Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications
Abstract
:1. Introduction
Historical Background of DLC
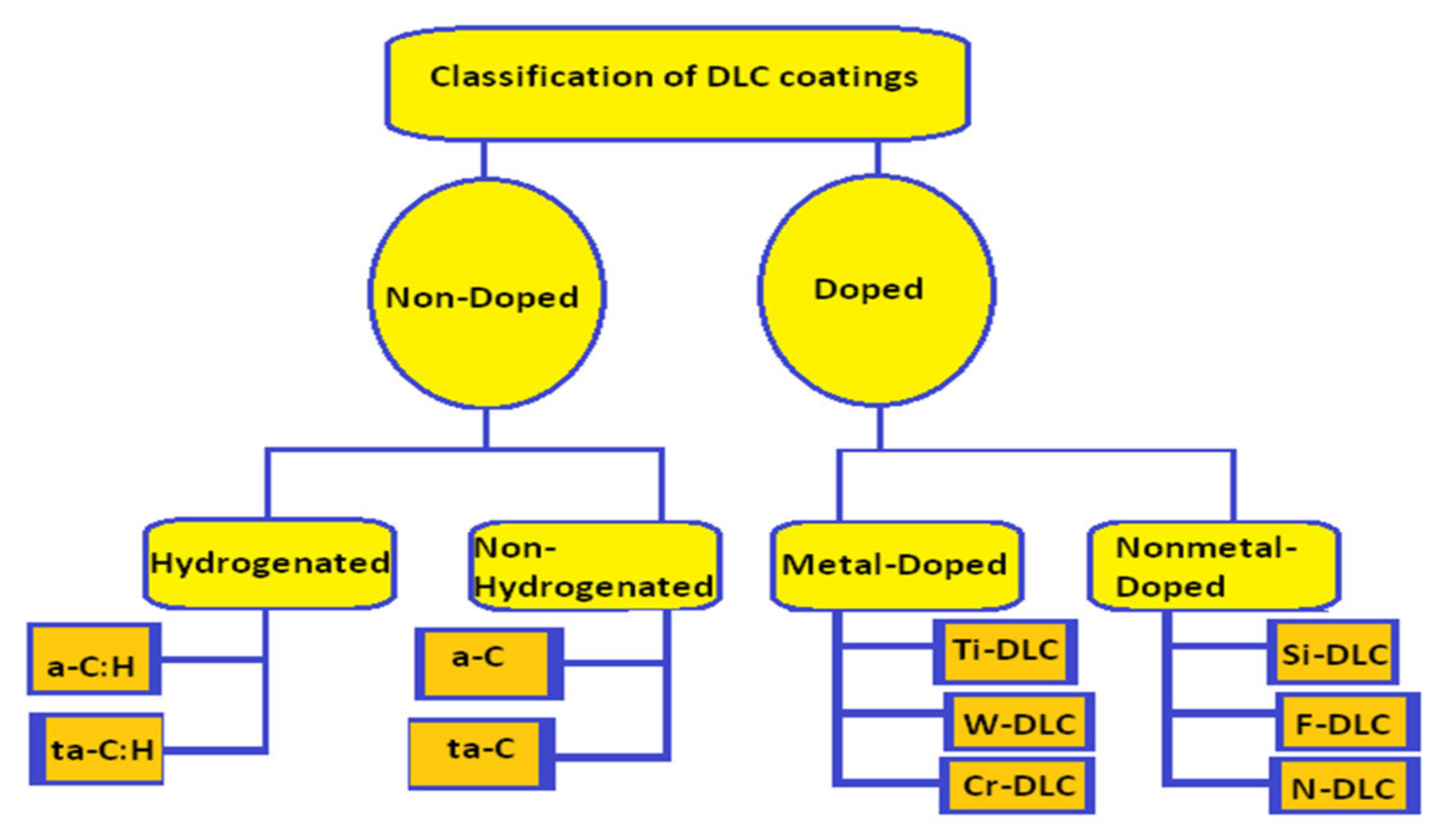
2. Classification of DLC Coatings
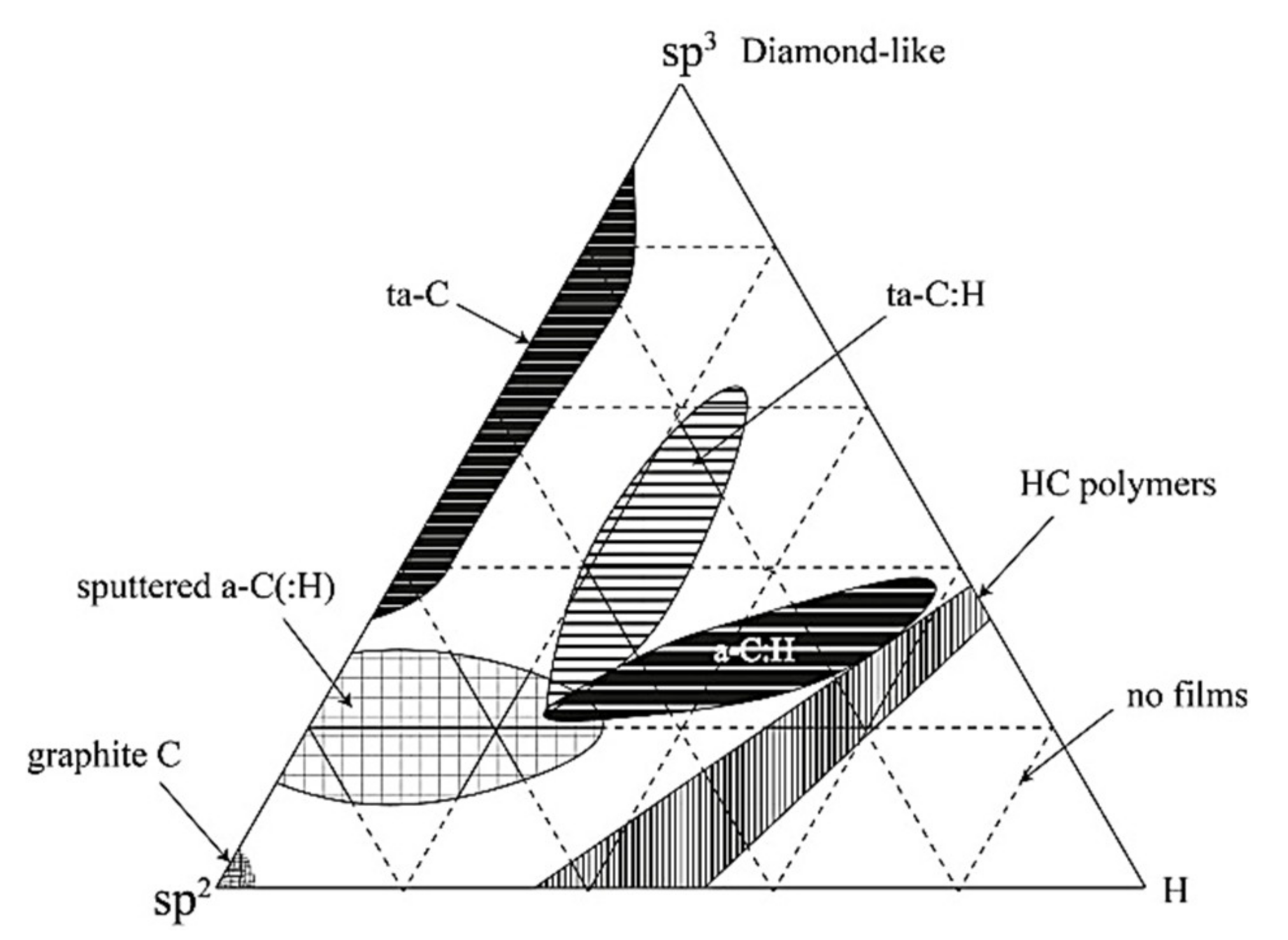
2.1. Stage Diagram
2.2. Structural Arrangement
2.3. Processing
2.4. Properties
2.4.1. Film Density
2.4.2. Sp3—Density Curvature
2.4.3. Refractive Index
2.4.4. Growth Rates in PECVD
2.4.5. Stress
2.4.6. Alloyed DLCs
3. Characterizations/Properties of DLC Coatings
3.1. DLC Substrate Compatibility
3.2. Tribological Properties of DLC Coatings
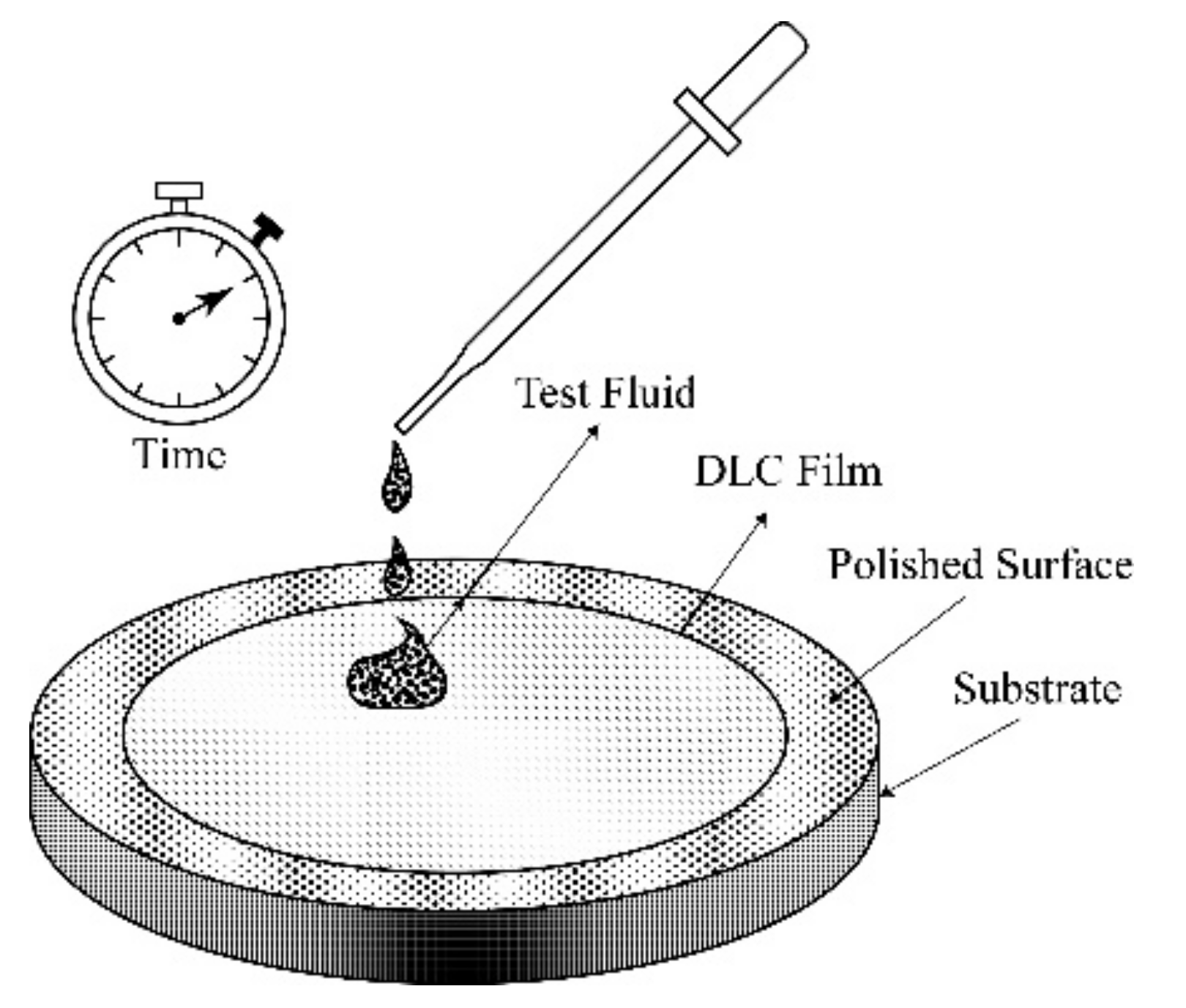
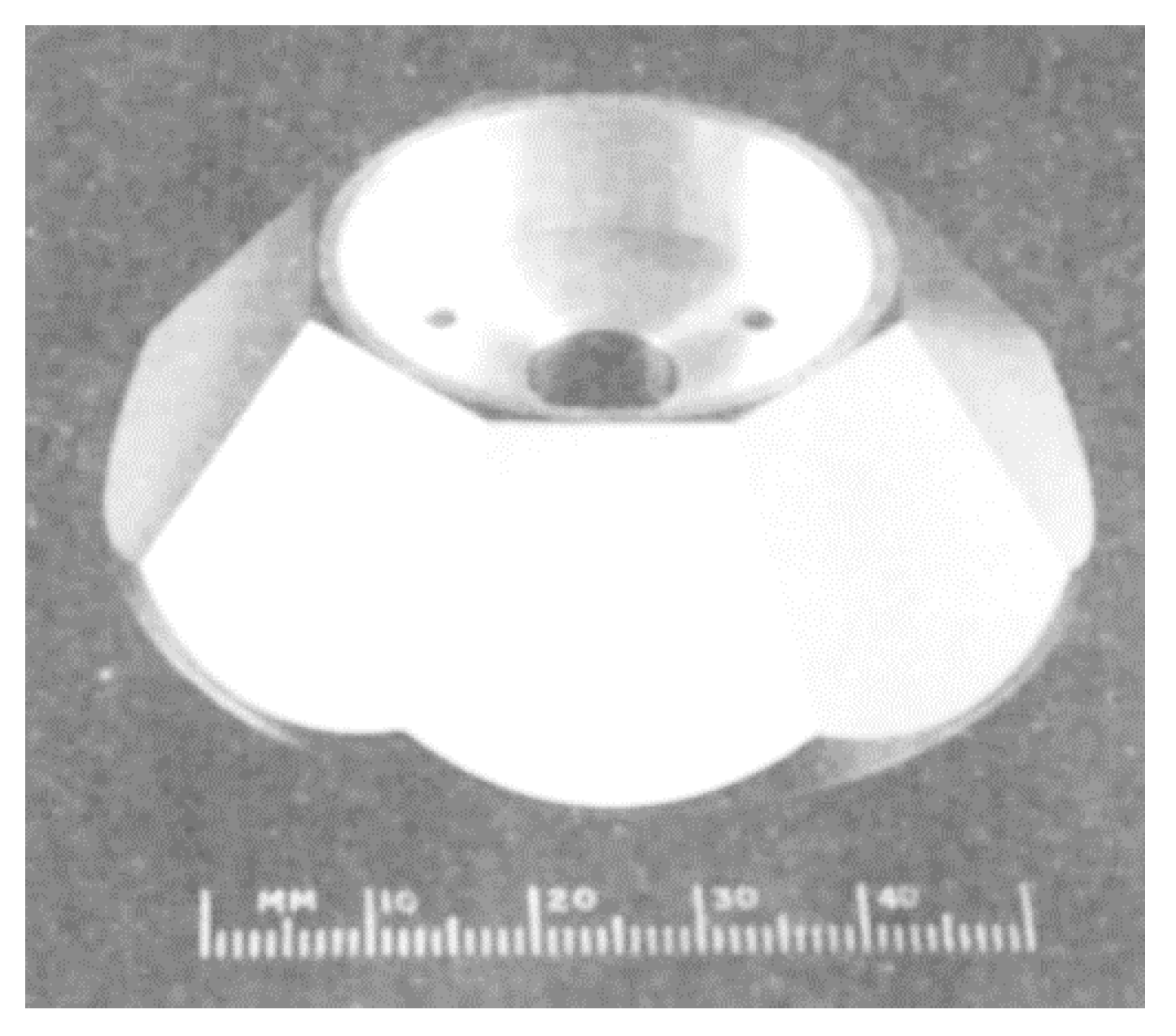
3.3. Chemical Resistance of DLC Coatings
3.4. Thermal Stress: DLC Films
3.5. DLC Film Adhesion
3.6. Recent Manufacturing Process
4. Applications of DLC Coatings
- Automotive: piston pins, rocker arms
- Medical: surgical tools, prosthetic applications
- Firearms: firearm slides, barrels, bolt carriers
- Industrial parts and machinery: pistons, plungers, gears, mechanical seals
- Injection molding: dies, ejector pins, slippery machine components
- Consumer products: wrist joint watches, jewelry, golf clubs
4.1. Carbon Coatings on Front Surface of Al Mirrors
4.2. DLC Coatings for Photothermal Conversion of Solar Energy
4.3. Mechanical Applications of DLC Layers
4.4. Electronic Device Applications of DLC
4.5. Medical Applications of DLC Layers
4.6. Others Applications of DLC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robertson, J. Diamond-Like Amorphous Carbon. Mater. Sci. Eng. R. Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Kržan, B.; Novotny-Farkas, F.; Vižintin, J. Tribological behavior of tungsten-doped DLC coating under oil lubrication. Tribol. Int. 2009, 42, 229–235. [Google Scholar] [CrossRef]
