Estrogen Signaling in Bone
Abstract
1. Introduction
2. Estrogen in Bone
2.1. Estrogen in Bone
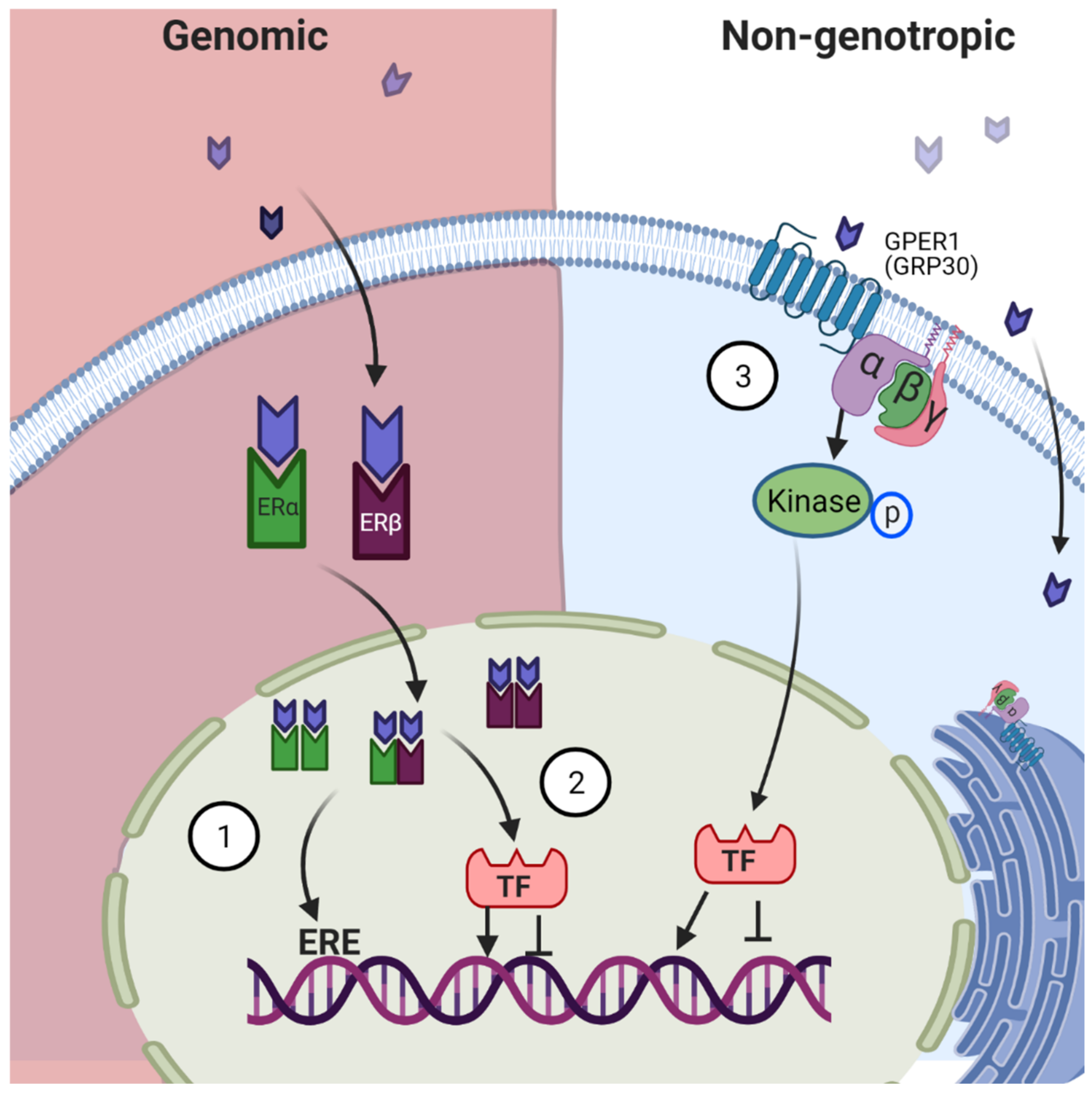
2.2. Mechanism of Action
3. Estrogen Receptors
3.1. Identification
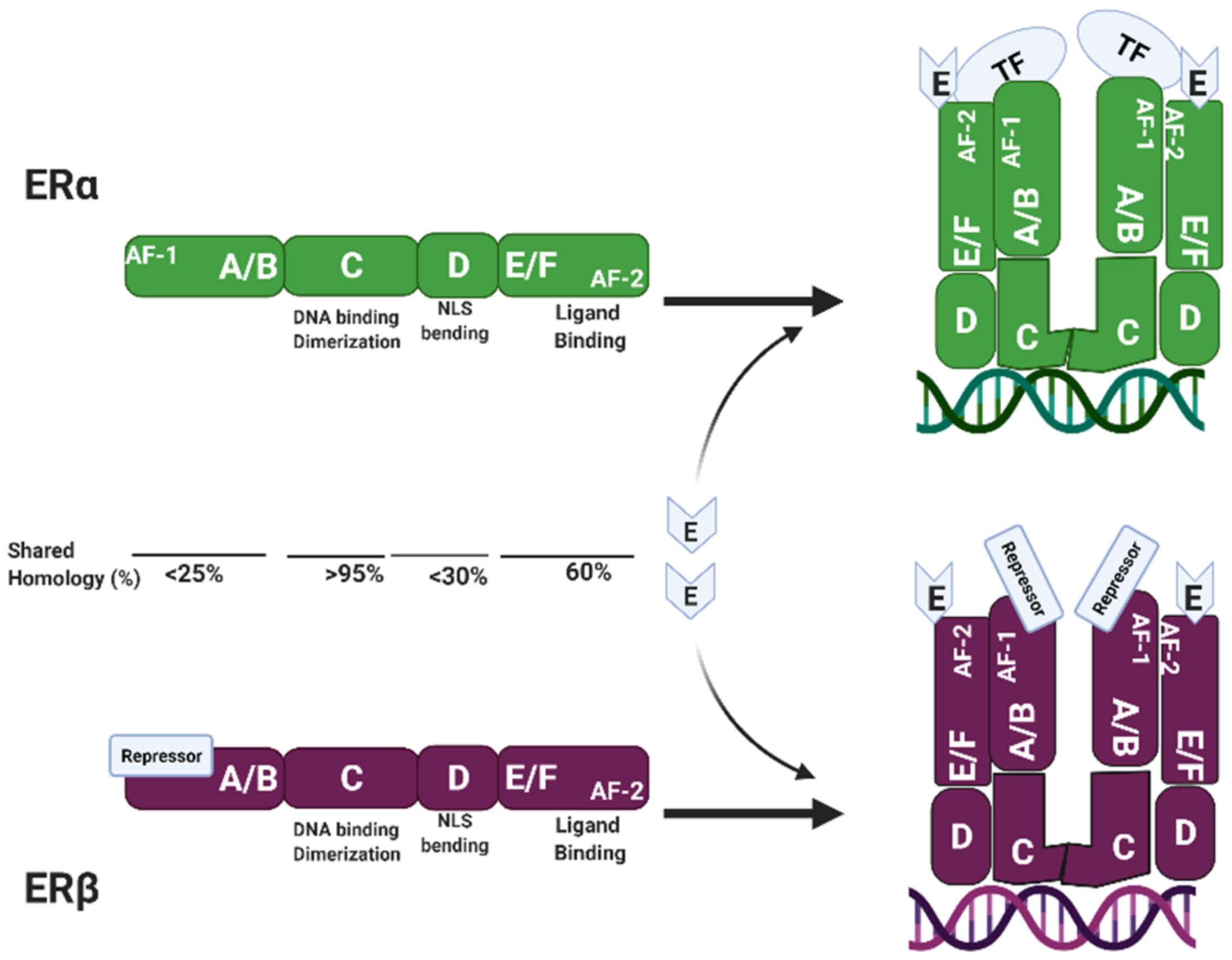
3.2. Estrogen Receptor Structure
3.3. Differential Expression of Estrogen Receptors in Bone
3.4. Role of ERα in Bone
3.5. Role of ER Beta in Bone
3.6. Role of GPER1/GP30 in Bone
3.7. Estrogen Receptor Mutation in Humans
3.8. Role of Estrogen and Its Receptors in Bone Regeneration
4. Discussion and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, M.P.; Potter, B.V. The structural biology of oestrogen metabolism. J. Steroid Biochem. Mol. Biol. 2013, 137, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [CrossRef]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.E.; Abdelgadir, S.E.; Resko, J.A. Regulation of aromatase gene expression in the adult rat brain. Brain Res. Bull. 1997, 44, 351–357. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Rossouw, J.E. Effect of postmenopausal hormone therapy on cardiovascular risk. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 2002, 20, S62–S65. [Google Scholar]
- Ottesen, B.; Pedersen, A.T. Physiological effects of ovarian hormones: Clinical aspects and compliance. Eur. Heart J. 1996, 17 (Suppl. D), 20–26. [Google Scholar] [CrossRef]
- Gillies, G.E.; McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 2010, 62, 155–198. [Google Scholar] [CrossRef] [PubMed]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar] [CrossRef]
- Lobo, R.A. Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Zachmann, M.; Ferrandez, A.; Můrset, G.; Prader, A. Estrogen treatment of excessively tall girls. Helv. Paediatr. Acta 1975, 30, 11–30. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Morishima, A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Carani, C.; Qin, K.; Simoni, M.; Faustini-Fustini, M.; Serpente, S.; Boyd, J.; Korach, K.S.; Simpson, E.R. Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 1997, 337, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.; Yang, M.X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol. 1995, 15, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Bellido, T.; Plotkin, L.I.; O’Brien, C.A.; Bodenner, D.L.; Han, L.; Han, K.; DiGregorio, G.B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 2001, 104, 719–730. [Google Scholar] [CrossRef]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gururaj, A.E.; Vadlamudi, R.K.; Rayala, S.K. The clinical relevance of steroid hormone receptor corepressors. Clin. Cancer Res. 2005, 11, 2822–2831. [Google Scholar] [CrossRef]
- Garcia-Bassets, I.; Kwon, Y.S.; Telese, F.; Prefontaine, G.G.; Hutt, K.R.; Cheng, C.S.; Ju, B.G.; Ohgi, K.A.; Wang, J.; Escoubet-Lozach, L.; et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell 2007, 128, 505–518. [Google Scholar] [CrossRef]
