1. Introduction
The GEMPix is an innovative detector developed at CERN a few years ago within a Marie Curie Initial Training Network (ITN) funded by the EU under FP7 between 2012 and 2016. ARDENT (Advanced Radiation Dosimetry European Network Training initiative) [
1] was coordinated by CERN and aimed at developing advanced detector technologies for radiation dosimetry. It involved 13 universities, research organisations and industries and recruited 15 Early Stage Researchers (ESR) on 3-year contracts, most of whom obtained a doctorate at the end of the project. The technologies that were part of the program of work were (1) gaseous detectors such as Gas Electron Multipliers (GEM) and tissue equivalent proportional counters (TEPC), (2) solid state detectors such as Medipix and silicon microdosimeters and (3) track detector techniques such as CR-39.
The main objectives of ARDENT targeted radiation dosimetry, microdosimetry and photon/neutron spectrometry. The envisaged applications were the characterization of radiation fields at particle accelerators used in research, industry and medicine, the characterization of radiation fields on-board aircrafts and in space, the assessment of secondary dose to radiation therapy patients and the measurement of the properties of clinical hadron beams used in particle therapy (hadron therapy).
The seed ideas that brought to the development of the GEMPix lie within a workshop held at CNAO, the National Centre for Oncological Hadrontherapy sited in Pavia, Italy [
2], soon after the start of ARDENT, in October 2012 [
3]. A very fruitful exchange of ideas took place around current and envisaged Quality Assurance (QA) instrumentation for particle therapy. The discussion focussed on:
- (1)
commissioning of the Treatment Planning System (TPS). This includes experimental data on integral depth dose distributions of monoenergetic pencil beams using the PTW Peakfinder (Physikalisch-Technische Werkstätten Dr. Pychlau GmbH, Freiburg, Germany, [
4]), transversal dose profiles in air measured with External Beam Therapy (EBT3) radiochromic films [
5], transversal dose profiles in a water phantom with pin-point Ionisation Chamber (IC) like the PTW 31014 [
6], uniformity tests of the scanned beam using EBT3 films;
- (2)
determination of absorbed dose to water under reference conditions with Farmer-type IC like the PTW 30013 [
7], and calibration of beam monitor chambers used to integrate the dose to patient;
- (3)
determination of procedures and reference values for periodic QA checks. Daily QA checks include spot position accuracy and size checks, and beam energy constancy check, using EBT3 films. Patient-specific pre-treatment QA includes TPS verification plan.
The current commissioning and QA procedures at CNAO are described in [
8]. The concept of GEM detectors [
9,
10], which are mostly used in high-energy physics, was presented, describing the Triple-GEM construction [
11] with the standard pad readout made of 128 channels allowing spatial resolution of typically a few mm, the FPGA-based data acquisition system (DAQ) and a specifically designed High Voltage (HV) power supply called HVGEM to supply the required power via seven HV channels. GEMs allow real time track reconstruction, from single particle to high intensity beams. At the same time, the single particle counting pixel detector Timepix [
12] was introduced by representatives of the Medipix collaboration [
13,
14,
15], explaining its particle tracking capabilities and particle discrimination properties by cluster analysis.
The idea to merge these two technologies and combine their advantages to build a gaseous detector with a highly pixelated readout and achieve superior spatial resolution unfolded rapidly, and yielded the design and construction of the first GEMPix prototypes in March 2013. Only a few years later the detector has proven very successful and has been used for several applications. Its use as a tracking detector operated as a highly granular and compact Time Projection Chamber (TPC) has been shown [
16]: the device can be operated in a hybrid mode with some pixels measuring deposited charge and others measuring the drift time of the charge in order to obtain a complete 3D reconstruction of the track. It has been introduced as a novel method to detect the very weak (5.9 keV) X-ray emission of
55Fe in radioactive waste produced by the operation of particle accelerators and experimental facilities, to determine its specific activity (Bq/g) in metallic waste characterization [
17]. Furthermore, the GEMPix has been used as a soft X-rays diagnostic tool for laser produced plasmas [
18,
19] and for studies on a dark matter detector with a negative ion TPC read by a GEMPix [
20].
The present paper will review the medical applications of the GEMPix. It is mostly a review paper, but the last sections describe on-going work and anticipate the most recent results that will be the subject of two dedicated publications currently under preparation. After illustrating the design and operating principle of the detector (
Section 2),
Section 3 and
Section 4 describe its imaging application in photon and electron radiation therapy (that we call ‘conventional radiation therapy’ in the following) and particle therapy. The results presented in these two sections have been published in [
21] and in [
22,
23,
24], respectively. A proof-of-principle of two GEMPix detectors as TPCs for proton tomography [
25] is discussed in
Section 5.
