-tuitive review of supercapacitors with recent progress and novel device applications. J. Energy Storage 2020, 31, 101652. [Google Scholar] [CrossRef]
- Chime, U.K.; Nkele, A.C.; Ezugwu, S.; Nwanya, A.C.; Shinde, N.; Kebede, M.; Ejikeme, P.M.; Maaza, M.; Ezema, F.I. Recent progress in nickel oxide-based electrodes for high-performance supercapacitors. Curr. Opin. Electrochem. 2020, 21, 175–181. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Wang, S.; Liu, H.K.; Li, L. Transition metal based battery-type electrodes in hybrid supercapacitors: A review. Energy Storage Mater. 2020, 28, 122–145. [Google Scholar] [CrossRef]
- Oloore, L.E.; Gondal, M.A.; Popoola, A.J.; Popoola, I. Pseudocapacitive contributions to enhanced electrochemical energy storage in hybrid perovskite-nickel oxide nanoparticles composites electrodes. Electrochim. Acta 2020, 361, 137082. [Google Scholar] [CrossRef]
- Padmanathan, N.; Selladurai, S. Electrochemical capacitance of porous NiO–CeO2 binary oxide synthesized via sol–gel technique for supercapacitor. Ionics 2013, 20, 409–420. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Sakthivel, P.; Asaithambi, S.; Murugan, R.; Yuvakkumar, R.; Ravi, G. Solvent dependent mor-phological modification of micro-nano assembled Mn2O3/NiO composites for high performance supercapacitor applications. Ceram. Int. 2019, 45, 4298–4307. [Google Scholar] [CrossRef]
- Xie, A.; Tao, F.; Li, T.; Wang, L.; Chen, S.; Luo, S.; Yao, C. Graphene-cerium oxide/porous polyaniline composite as a novel electrode material for supercapacitor. Electrochim. Acta 2018, 261, 314–322. [Google Scholar] [CrossRef]
- Maheswari, N.; Muralidharan, G. Hexagonal CeO2 nanostructures: An efficient electrode material for supercapac-itors. Dalton Trans. 2016, 45, 14352–14362. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, H. A simple synthesis method of nanocrystals CeO2 modified rGO composites as electrode materials for supercapacitors with long time cycling stability. Powder Technol. 2018, 327, 275–281. [Google Scholar] [CrossRef]
- Sivasakthi, P.; Bapu, G.R.; Murugavel, K.; Mohan, S. Facile method of pulse electrodeposited NiO-CeO2Sm doped nanocomposite electrode on copper foam for supercapacitor application. J. Alloys Compd. 2017, 709, 240–247. [Google Scholar] [CrossRef]
- Deng, D.; Chen, N.; Li, Y.; Xing, X.; Liu, X.; Xiao, X.; Wang, Y. Cerium oxide nanoparticles/multi-wall carbon nanotubes composites: Facile synthesis and electrochemical performances as supercapacitor electrode materials. Phys. E Low Dimens. Syst. Nanostruct. 2017, 86, 284–291. [Google Scholar] [CrossRef]
- Ahmad, N.; Alam, M.; Wahab, R.; Ahmad, J.; Ubaidullah, M.; Ansari, A.A.; Alotaibi, N.M. Synthesis of NiO–CeO2 nanocomposite for electrochemical sensing of perilous 4-nitrophenol. J. Mater. Sci. Mater. Electron. 2019, 30, 17643–17653. [Google Scholar] [CrossRef]
- Ahmad, N.; Umar, A.; Kumar, R.; Alam, M. Microwave-assisted synthesis of ZnO doped CeO2 nanoparticles as potential scaffold for highly sensitive nitroaniline chemical sensor. Ceram. Int. 2016, 42, 11562–11567. [Google Scholar] [CrossRef]
- Ansari, A.A.; Labis, J.; Alam, M.; Ramay, S.M.; Ahmad, N.; Mahmood, A. Influence of copper ion doping on structural, optical and redox properties of CeO2 nanoparticles. J. Electroceram. 2016, 36, 150–157. [Google Scholar] [CrossRef]