- Caizzi, L.; Ferrero, G.; Cutrupi, S.; Cordero, F.; Ballaré, C.; Miano, V.; Reineri, S.; Ricci, L.; Friard, O.; Testori, A.; et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4892–4897. [Google Scholar] [CrossRef]
- Jensen, E.V.; Jacobson, H.I.; Walf, A.A.; Frye, C.A. Estrogen action: A historic perspective on the implications of considering alternative approaches. Physiol. Behav. 2010, 99, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Toft, D.; Gorski, J. A receptor molecule for estrogens: Isolation from the rat uterus and preliminary characterization. Proc. Natl. Acad. Sci. USA 1966, 55, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Walter, P.; Greene, G.; Krust, A.; Goffin, C.; Jensen, E.; Scrace, G.; Waterfield, M.; Chambon, P. Cloning of the human oestrogen receptor cDNA. J. Steroid Biochem. 1986, 24, 77–83. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef]
- Hall, J.M.; McDonnell, D.P. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 1999, 140, 5566–5578. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Börjesson, A.E.; Lagerquist, M.K.; Windahl, S.H.; Ohlsson, C. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell. Mol. Life Sci. 2013, 70, 4023–4037. [Google Scholar] [CrossRef]
- Kusec, V.; Virdi, A.S.; Prince, R.; Triffitt, J.T. Localization of estrogen receptor-alpha in human and rabbit skeletal tissues. J. Clin. Endocrinol. Metab. 1998, 83, 2421–2428. [Google Scholar] [CrossRef]
- Kennedy, J.; Baris, C.; Hoyland, J.A.; Selby, P.L.; Freemont, A.J.; Braidman, I.P. Immunofluorescent localization of estrogen receptor-alpha in growth plates of rabbits, but not in rats, at sexual maturity. Bone 1999, 24, 9–16. [Google Scholar] [CrossRef]
- Gruber, R.; Czerwenka, K.; Wolf, F.; Ho, G.M.; Willheim, M.; Peterlik, M. Expression of the vitamin D receptor, of estrogen and thyroid hormone receptor alpha- and beta-isoforms, and of the androgen receptor in cultures of native mouse bone marrow and of stromal/osteoblastic cells. Bone 1999, 24, 465–473. [Google Scholar] [CrossRef]
- Oreffo, R.O.; Kusec, V.; Virdi, A.S.; Flanagan, A.M.; Grano, M.; Zambonin-Zallone, A.; Triffitt, J.T. Expression of estrogen receptor-alpha in cells of the osteoclastic lineage. Histochem. Cell Biol. 1999, 111, 125–133. [Google Scholar] [CrossRef]
- Arts, J.; Kuiper, G.G.; Janssen, J.M.; Gustafsson, J.A.; Löwik, C.W.; Pols, H.A.; van Leeuwen, J.P. Differential expression of estrogen receptors alpha and beta mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology 1997, 138, 5067–5070. [Google Scholar] [CrossRef]
- Onoe, Y.; Miyaura, C.; Ohta, H.; Nozawa, S.; Suda, T. Expression of estrogen receptor beta in rat bone. Endocrinology 1997, 138, 4509–4512. [Google Scholar] [CrossRef]