Section 6 illustrates a very recent version of the detector for microdosimetry called GEMTEQ, whereas
Section 7 discusses the latest development for a larger area version of the GEMPix (LaGEMPix) for use in QA in hadrontherapy. Finally, future developments are outlined in
Section 8.
2. The GEMPix
The GEMPix couples two CERN technologies, the Gas Electron Multiplier [
9,
10] as amplifier for electric charges with four highly pixelated Timepix ASICs (the so-called quad or quadboard) as readout [
12]. The concept for gas pixel detectors is not new [
26]; it was first developed by Bellazzini et al. [
27] as an X-ray polarimeter using a custom ASIC [
28] for X-ray astronomy [
29,
30]. Other examples include the GRIDPIX designed for high-energy physics applications [
31], but sparks and discharges have proven to be persistent problems with these devices. Thanks to a specially designed High Voltage power supply (HVGEM) [
32] and a carefully designed GEM electrode layout, the GEMPix demonstrates good reliability and discharge resistance.
Figure 1 shows the GEMPix. It consists of (1) a drift gap of about 1 cm, (2) a triple-GEM setup for charge amplification and (3) an array of 2 × 2 ‘naked’ Timepix ASICs, i.e., Timepix ASICs without the usual silicon sensor layer. The GEMPix is thoroughly described in [
33,
34]. Its design and operating principles are recalled here, first illustrating those of its two main components, and then their combination in the GEMPix.
The GEM [
9] is a thin (50 µm) kapton foil, copper cladded on each side and chemically perforated by a high density of holes having bi-conical structure with an external (internal) diameter of 70 µm (50 µm) and a pitch of 140 µm, as shown in
Figure 2. By applying a suitable voltage difference (typically 400 ÷ 500 V) between the two metallic sides, an electric field with a field strength as large as 100 kV/cm is produced inside the holes. The holes act as spatially well-confined electron multiplication channels for the electrons released by ionizing radiation in the drift gap. The maximum gain reachable with a single GEM foil detector is of the order of 10
3. In safe operating conditions, effective gas gains (i.e., the product between the electron multiplication and the transparency, which is the overall efficiency of transporting the electrons through the GEM holes [
11]) of up to 10
4–10
5 are reachable by assembling several GEM foils close to each other. The distance between the three GEM foils of the GEMPix is the same as that used by the LHCb muon chambers in order to optimize the time resolution (the first gap is 1 mm) and to reduce the probability of discharge as much as possible (the second gap is 2 mm, which results in the electron clouds being shared by more holes of the third GEM foil). Typically, the electric field applied between two GEMs to transfer the electrons to the next GEM range between 0.5 and 3 kV/cm depending on the gas and the performance required by the detector application.
Figure 3 shows a scheme of the Timepix chip. The movement of the electric charge produced by the last GEM induces a signal on a group of pads of the chip underlying the charge cluster. The Timepix is able to collect and measure the information of this charge in three different modes, always referring to the time window defined a priori. The first mode (‘Medipix mode’) allows measuring the number of hits per pixel in the time window. The second mode is the Time-over-Threshold (‘ToT’): the Timepix is able to measure with a precision on the order of 10 ns the time spent above the threshold by the analog signal induced on the single pixel; this time is proportional to the collected charge and, therefore, if the detector is calibrated, to the energy deposited by the radiation. This mode is typically used for spectroscopy of X-rays, dosimetry, microdosimetry and for dE/dX measurements. The third mode is the Time-of-Arrival (‘ToA’): the Timepix measures the time of arrival of the electron cloud with respect to an external trigger for a maximum of a 2 ms time window. This mode is typically used for 3D reconstruction of a track inside the detector. The readout mode can be chosen for each pixel individually, such that a combined ToT and ToA analysis of a charge cluster becomes possible when using a mixed mode by operating some pixels in ToA mode and the rest of the pixels in ToT mode.
The Timepix ASICs are read out by the FITPix, an FPGA based module shown in
Figure 1 [
35] using the Pixelman software [
36]. This software, developed by the University of Prague, is able to save the time and charge information of each pixel within the preset time interval in matrix form, showing the image obtained online and saving the data to a file. It is also possible to use an online cluster analysis in order to write a file with the list of reconstructed pixel clusters, reporting for each one the main information such as start time of the event, the total number of clusters, and for each of them the total charge, some geometric parameters and the position.