- Xiong, S.; Jiang, S.; Wang, J.; Lin, H.; Lin, M.; Weng, S.; Chen, J. A high-performance hybrid supercapacitor with NiO derived NiO@ Ni-MOF composite electrodes. Electrochim. Acta 2020, 135956. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, J.; Tong, J.; Hu, Y.; Guan, B.; Hu, B.; Wang, C. Hierarchical porous MnO2/CeO2 with high per-formance for supercapacitor electrodes. Chem. Eng. J. 2016, 286, 139–149. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, C.; Rajkumar, M.; Rajavel, K.; Zhu, P.; Xuan, W.; Wang, F. Porous nickel oxide mi-crosphere and Ti3C2Tx hybrid derived from metal-organic framework for battery-type supercapacitor electrode and non-enzymatic H2O2 sensor. Electrochim. Acta 2019, 322, 134771. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadossd, G.; Thangamuthu, R.; Ragupathy, P.; Kumar, M.R.; Kumar, R.M.; Jayavel, R. High Electrochemical Performance and Enhanced Electrocatalytic Behavior of a Hydrothermally Synthesized Highly Crystalline Heterostructure CeO2@NiO Nanocomposite. Inorg. Chem. 2019, 58, 13843–13861. [Google Scholar] [CrossRef] [PubMed]
- Racik, K.M.; Guruprasad, K.; Mahendiran, M.; Madhavan, J.; Maiyalagan, T.; Raj, M.V.A. Enhanced electro-chemical performance of MnO2/NiO nanocomposite for supercapacitor electrode with excellent cycling stability. J. Mater. Sci. Mater. Electron. 2019, 30, 5222–5232. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Ragupathy, P.; Kumar, R.M.; Jayavel, R. Enhanced electro-chemical supercapacitor and excellent amperometric sensor performance of heterostructure CeO2-CuO nanocomposites via chemical route. Appl. Surf. Sci. 2018, 456, 104–113. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Muralidharan, G. Nanostructured NiO based all solid state electrochromic device. J. Sol Gel Sci. Technol. 2007, 46, 190–194. [Google Scholar] [CrossRef]
- Korošec, R.C.; Bukovec, P. The role of thermal analysis in optimization of the electrochromic effect of nickel oxide thin films, prepared by the sol–gel method: Part II. Thermochim. Acta 2004, 410, 65–71. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Manikandan, E.; Nuru, Z.Y.; Maaza, M. Investigation on the structural properties of CeO2 nano-fibers via CTAB surfactant. Mater. Lett. 2015, 160, 61–63. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Akhtar, K.; Abdullah, M.M. Role of ZnO-CeO2 nanostructures as a photo-catalyst and chemi-sensor. J. Mater. Sci. Technol. 2011, 27, 594–600. [Google Scholar] [CrossRef]
- Tian, J.; Shao, Q.; Dong, X.; Zheng, J.; Pan, D.; Zhang, X.; Guo, Z. Bio-template synthesized NiO/C hollow mi-crospheres with enhanced Li-ion battery electrochemical performance. Electrochim. Acta 2018, 261, 236–245. [Google Scholar] [CrossRef]
- Wu, W.; Qi, W.; Zhao, Y.; Tang, X.; Qiu, Y.; Su, D.; Fan, H.; Wang, G. Hollow CeO2 spheres conformally coated with graphitic carbon for high-performance supercapacitor electrodes. Appl. Surf. Sci. 2019, 463, 244–252. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.-C. Surface Characterization of CeO2/SiO2and V2O5/CeO2/SiO2Catalysts by Raman, XPS, and Other Techniques. J. Phys. Chem. B 2002, 106, 10964–10972. [Google Scholar] [CrossRef]
- Oskueyan, G.; Lakouraj, M.M.; Mahyari, M. Nitrogen and sulfur Co-Doped graphene quantum dots decorated CeO2 nanoparticles/polyaniline: As high efficient hybrid supercapacitor electrode materials. Electrochim. Acta 2019, 299, 125–131. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Marzolo, F.; Rubino, A.; Zanoni, R.; Pagnanelli, F. Optimizing the structure of Ni–Ni (OH) 2/NiO core-shell nanowire electrodes for application in pseudocapacitors: The influence of metallic core, Ni (OH) 2/NiO ratio and nanowire length. J. Alloys Compd. 2020, 157718. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Zanoni, R.; Pagnanelli, F. Full recycling of spent lithium ion batteries with production of core-shell nanowires//exfoliated graphite asymmetric supercapacitor. J. Energy Chem. 2020, 58C, 336–344. [Google Scholar] [CrossRef]
- Maheswari, N.; Muralidharan, G. Supercapacitor behavior of cerium oxide nanoparticles in neutral aqueous elec-trolytes. Energy Fuels 2015, 29, 8246–8253. [Google Scholar] [CrossRef]