- Braidman, I.P.; Hainey, L.; Batra, G.; Selby, P.L.; Saunders, P.T.; Hoyland, J.A. Localization of estrogen receptor beta protein expression in adult human bone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 214–220. [Google Scholar] [CrossRef]
- Bord, S.; Horner, A.; Beavan, S.; Compston, J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J. Clin. Endocrinol. Metab. 2001, 86, 2309–2314. [Google Scholar] [CrossRef][Green Version]
- Chagin, A.S.; Sävendahl, L. GPR30 estrogen receptor expression in the growth plate declines as puberty progresses. J. Clin. Endocrinol. Metab. 2007, 92, 4873–4877. [Google Scholar] [CrossRef]
- Chuang, S.C.; Chen, C.H.; Chou, Y.S.; Ho, M.L.; Chang, J.K. G Protein-Coupled Estrogen Receptor Mediates Cell Proliferation through the cAMP/PKA/CREB Pathway in Murine Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 6490. [Google Scholar] [CrossRef]
- Heino, T.J.; Chagin, A.S.; Sävendahl, L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J. Endocrinol. 2008, 197, R1–R6. [Google Scholar] [CrossRef]
- Dohi, O.; Hatori, M.; Suzuki, T.; Ono, K.; Hosaka, M.; Akahira, J.; Miki, Y.; Nagasaki, S.; Itoi, E.; Sasano, H. Sex steroid receptors expression and hormone-induced cell proliferation in human osteosarcoma. Cancer Sci. 2008, 99, 518–523. [Google Scholar] [CrossRef]
- Lillo Osuna, M.A.; Garcia-Lopez, J.; El Ayachi, I.; Fatima, I.; Khalid, A.B.; Kumpati, J.; Slayden, A.V.; Seagroves, T.N.; Miranda-Carboni, G.A.; Krum, S.A. Activation of Estrogen Receptor Alpha by Decitabine Inhibits Osteosarcoma Growth and Metastasis. Cancer Res. 2019, 79, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Lau, K.M.; Adams, J.Y.; McNeal, J.E.; Taplin, M.E.; Wang, J.; Singh, H.; Ho, S.M. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am. J. Pathol. 2001, 159, 79–92. [Google Scholar] [CrossRef]
- Zhu, X.; Leav, I.; Leung, Y.K.; Wu, M.; Liu, Q.; Gao, Y.; McNeal, J.E.; Ho, S.M. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am. J. Pathol. 2004, 164, 2003–2012. [Google Scholar] [CrossRef]
- Lindberg, M.K.; Alatalo, S.L.; Halleen, J.M.; Mohan, S.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J. Endocrinol. 2001, 171, 229–236. [Google Scholar] [CrossRef]
- Movérare, S.; Venken, K.; Eriksson, A.L.; Andersson, N.; Skrtic, S.; Wergedal, J.; Mohan, S.; Salmon, P.; Bouillon, R.; Gustafsson, J.A.; et al. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13573–13578. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Clément-Lacroix, P.; Minet, D.; Fraslon-Vanhulle, C.; Gaillard-Kelly, M.; Resche-Rigon, M.; Baron, R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J. Clin. Investig. 2003, 111, 1319–1327. [Google Scholar] [CrossRef]