The GEMPix can be assembled with different drift gap sizes according to the type of particle to be detected. In the case of charged particles, a few millimeters are sufficient to produce a dozen electrons distributed along the gap, which are sufficient to generate a detectable signal. There are two possible configurations visible in
Figure 4: one in which the particle enters perpendicularly to the GEM foils (“head-on”) and one in which the particle enters parallel to them (“side-on”). In the second case, it is possible to expand the drift area up to a few centimeters in order to create a small TPC with the possibility to reconstruct the particle track in three dimensions, as for example used in proton tomography (
Section 5). In the case of soft X-rays, a gap of about 1 cm is preferred, both to increase the detection efficiency and to contain all the tracks of the primary electron produced. In addition, metal layers suitably designed for the detection of gamma rays can be inserted in the drift area. Recently, thin layers of B
4C have been used for the detection of thermal neutrons: the lithium ion and alpha particle produced in the
10B(n,α) reaction strongly ionize the gas in the drift zone, allowing a good discrimination from the gamma signal.
In some applications with high intensity beams or highly ionizing particles, it is possible to reduce the detector configuration to two GEM foils with the advantage of a lower lateral diffusion of the electron cloud and therefore of a better spatial resolution (measurements with only two GEMs turned on are described in
Section 3). A detailed study on the gas mixture has been performed in the past [
37] showing that a time resolution of better than 5 ns is achieved with CF
4 and iso-C
4H
10 based gas mixtures, considerably improving the results obtained with the standard Ar/CO
2 (70/30) mixture. Regarding the discharge probability, the use of a small fraction of iso-C
4H
10 or a large amount of CF
4 results in a very stable detector operation. Therefore, an Ar/CO
2/CF
4 (45/15/40) gas mixture is often used with the GEMPix detector.
The HVGEM [
32], designed at the Frascati National Laboratories (LNF) of the National Institute of Nuclear Physics (INFN) in Italy, is built with seven floating power supplies with a maximum of 1200 V each, controlled via CANbus and a LabVIEW based software for voltage settings and current monitoring. In this way, each GEM foil has its own power supply allowing a safe operation of the detector, avoiding dangerous discharges versus the front-end electronics. A second shrewdness is the particular electrode path for each GEM foil trying to stave off the High Voltage from the wire bonding of the ASIC readout.
Like all gas detectors, the gain of the GEMPix also depends on the temperature, pressure and humidity of the gas. Therefore, a sensor by yoctopuce [
38] reading the thermodynamic variables has been installed inside the detector with the aim of compensating the gain variation by changing the voltages applied to the GEM foils, thus keeping the detector response stable within less than ±5% as shown in
Figure 5 (changes of more than 30% were observed without any correction).
The GEMPix is able to measure position and charge of the electron clusters produced by the primary ionization of the particles incident on the detector with great precision, thanks to the four Timepix ASICs.
3. Conventional Radiation Therapy
The daily QA and treatment plan verification measurements in radiation therapy facilities are typically performed with gafchromic films or a matrix of small ion chambers [
39]. The first method shows very good spatial resolution but needs relatively long time for data analysis, while the second has an online data acquisition but comes with dead space (i.e., non-active detection area) in the treatment area. The GEMPix is a detector that could be used for this application offering advantages on both spatial resolution without dead space and online monitoring capabilities.
Several measurements have been performed with GEM and GEMPix detectors at the Tor Vergata Radiotherapy Centre in Italy (
Figure 6) using two electric field configurations [
21]: the GEMPix in a standard configuration with all electric fields on, and the triple GEM operated with zero electric drift field and the first GEM foil as a gamma ray converter. In the latter case, the spatial resolution is expected to be better, as the gap between the first and the second GEM now serving as the drift gap is only 1 mm and, therefore, the charge spread is reduced. The conversion efficiency was certainly very low but still reasonably good to obtain a 2D image in 10 times less time as compared with gafchromic films. Moreover, with the GEMPix used in ToT mode, the measured charge is correlated more precisely to the dose released in the gas.
To compare the results obtained with the GEMPix and with gafchromic films quantitatively, the gamma index [
40,
41] was used, one of the most common quantities for comparison of 2D dose distributions. The gamma index takes into account the dose difference and the distance difference of the two dose distributions to calculate a dimensionless metric for each point. It is commonly accepted that the first must be maximum 3% and the second maximum 3 mm. When the gamma index is less than one, both criteria are satisfied and this condition must be valid for at least 90% of the reference dose points to which a measured dose is compared.