- Evtukh, A.A.; Litovchenko, V.G.; Litvin, Y.M.; Fedin, D.V.; Dzyan, O.S.; Pedchenko, Y.N.; Chakhovskoi, A.G.; Felter, T.E. Silicon Doped Diamond-Like C Films as a Coating for Improvement of Electron Field Emission. In Proceedings of the 14th International Vacuum Microelectronics Conference, Davis, CA, USA, 12–16 August 2001; p. 295. [Google Scholar]
- Louis, B. Amorphous Diamond, a New Super-Hard Form of C Created under Ultrahigh Pressure; Science Daily: Rockville, MD, USA, 2011. [Google Scholar]
- Treutler, C.P.O. Industrial use of plasma-deposited coatings for components of automotive fuel injection systems. Surf. Coat. Technol. 2005, 200, 1969–1975. [Google Scholar] [CrossRef]
- Van Der Kolk, G.J. Tribology of Diamond-Like C Films, Springer Science + Business Media; Donnet, C., Erdemir, A., Eds.; LLC: New York, NY, USA, 2008; pp. 484–493. [Google Scholar]
- Klaus, B.; Dieter, H. History of Diamond-Like C Films—From First Experiments to Worldwide Applications. Surface Coat. Technol. 2014, 242, 214–225. [Google Scholar]
- Goglia, P.; Berkowitz, J.; Hoehn, J.; Xidis, A. Diamond-like carbon applications in high density hard disc recording heads. Diam. Relat. Mater. 2001, 10, 271–277. [Google Scholar] [CrossRef]
- Ferrari, A. Diamond-like carbon for magnetic storage disks. Surf. Coat. Technol. 2004, 180, 180–181. [Google Scholar] [CrossRef]
- Schmellenmeier, H. Die Beeinflussung von festen Oberflachen durch eine ionisierte. Exp. Tech. Phys. 1953, 1, 49–68. [Google Scholar]
- Schmellenmeier, H. Carbon layers with diamond structure. Phys. Chem. 1956, 205, 349–360. [Google Scholar]