- Kumar, R.; Agrawal, A.; Nagarale, R.K.; Sharma, A. High Performance Supercapacitors from Novel Metal-Doped Ceria-Decorated Aminated Graphene. J. Phys. Chem. C 2016, 120, 3107–3116. [Google Scholar] [CrossRef]
- Araújo, A.J.M.; Silva, V.D.; Sousa, A.R.; Grilo, J.P.; Simões, T.A.; Macedo, D.A.; Nascimento, R.M.; Paskocimas, C.A. Battery-like behavior of Ni-ceria based systems: Synthesis, surface defects and electrochemical assessment. Ceram. Int. 2019, 45, 7157–7165. [Google Scholar] [CrossRef]
- Godlaveeti, S.K.; Maseed, H.; Reddy, S.A.; Nagireddy, R.R. Electrochemical performance of ternary RGO/β-Ni (OH) 2/CeO2 composite in addition with different metal oxides for supercapacitor application. Adv. Nat. Sci. Nosci. Nanotechnol. 2020, 11, 025021. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H. Study on the construction of YMnO3/CeO2 composite photocatalyst heterostructure and photo-catalytic degradation of methyl red. Optik 2020, 201, 163524. [Google Scholar] [CrossRef]
- Phokha, S.; Hunpratub, S.; Usher, B.; Pimsawat, A.; Chanlek, N.; Maensiri, S. Effects of CeO2 nanoparticles on electrochemical properties of carbon/CeO2 composites. Appl. Surf. Sci. 2018, 446, 36–46. [Google Scholar] [CrossRef]
- Phokha, S.; Hunpratub, S.; Chanlek, N.; Sonsupap, S.; Maensiri, S. Synthesis, characterization and electrochemical performance of carbon/Ni-doped CeO2 composites. J. Alloys Compd. 2018, 750, 788–797. [Google Scholar] [CrossRef]


| Area (VA) | Scan Rate (V/s) | Potential Window (V) | Mass (g) | Specific Capacitance (F/g) |
|---|---|---|---|---|
| 8.24 × 10−6 | 0.005 | 0.5 | 0.001 | 1.6478 |
| 1.19 × 10−5 | 0.01 | 0.5 | 0.001 | 1.1893 |
| 3.79 × 10−5 | 0.05 | 0.5 | 0.001 | 0.7571 |
| 6.32 × 10−5 | 0.1 | 0.5 | 0.001 | 0.6322 |
| Current Density (A/g) | Discharge Time (s) | Potential Drop (V) | Specific Capacitance (mF/g) | Energy Density (mF/g*V2) | Power Density (mF/s.g*V2) |
|---|---|---|---|---|---|
| 1 × 10−4 | 42.09 | 0.5 | 8.418 | 1.052 | 0.025 |
| 2 × 10−4 | 16.44 | 0.5 | 6.576 | 0.822 | 0.05 |
| 3 × 10−4 | 12.83 | 0.5 | 7.698 | 0.962 | 0.075 |
| 4 × 10−4 | 8.87 | 0.5 | 7.096 | 0.887 | 0.1 |
| 6 × 10−4 | 5.51 | 0.5 | 6.612 | 0.826 | 0.15 |
| 8 × 10−4 | 3.81 | 0.5 | 6.096 | 0.726 | 0.20 |
| 1 × 10−3 | 2.91 | 0.5 | 2.962 | 0.229 | 0.0786 |
| Composition | Synthesis Method | Reported Year | Charge Transferred Resistance (Ω) | Reference |
|---|---|---|---|---|
| RGO/β-Ni(OH)2/CeO2 | Hydrothermal | 2020 | 1.99 | [38] |
| YMnO3/CeO2 | sonochemistry | 2020 | 320 | [39] |
| carbon/CeO2 | polymer pyrolysis method | 2018 | 1.2 | [40] |
| CeO2/NiO | Hydrothermal | 2018 | 1.9 | [41] |
| CeO2/NiO | Hydrothermal | 0.4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Ali Alghamdi, A.; AL-Abdulkarim, H.A.; Mustafa, G.M.; Baghdadi, N.; Alharthi, F.A. Structural, Morphological, and Electrochemical Performance of CeO2/NiO Nanocomposite for Supercapacitor Applications. Appl. Sci. 2021, 11, 411. https://doi.org/10.3390/app11010411
Ahmad N, Ali Alghamdi A, AL-Abdulkarim HA, Mustafa GM, Baghdadi N, Alharthi FA. Structural, Morphological, and Electrochemical Performance of CeO2/NiO Nanocomposite for Supercapacitor Applications. Applied Sciences. 2021; 11(1):411. https://doi.org/10.3390/app11010411
Chicago/Turabian StyleAhmad, Naushad, Abdulaziz Ali Alghamdi, Hessah A. AL-Abdulkarim, Ghulam M. Mustafa, Neazar Baghdadi, and Fahad A. Alharthi. 2021. "Structural, Morphological, and Electrochemical Performance of CeO2/NiO Nanocomposite for Supercapacitor Applications" Applied Sciences 11, no. 1: 411. https://doi.org/10.3390/app11010411
APA StyleAhmad, N., Ali Alghamdi, A., AL-Abdulkarim, H. A., Mustafa, G. M., Baghdadi, N., & Alharthi, F. A. (2021). Structural, Morphological, and Electrochemical Performance of CeO2/NiO Nanocomposite for Supercapacitor Applications. Applied Sciences, 11(1), 411. https://doi.org/10.3390/app11010411