In case of the first, standard GEMPix configuration with three active GEMs, the gamma index shows the best results with the lower energy spectrum (6 MV, gamma index <1 in 96.6% of the points). For higher energy spectra, the percentage of the gamma index decreases (69 and 40% for 10 and 18 MV spectra, respectively). An analogous result is obtained for an Intensity-Modulated Radiation Therapy (IMRT) field. High energy fields are affected by large halos which have no counterpart on the gafchromic films.
A significant optimization of the results has been obtained working in a double-GEM configuration with zero voltage applied to the first GEM foil, i.e., with zero drift field; in this way only two GEMs are used as charge amplifiers. The gamma index percentage improves to better than 90% as shown in
Figure 7. Some tests with IMRT fields have shown an excellent agreement, especially when a threshold cut is applied.
The GEMPix presents some advantages with respect to the traditional methods used in radiation therapy:
It is more sensitive and the gain has been even reduced working with only two GEM foils. The lower limit is represented by the single dose pulse, a limit that gafchromic films cannot reach [
5]. The upper limit is the storage bit limit of the read-out register.
It has an optimal linearity with dose rate and this result is confirmed by the currents measured on the GEM electrodes.
Compared to gafchromic films, it does not need a scanning process to read the measured doses. This also means no fading and no UV sensitivity problems.
The spatial resolution obtained with GEM detectors is at least an order of magnitude better than with ion chamber (IC) matrix arrays. With this spatial resolution, no interpolation software is needed to calculate the intermediate dose values as it is the case for IC arrays.
The pixel pitch of the GEMPix is even better (55 μm) than the one of EBT gafchromic films (340 μm) [
5]. The gamma index showed excellent agreement between the GEMPix and gafchromic films.
The GEMPix is able to perform fast real-time IMRT treatment plan verifications. It has been demonstrated that it is also able to measure single pulses. Then, IMRT fields both in ‘step and shoot’ and in continuous mode can be reconstructed in time with a very high resolution [
21]. No other devices can reach these performances.
The drawback is represented by the active area which is limited to a few cm
2 essentially due to the wire bonding of the chip, while larger fields are used in radiation therapy (for example, 16 × 21 cm
2 is the maximum field size for the Elekta Synergy Linac used in Tor Vergata). A large area GEMPix is under development, as discussed in
Section 7 and
Section 8.
4. Hadron Therapy
Hadron therapy is an advanced radiation modality for cancer treatment. The inherent advantage of hadron therapy is its inverted depth dose curve—the so-called Bragg curve—that allows for highly conformal treatment plans with large dose gradients. The dose is well confined in depth with a moderate lateral spread [
42]. Therefore, detectors for beam dosimetry and quality assurance should offer a spatial resolution of the order of 1 mm or better. For patient-specific treatment plan verification, arrays of ionization chambers in a water phantom are often used [
43]. However, the spatial resolution is limited to the size of each ionization chamber, which is currently around 5 mm.
The GEMPix potentially offers a much better spatial resolution due to its highly pixelated readout with a pixel pitch of 55 µm. Therefore, it provides new information such as 2D images and 3D data representation. As a first step, a triple-GEM detector coupled to an earlier, coarser readout was irradiated with protons and carbon ions in air [
44]. Lateral beam profiles of the GEMPix for one carbon ion beam energy were found in agreement with those obtained by radiochromic EBT3 films. Then, the GEMPix was placed inside a watertight box and mounted on the 3D positioning system of a water phantom used for QA at CNAO in order to measure the 3D dose distribution. Bragg curves were calculated by normalizing the integrated GEMPix response at certain depth positions to the respective delivered dose measured by the Dose Delivery System (DDS) of CNAO [
45]. Promising results obtained with this setup [
22] led to the development of an integrated system consisting of an IBA Scanditronix Wellhöfer Blue Phantom type 2001 water phantom, a PTW model 34080 reference ionization chamber, the GEMPix, a trigger system and other auxiliary equipment such as the high-voltage supply, and the control and data acquisition software (
Figure 8). The system can be set up relatively fast by keeping all equipment on trolleys. It is a stand-alone system as the normalization to the delivered dose is performed with the reference ionization chamber [
23,
24]. Measurements were performed with carbon ions (C
6+) at one of the fixed horizontal beam lines at CNAO, where a synchrotron delivers scanning proton and carbon ion beams to three treatment rooms. The smallest intensity characterized for clinical applications of 2 × 10
6 ions per spill and three beam energies (280, 332 and 380 MeV/u resulting in ranges in water of 150, 200 and 250 mm, respectively) were used. 2D images in the plane perpendicular to the beam axis were acquired at different depths in water. From the 2D images, lateral beam profiles were obtained and compared to radiochromic films showing very good agreement. The Bragg curve was calculated from the 2D images by summing over all pixel values in a single image, normalizing this value to the corresponding reference ionization chamber measurement, then averaging this normalized sum value over all measurements at the same depth and finally plotting this number versus depth. Bragg curves obtained with the integrated system were compared to a dedicated FLUKA Monte Carlo simulation [
46,
47] and found to match within ±15% (
Figure 9).