- König, H.; Helwig, G.Z. Thin Layers Formed from Hydrocarbons by Electron or Ion Bombardment. Physik 1951, 129, 491–503. [Google Scholar] [CrossRef]
- Heisen, A. Über die Bildung dünner Kohleschichten in einer in Benzolatmosphäre brennenden Glimmentladung. Ann. Phys. 1958, 457, 23–35. [Google Scholar] [CrossRef]
- Heisen, A. Colour vision in Man and Animals. Optik 1961, 18, 59–68. [Google Scholar]
- Aisenberg, S.; Chabot, R. Ion-beam deposition of thin films of diamondlike carbon. J. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Aisenberg, S.; Chabot, R. Physics of ion plating and ion beam deposition. J. Vac. Sci. Technol. 1973, 10, 104–107. [Google Scholar] [CrossRef]
- Spencer, E.G.; Schmidt, P.H.; Joy, D.C.; Sansalone, F. Ion-beam-deposited polycrystalline diamondlike films. J. Appl. Phys. Lett. 1976, 29, 118–120. [Google Scholar] [CrossRef]
- Whitmell, D.S.; Williamson, R. The deposition of hard surface layers by hydrocarbon cracking in a glow discharge. Thin Solid Films 1976, 35, 255–261. [Google Scholar] [CrossRef]
- Holland, L. Some characteristics and uses of low-pressure plasmas in materials science. J. Vac. Sci. Technol. 1977, 14, 5–15. [Google Scholar] [CrossRef]
- Bewilogua, K.; Wagner, D. The effect of secondary electrons in the ion plating deposition of amorphous hydrogenated carbon (aC: H) films. Vacuum 1991, 42, 473–476. [Google Scholar] [CrossRef]
- Allen, S.M.; Thomas, E.L. The Structure of Materials. Wiley: New York, NY, USA, 1998; p. 2. [Google Scholar]
- Carbon Based Films-Classification and Designations ISO 20523:2017; The International Organization for Standardization: Geneva, Switzerland, 2017.