- Börjesson, A.E.; Windahl, S.H.; Lagerquist, M.K.; Engdahl, C.; Frenkel, B.; Movérare-Skrtic, S.; Sjögren, K.; Kindblom, J.M.; Stubelius, A.; Islander, U.; et al. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc. Natl. Acad. Sci. USA 2011, 108, 6288–6293. [Google Scholar] [CrossRef]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef]
- Rooney, A.M.; van der Meulen, M.C.H. Mouse models to evaluate the role of estrogen receptor α in skeletal maintenance and adaptation. Ann. N. Y. Acad. Sci. 2017, 1410, 85–92. [Google Scholar] [CrossRef]
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Investig. 2013, 123, 394–404. [Google Scholar] [CrossRef]
- Määttä, J.A.; Büki, K.G.; Gu, G.; Alanne, M.H.; Vääräniemi, J.; Liljenbäck, H.; Poutanen, M.; Härkönen, P.; Väänänen, K. Inactivation of estrogen receptor α in bone-forming cells induces bone loss in female mice. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 478–488. [Google Scholar] [CrossRef]
- Windahl, S.H.; Börjesson, A.E.; Farman, H.H.; Engdahl, C.; Movérare-Skrtic, S.; Sjögren, K.; Lagerquist, M.K.; Kindblom, J.M.; Koskela, A.; Tuukkanen, J.; et al. Estrogen receptor-α in osteocytes is important for trabecular bone formation in male mice. Proc. Natl. Acad. Sci. USA 2013, 110, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, S.; Inoue, K.; Igarashi, K.; Sugizaki, H.; Shirode-Fukuda, Y.; Inoue, E.; Yu, T.; Takeuchi, J.K.; Kanno, J.; Bonewald, L.F.; et al. Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone 2014, 60, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Windahl, S.H.; Vidal, O.; Andersson, G.; Gustafsson, J.A.; Ohlsson, C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J. Clin. Investig. 1999, 104, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Windahl, S.H.; Hollberg, K.; Vidal, O.; Gustafsson, J.A.; Ohlsson, C.; Andersson, G. Female estrogen receptor beta-/- mice are partially protected against age-related trabecular bone loss. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 1388–1398. [Google Scholar] [CrossRef]
- Sims, N.A.; Dupont, S.; Krust, A.; Clement-Lacroix, P.; Minet, D.; Resche-Rigon, M.; Gaillard-Kelly, M.; Baron, R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 2002, 30, 18–25. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.A.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef]
- Varshney, M.K.; Inzunza, J.; Lupu, D.; Ganapathy, V.; Antonson, P.; Ruegg, J.; Nalvarte, I.; Gustafsson, J.A. Role of estrogen receptor beta in neural differentiation of mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 2017, 114, E10428–E10437. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Askew, G.R.; Dellovade, T.L.; Merchenthaler, I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology 2002, 143, 1643–1650. [Google Scholar] [CrossRef]