Figure 10 shows a 3D data representation of the measured dose. The average value per pixel per position is calculated and a linear interpolation between positions is used. The figure shows the lateral beam spreading, the Bragg peak and the fragmentation tail of the carbon ion. This information cannot be acquired with commercially available QA detectors.
5. Proton Tomography
With the increasing number of proton therapy centres there is a renewed interest in proton tomography [
48,
49,
50]. Preliminary tests with two GEMPix operated as TPCs were performed to measure the position, direction and energy of protons traversing an object in order to reconstruct an image of it. The setup consists of two GEMPix-based TPCs with a Timepix3 (instead of the usual Timepix) quadboard as tracker and a BaF
2 crystal for measurement of the residual proton energy. The results presented in this section are published in [
25].
Figure 11 shows the setup. A phantom was placed between the two tracking devices to try and measure the performance and spatial resolution of the system. The phantom and the reconstructed image are shown in
Figure 12. Although the sensitive volume of the TPC is 5 × 5 × 5 cm
3 (yielding sufficiently long tracks for measuring the incident angle of the particle) and the improved temporal resolution of the Timepix3 is included (drift coordinate precision), the angular resolution is still not optimal for the phantom image reconstruction. These uncertainties on the track reconstruction are dominated by the clustering effect of the triple-GEM structure and the inhomogeneity of the electric field inside the TPC.
In order to improve the performance of the system, an algorithm finding the location of the primary ionization would be needed. The track fit using only the location of the primary ionization will be more realistic than using all the hits of the clusters. Another possible improvement could be an optimized triple-GEM configuration and the use of a gas mixture generating less lateral diffusion. The final angular resolution obtained with the current set-up is 30.7 mrad in the xy-plane and 23.4 mrad in the zy-plane. Once the above-mentioned issues are solved and an angular resolution below 1 mrad is reached, a step towards proton-CT can be investigated by irradiating the phantom from different angles. A 3-dimensional energy density map could then be reconstructed to improve future proton therapy treatment plans.
6. Microdosimetry
The most recent application of the GEMPix to medical physics lies in the field of microdosimetry. Microdosimetry is the ‘systematic study of the spatial and temporal distributions of absorbed energy in irradiated matter’ [
51]. The golden standard for detectors in microdosimetry is the Tissue Equivalent Proportional Counter (TEPC). A TEPC is a device consisting of an active volume filled with tissue equivalent (TE) gas, with TE walls and a thin anode wire. The gas pressure is only a few percent of the atmospheric pressure and therefore the ionization of a particle in the active volume is the same as in a small piece of tissue. Both the lineal energy and the absorbed dose to tissue can be measured [
52]. It is a well-established technology that can measure the microdosimetric distributions accurately and reliably, but it has important limitations that the GEMPix could in principle overcome. For example, the equivalent mean path length in a TEPC can be selected by adjusting the operational pressure, but it is typically limited to a few micrometres, with a lower limit of operation of 0.3 µm in case of special design [
53], which is not enough to obtain details on the particle track structure. Detectors with the best spatial resolutions tend to be complex and bulky [
54]. Furthermore, conventional TEPCs feature only a single readout channel in a detection volume of fixed dimensions.
While GEMs had been used before in microdosimetry [
55], the combination with a highly pixelated readout is unique to the GEMPix. In case of the GEMPix detector, it is possible to achieve a resolution down to the scale of tens of nanometres because the inherently good spatial resolution (pixel pitch of 55 µm) is scaled down using a gas instead of a solid material by a factor of approximately 1000. The particle track imaging capability of the GEMPix is completely new to microdosimetry.
The version of GEMPix for use as microdosimeter is called GEMTEQ (‘GEMPix detector for microdosimetry with tissue equivalent gas’). For this application, the GEMTEQ is operated with a so-called propane-based TE gas (C
3H
8:CO
2:N
2, 55:39.6:5.4). It was calibrated using an
55Fe source and its performance was tested in various radiation fields (photons from an X-ray generator and from radioactive sources, neutrons from a neutron generator and a mixed photon/neutron field from an AmBe radioactive source). One of the GEMTEQ prototypes is equipped with a tissue equivalent cathode replacing the Mylar foil by an A150 conductive plastic.
Figure 13 shows the GEMTEQ detector setup for measurements in a neutron field.