- Ohgoe, Y.; Hirakuri, K.K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC films in terms of biological response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Nakamori, H.; Kogo, Y.; Sakurai, M.; Ohtake, N.; Saitoh, H. Correlation between Optical Properties and Hardness of Diamond-Like Carbon Films. Solid Mech. Mater. Eng. 2013, 7, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Jacob, W.; Moller, W. On the structure of thin hydrocarbon films. App. Phys. Lett. 1993, 63, 1771–1773. [Google Scholar] [CrossRef]
- Fallon, P.J.; Veerasamy, V.S.; Davis, C.A.; Robertson, J.; Amaratunga, G.A.J.; Milne, W.I.; Koskinen, J. Properties of filtered-ion-beam-deposited diamondlike carbon as a function of ion energy. Phys. Rev. B 1993, 48, 4777. [Google Scholar] [CrossRef] [PubMed]
- Polo, M.C.; Andujar, J.L.; Robertson, J.; Milne, W.I. Preparation of tetrahedral amorphous carbon films by filtered cathodic vacuum arc deposition. Diam. Relat. Mater. 2000, 9, 663–667. [Google Scholar] [CrossRef]
- Lifshitz, Y.; Lempert, G.D.; Grossman, E.; Avigal, I.; Uzan-Saguy, C.; Kalish, R.; Kulik, J.; Marton, D.; Rabalais, J.W. Growth mechanisms of DLC films from C+ ions: Experimental studies. Diam. Relat. Mater. 1995, 4, 318–323. [Google Scholar] [CrossRef]
- Merkulov, V.I.; Lowndes, D.H.; Jellison, G.E.; Puretzky, A.A.; Geohegan, D.B. Field emission studies of smooth and nanostructured carbon films. App. Phys. Lett. 1999, 73, 1228. [Google Scholar] [CrossRef]
- Koidl, C.; Wagner, B.; Dischler, J.; Wagner, M. Plasma deposition, properties and structure of amorphous hydrogenated carbon films. Mat. Sci. Forum. 1990, 52, 41–70. [Google Scholar] [CrossRef]