- Antal, M.C.; Krust, A.; Chambon, P.; Mark, M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc. Natl. Acad. Sci. USA 2008, 105, 2433–2438. [Google Scholar] [CrossRef]
- Mårtensson, U.E.; Salehi, S.A.; Windahl, S.; Gomez, M.F.; Swärd, K.; Daszkiewicz-Nilsson, J.; Wendt, A.; Andersson, N.; Hellstrand, P.; Grände, P.O.; et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009, 150, 687–698. [Google Scholar] [CrossRef]
- Ford, J.; Hajibeigi, A.; Long, M.; Hahner, L.; Gore, C.; Hsieh, J.T.; Clegg, D.; Zerwekh, J.; Oz, O.K. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 298–307. [Google Scholar] [CrossRef]
- Merenbakh-Lamin, K.; Ben-Baruch, N.; Yeheskel, A.; Dvir, A.; Soussan-Gutman, L.; Jeselsohn, R.; Yelensky, R.; Brown, M.; Miller, V.A.; Sarid, D.; et al. D538G mutation in estrogen receptor-α: A novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res. 2013, 73, 6856–6864. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Zundelevich, A.; Dadiani, M.; Kahana-Edwin, S.; Itay, A.; Sella, T.; Gadot, M.; Cesarkas, K.; Farage-Barhom, S.; Saar, E.G.; Eyal, E.; et al. ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res. 2020, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Jeselsohn, R.; Yelensky, R.; Buchwalter, G.; Frampton, G.; Meric-Bernstam, F.; Gonzalez-Angulo, A.M.; Ferrer-Lozano, J.; Perez-Fidalgo, J.A.; Cristofanilli, M.; Gómez, H.; et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. 2014, 20, 1757–1767. [Google Scholar] [CrossRef]
- Takeshita, T.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Inao, T.; Sueta, A.; Fujiwara, S.; Omoto, Y.; Iwase, H. Droplet digital polymerase chain reaction assay for screening of ESR1 mutations in 325 breast cancer specimens. Transl. Res. 2015, 166, 540–553.e542. [Google Scholar] [CrossRef]
- Lang-Muritano, M.; Sproll, P.; Wyss, S.; Kolly, A.; Hürlimann, R.; Konrad, D.; Biason-Lauber, A. Early-Onset Complete Ovarian Failure and Lack of Puberty in a Woman With Mutated Estrogen Receptor β (ESR2). J. Clin. Endocrinol. Metab. 2018, 103, 3748–3756. [Google Scholar] [CrossRef]
- Baetens, D.; Güran, T.; Mendonca, B.B.; Gomes, N.L.; De Cauwer, L.; Peelman, F.; Verdin, H.; Vuylsteke, M.; Van der Linden, M.; Atay, Z.; et al. Biallelic and monoallelic ESR2 variants associated with 46,XY disorders of sex development. Genet. Med. Off. J. Am. Coll. Med Genet. 2018, 20, 717–727. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Reviews. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Tsunoda, M.; Kinoshita, S.; Saura, R. Role of fracture hematoma and periosteum during fracture healing in rats: Interaction of fracture hematoma and the periosteum in the initial step of the healing process. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2000, 5, 64–70. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cho, T.J.; Kon, T.; Aizawa, T.; Tsay, A.; Fitch, J.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Impaired fracture healing in the absence of TNF-alpha signaling: The role of TNF-alpha in endochondral cartilage resorption. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003, 18, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Takayanagi, H. Osteoimmunology in Bone Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, H.; Dennis, J.E.; Bruder, S.P.; Haynesworth, S.E.; Lennon, D.P.; Caplan, A.I. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells. Exp. Cell Res. 1991, 195, 492–503. [Google Scholar] [CrossRef]
- Hayashi, O.; Katsube, Y.; Hirose, M.; Ohgushi, H.; Ito, H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif. Tissue Int. 2008, 82, 238–247. [Google Scholar] [CrossRef]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Tatsuyama, K.; Maezawa, Y.; Baba, H.; Imamura, Y.; Fukuda, M. Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur. J. Histochem. 2000, 44, 269–278. [Google Scholar]
- Lee, P.A.; Witchel, S.F. The influence of estrogen on growth. Curr. Opin. Pediatrics 1997, 9, 431–436. [Google Scholar] [CrossRef]
- Beil, F.T.; Barvencik, F.; Gebauer, M.; Seitz, S.; Rueger, J.M.; Ignatius, A.; Pogoda, P.; Schinke, T.; Amling, M. Effects of estrogen on fracture healing in mice. J. Trauma 2010, 69, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Chen, J.T.; Lin, P.I.; Cherng, Y.G.; Yang, S.T.; Chen, R.M. Inhibition of the estrogen receptor alpha signaling delays bone regeneration and alters osteoblast maturation, energy metabolism, and angiogenesis. Life Sci. 2020, 258, 118195. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, E.; Liedert, A.; Heilmann, A.; Wehner, T.; Bindl, R.; Fischer, L.; Haffner-Luntzer, M.; Jakob, F.; Schinke, T.; Amling, M.; et al. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis. Models Mech. 2015, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Mohamad, N.V.; Jayusman, P.A.; Shuid, A.N.; Ima-Nirwana, S.; Chin, K.Y. The use of selective estrogen receptor modulators on bone health in men. Aging Male Off. J. Int. Soc. Study Aging Male 2019, 22, 89–101. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Castillo, N. Estrogen Signaling in Bone. Appl. Sci. 2021, 11, 4439. https://doi.org/10.3390/app11104439
Lara-Castillo N. Estrogen Signaling in Bone. Applied Sciences. 2021; 11(10):4439. https://doi.org/10.3390/app11104439
Chicago/Turabian StyleLara-Castillo, Nuria. 2021. "Estrogen Signaling in Bone" Applied Sciences 11, no. 10: 4439. https://doi.org/10.3390/app11104439
APA StyleLara-Castillo, N. (2021). Estrogen Signaling in Bone. Applied Sciences, 11(10), 4439. https://doi.org/10.3390/app11104439