Standard dose spectra as with a TEPC were obtained. These results were achieved by grouping together several pixels in a ‘superpixel’: the superpixels can be defined offline to adjust the equivalent mean path length. The energy deposition of all pixels in a superpixel is summed and these values are used to populate a histogram similar to the pulse height histograms obtained in standard microdosimetry.
Figure 14 shows the results of measurements with an AmBe source (solid black line). In order to show one of the potential advantages of the pixelated readout of the GEMPix, an analysis to discriminate photons and neutrons in the mixed radiation field provided by the AmBe source was set up: a particle track in the GEMPix often stretches over several superpixels. While for the standard dose distributions, the energy deposition in each superpixel is used to populate the histogram (as shown for the complete data set in
Figure 14), the total energy deposition of the entire track is used to discriminate neutrons and photons. The value of the discrimination parameter—the total energy deposition of a particle in the GEMPix—was chosen to be the maximum energy deposition observed in the measurements with an Am-241 source. Any particle track in the AmBe data set with an energy below this limit was assigned as ‘photon-like’, the remaining tracks were assigned as ‘neutron-like’. The dose distributions of the ‘photon-like’ component of the AmBe data set and the pure Am-241 data set match well. In conclusion, this analysis makes use of the fact that the GEMPix provides multi-channel results (i.e., 2D images of particle tracks), while this is not possible in a TEPC as it features only a single channel.
Two-dimensional particle track images were obtained from the measurements using the same data set as for the dose spectra (
Figure 15). This is impossible with conventional TEPCs and opens the door for many new applications in microdosimetry. These include: particle tracks can be studied in detail (track structure microdosimetry), particle identification becomes possible (helping to disentangle contributions from different types of radiation in a mixed field) and effects of the size of sensitive volumes can be studied offline on a single data set. An outline of the results recently obtained can be found in [
56], and the full results are given in [
57].
7. LaGEMPix, a Large Area GEMPix
The superior imaging and particle discrimination capabilities of the GEMPix have been shown in the previous sections, but the current sensitive area of the detector (2.8 × 2.8 cm) is too small for a practical application as QA tool in hadron therapy. This section focuses on the application in hadron therapy, but a larger area detector is also needed in other fields such as conventional radiation therapy with photon beams. A large area GEMPix (LaGEMPix) with a 20 × 20 cm2 size (the typical maximum clinical field size with scanned hadron therapy beams) cannot be easily (and economically) achieved by simply scaling up the current design by tiling more Timepix ASICs. First, the current Timepix chip has wire bonds located on one side, so the quad configuration (2 × 2 chips) used in the GEMPix cannot be enlarged to an arbitrary n x n chip configuration. For this, one would need to use the latest Timepix4 version that is about four times larger than the Timepix and in which the connection for data transfer is from below the chip by Through-silicon Via (vertical interconnect access). However, such a solution would be very expensive just for the cost of the Timepix4 ASICs. In addition, the 55 µm pixel size would require complex electronics to handle the large number of signals and each acquisition would generate a huge amount of data, which are actually not needed as spatial resolution of the order of 1 mm is sufficient for application in QA.
We have considered a few alternative readouts for a large area detector. In the present section, we describe the one currently being explored; alternative solutions are briefly illustrated in the next section. The on-going development uses a novel readout based on the detection of the scintillation photons generated in the GEM holes. A first LaGEMPix prototype with a six time larger active area than the GEMPix (6 × 8 cm
2) has been developed in a collaboration between CERN and the Holst Centre/TNO (Eindhoven, The Netherlands) within an EU ATTRACT funded project [
58]. The LaGEMPix combines a triple-GEM detector and an optical readout based on three main building blocks: a Thin Film Transistor (TFT) backplane, a light sensitive OPD (optical photo detectors) frontplane and a transparent thin-film encapsulation, serving as a protection against ambient conditions [
59]. The triple-GEM detector includes a 20 μm thick Mylar window used as the cathode at 3.5 mm from the first GEM. On the bottom of the third GEM, a 1.1 mm thick ITO (Indium Tin Oxide) coated glass anode is placed at 1.9 mm distance to collect the electrons produced during the amplification process while allowing the optical photons to pass through [
60,
61].