- Zou, J.W.; Reichelt, K.; Schmidt, K.; Dischler, B. The deposition and study of hard carbon films. J. App. Phys. 1989, 65, 3914–3918. [Google Scholar] [CrossRef]
- Kessels, W.M.M.; Gielen, J.W.A.M.; van de Sanden, M.C.M.; van Ijzendoorn, L.J.; Schram, D.C. A model for the deposition of aC: H using an expanding thermal arc. Surf. Coat. Technol. 1998, 98, 1584–1589. [Google Scholar] [CrossRef]
- Schwarz-Selinger, T.; Von Keudell, A.; Jacob, W. Plasma chemical vapor deposition of hydrocarbon films: The influence of hydrocarbon source gas on the film properties. J. App. Phys. 1999, 86, 3968. [Google Scholar] [CrossRef]
- Tamor, M.A.; Vassell, W.C.; Carduner, K.R. Atomic constraint in hydrogenated “diamond-like” carbon. App. Phys. Lett. 1991, 58, 592–594. [Google Scholar] [CrossRef]
- Donnet, C.; Fontaine, J.; Lefebvre, F.; Grill, A.; Patel, V.; Jahnes, C. Solid state 13C and 1H nuclear magnetic resonance investigations of hydrogenated amorphous carbon. J. App. Phys. 1999, 85, 3264–3270. [Google Scholar] [CrossRef]
- Weiler, M.; Sattel, S.; Giessen, T.; Jung, K.; Ehrhardt, H.; Veerasamy, V.S.; Robertson, J. Preparation and properties of highly tetrahedral hydrogenated amorphous carbon. Phys. Rev. B 1996, 53, 1594. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Lang, K.; Li, E.; Robertson, J. Deposition of tetrahedral hydrogenated amorphous carbon using a novel electron cyclotron wave resonance reactor. App. Phys. Lett. 1998, 72, 1314–1316. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Wu, H.; Liu, X.; Wang, L.; Yu, Q.; Li, A.; Wang, H.; Song, C.; Gao, Z.; et al. Construction Of Sp3/Sp2C Interface in 3D N-Doped NanoC For The O Reduction Reaction. Angew Chem. Int. Ed. 2019, 58, 15089. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Xu, D.; Ge, J. Construction of Nitrogen-Doped C Nanosheets for Efficient and Stable O Reduction Electrocatalysis. J. Electron. Mater. 2021, 50, 1349–1357. [Google Scholar] [CrossRef]