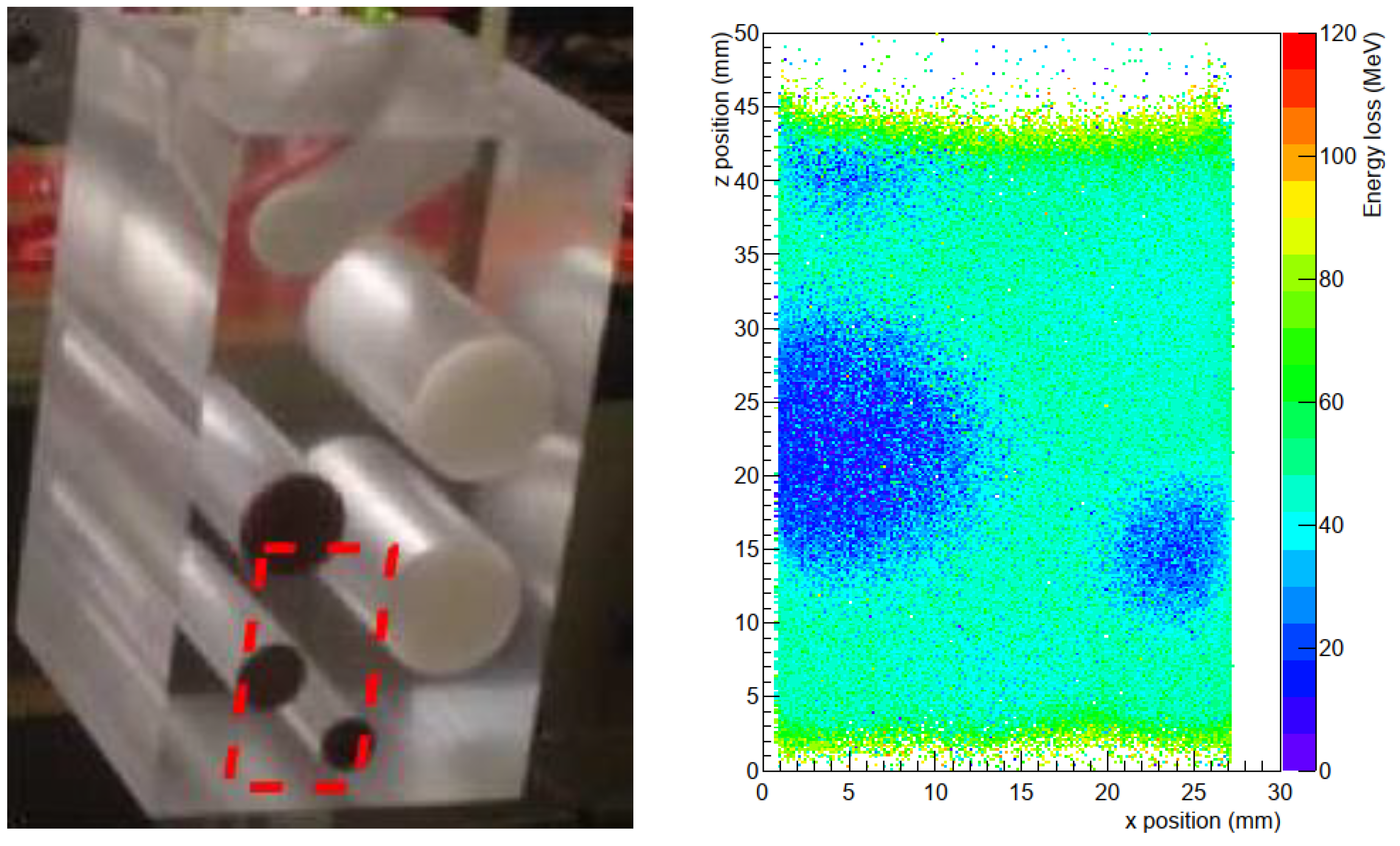
A LaGEMPix prototype (
Figure 16) has been built. A first account of the results obtained are given in [
62], the full results can be found in [
63]. A number of tests were performed to characterize the detector, placed inside a custom-made black Polymethylmethacrylate (PMMA) box to shield the ambient light and provide a well aligned set-up. A gain scan was performed while exposing the device to photons from a 3 TBq
137Cs source, varying the sum of the GEM voltages from 0 to 1030 V, to define the optimal operating conditions. The spatial resolution was determined with 40 kV X-rays from an X-Ray irradiator employing a 3 mm thick copper plate placed on the inner wall of the box at 7 mm from the Mylar window, with a set of holes of different spacing (from 3 to 20 mm) drilled in it. An image obtained with this copper plate is shown in
Figure 17. The FWHM of a single hole of 5 mm diameter is 6.7 ± 0.1 mm. Two holes at a distance of 3 mm (edge to edge) can be resolved in the sense that two peaks with a dip in the intensity between the peaks are visible. The current results are promising but also show that the required sub-millimeter spatial resolution is not yet achieved.
8. On-Going and Future Developments
The developments currently on-going focus on improvements of the GEMTEQ and LaGEMPix versions of the GEMPix. The long-term goal would be to merge the two detectors and integrate them into the motorized water phantom described in
Section 4, to achieve an all-in quality assurance tool for treatment planning and dose delivery in particle therapy, driven by a detailed knowledge of the radiobiological effectiveness (RBE) of the radiation.
8.1. GEMTEQ
The GEMTEQ discussed in
Section 6 is a version of the GEMPix specifically adapted for microdosimetry. The pixelated readout of the GEMTEQ is a key feature, which unlike the TEPC allows visualizing and analysing the full particle track. TEPCs are sealed detectors operating at low pressure so that the mean path length in the active gas volume is equivalent to typically a few µm in tissue, simulating the cell size. The current version of GEMTEQ works with flushing propane-based TE gas at atmospheric pressure (the way GEMs are usually operated). We are currently designing a sealed version of the GEMTEQ (
Figure 18), consisting of a vacuum chamber provided with a thin carbon window housing the detector, connected to a pumping station to adjust the operating pressure. The aim is to be able to run the GEMTEQ in sealed mode, to make it more portable, at pressure below atmospheric to achieve sub-micrometric spatial resolution and head towards microdosimetry at the sub-micron level.
The spatial information obtained from the pixelated readout will be used to perform data analyses beyond standard microdosimetry, i.e., particle track analysis, and achieve track-structure microdosimetry. Upgrading the readout to the latest generation of Timepix (Timepix3 or Timepix4) will allow for measurements in a data-driven acquisition mode with simultaneous acquisition of charge and time-of-arrival information, for operation as a TPC. In this way, 3D particle tracks will be reconstructed and possibly track lengths and the change of the energy deposition along the track could be measured for each particle.
8.2. LaGEMPix
The final goal of the LaGEMPix development is to achieve a large size detector for monitoring the typical clinical field size used in hadron therapy, up to 20 × 20 cm
2, allowing for a precise evaluation of the dose distribution with scanned ion beams. Two possible modifications of the LaGEMPix described in
Section 7 are under consideration and will be explored in the coming months to improve the current spatial resolution. The first implies slightly modifying the present design, by decreasing the distance between the last GEM and the readout plane to reduce the dispersion of the light before reaching the readout. The second focuses on increasing the light detection efficiency by replacing the current OPD active layer by a different one offering a better match between the emitted light and the OPD’s quantum efficiency.
The other options involve changing the readout technology. Excluding the approach of scaling up the current design by tiling more Timepix ASICs (either Timepix3 or Timepix4) for the reasons mentioned in the previous section, there are two alternatives that may be considered. The first alternative to the current readout system is to eliminate the OPD layer leaving a TFT-only electronic readout. With this approach, the readout would directly measure the secondary electrons produced in the electron avalanche. This TFT-only solution could yield a more compact and more efficient device with a higher signal-to-noise ratio, providing a spatial resolution potentially better than 1 mm, as the electrons are guided by the electric field and their diffusion is much smaller than unfocused light. The spatial resolution of the current LaGEMPix has been compared with that of the standard GEMPix with 40 kV X-rays from an X-Ray irradiator, using the “edge response” method [
64]. The resolution obtained with the GEMPix is about a factor of 2 better than that of the LaGEMPix, indicating that a better performance can be achieved with the charge readout option.
The other option still exploits the light emitted in the GEM holes, but detecting the optical photons using a CCD/CMOS camera or the TpxCam [
65,
66]. The TpxCam is a fast optical camera based on a silicon pixel sensor combined with the Timepix chip, providing nanosecond scale time resolution and high quantum efficiency for photons with wavelength between 400 and 1050 nm. In this version, the LaGEMPix would not be as compact as the version using a TFT readout, as it will have to include an optical system consisting of mirror and lenses to focus the light. On the other hand, it would offer the possibility to keep the electronics off the beam.
Another possible future development is to employ air instead of an argon-based gas mixture. This could be an optimal solution to simplify the present device. High photon fluences, like those used in radiation therapy, produce significant ionization in air and the GEMPix is highly sensitive to detect this ionization.
Finally, it is worth mentioning that QA checks are usually performed before patient treatment. Some preliminary attempts have been made to build devices for on-line transit dosimetry: from a measurement of the dose transmitted through the patient during a treatment session, a software would reconstruct the dose released inside the patient. Until now, conventional devices, like matrix array and EPID, have not guaranteed satisfactory and universally accepted results. This is a field where the GEMPix may also bring a contribution.