- Jiangwei, C.; Longlong, H.; Chang, Y.; Yiwang, D.; Chun, Y.; Jieshan, Q. Highly efficient & economic synthesis of CoS1.097/nitrogen-doped C for enhanced triiodide reduction. Carbon 2021, 174, 445–450. [Google Scholar]
- Xia, Y.; Zhang, Z.; Qin, F.; Gao, J.; Wang, H.; Xu, Z.; Tan, X.; Liu, X.; Li, X.; Yin, Z. Electrocatalytic activity enhancement of N, P-doped C nanosheets derived from polymerizable ionic liquids. J. Appl. Electrochem. 2021, 51, 669–679. [Google Scholar] [CrossRef]
- Yu, X.; Lu, X.; Qin, G.; Li, H.; Li, Y.; Yang, L.; Song, Z.; An, Y.; Yan, Y. Large-scale synthesis of flexible TiO2/N-doped C nanofibres. A highly efficient all-day-active photocatalyst with electron storage capacity. Ceram. Int. 2020, 46, 12538–12547. [Google Scholar] [CrossRef]
- Yu, X.; Lai, S.; Xin, S.; Chen, S.; Zhang, X.; She, X.; Zhan, T.; Zhao, X.; Yang, D. Coupling of Iron Phthalocyanine at C Defect Site via π-π Stacking for Enhanced O Reduction Reaction. Appl. Catal. B Environ. 2020, 119437. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, D.; Yi, D.; Yang, Y.; Wang, X.; Yao, J. sp2/sp3 Hybridized Carbon as an Anode with Extra Li-Ion Storage Capacity. Construction and Origin. ACS Central Sci. 2020, 6, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Suo, B.; Shi, Y.; Yuan, H.; Zhu, C.; Chen, Y. General Fabrication of 3D Hierarchically Structured Bamboo-like Nitrogen-Doped C Nanotube Arrays on 1D Nitrogen-Doped C Skeletons for Highly Efficient Electromagnetic Wave Energy Attenuation. ACS Appl. Mat. Interfaces 2020, 12, 40691–40701. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Kawaguchi, M.; Mizoguchi, I. Effects of sp2/sp3 Ratio and Hydrogen Content on In Vitro Bending and Frictional Performance of DLC-Coated Orthodontic Stainless Steels. Coatings 2018, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Yimin, L.; Guojun, H.; Sai, W.; Chaowei, M.; Shangfang, W.; Fangtao, T.; Wei, L.; Haiyuan, C.; Yong, C. A Review on Diamond-like C Films Grown by Pulsed Laser Deposition. Appl. Surf. Sci. 2020, 5, 148573. [Google Scholar]
- Zhong, W.X.; Zhao, X.R.; Qin, J.Y.; Yang, J. An Active Hybrid Electrocatalyst with Synergized Pyridinic Nitrogen - Cobalt and O Vacancies for Bifunctional O Reduction and Evolution. Chin. J. Chem. 2021, 39, 655–660. [Google Scholar] [CrossRef]
- Lemoine, P.; Quinn, J.P.; Maguire, P.D.; McLaughlin, J.A.D. Measuring the thickness of ultra-thin diamond-like carbon films. Carbon 2006, 44, 2617. [Google Scholar] [CrossRef]
- Sarakinos, K.; Braun, A.; Zilkens, C.; Mra, Z.S.; Schneider, J.M.; Zoubos, H.; Patsalas, P. Exploring the potential of high power impulse magnetron sputtering for growth of diamond-like carbon films. Surf. Coat. Technol. 2012, 206, 2706–2710. [Google Scholar] [CrossRef] [Green Version]
- Chhowalla, M.; Robertson, J.; Chen, C.W.; Silva, S.R.P.; Amaratunga, A.G.A. Influence of ion energy and substrate temperature on the optical and electronic properties of tetrahedral amorphous carbon (ta-C) films. J. Appl. Phys. 1997, 81, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Kamata, K.; Inoue, T.; Sugai, K.; Saitoh, H. Maruyama. J. Appl. Phys. 1995, 78, 1394. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Kleinsorge, B.; Adamopoulos, G.; Robertson, J.; Milne, W.I.; Stolojan, V.; Brown, L.M.; Libassi, A.K.A.B. Determination of bonding in amorphous carbons by electron energy loss spectroscopy, Raman scattering and X-ray reflectivity. J. Non-Cryst. Solids. 2000, 765, 266–269. [Google Scholar] [CrossRef]
- Libassi, A.C.; Ferrari, V.; Stolojan, B.K.; Tanner, J.; Robertson, L.; Brown, M. Density, sp3 content and internal layering of DLC films by X-ray reflectivity and electron energy loss spectroscopy. Diam. Relat. Mater. 2000, 9, 771. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.; Liu, A.; Jia, Z.; Han, J. Effects of mass density on the micro hardness and modulus of tetrahedral amorphous carbon films. Mater. Lett. 2007, 61, 4647. [Google Scholar] [CrossRef]
- Chua, H.C.; Teo, K.B.K.; Tsai, T.H.; Milne, W.I.; Sheeja, D.; Tay, B.K.D. A Correlation of surface, mechanical and micropropertiesof tetrahedral amorphous carbon films deposited under different magnetic confinement conditions. Schneider. Appl. Surf. Sci. 2004, 221, 455. [Google Scholar] [CrossRef]
- Pastorelli, R.; Ferrari, A.C.; Beghi, M.G.; Bottani, C.E.; Robertson, J. Elastic constants of ultrathin diamond-like carbon films. Diamond Relat. Mater. 2000, 9, 825. [Google Scholar] [CrossRef]
- Paik, N. High density DLC films prepared using a magnetron sputter type negative ion source. Diam. Relat. Mater. 2005, 14, 196. [Google Scholar] [CrossRef]
- Aijaz, A.; Sarakinos, K.; Lundin, D.; Brenning, N.; Helmersson, U. A strategy for increased carbon ionization in magnetron sputtering discharges. Diamond Relat. Mater. 2012, 23, 1. [Google Scholar] [CrossRef] [Green Version]
- Logothetidis, S.; Patsalas, P.; Gioti, M.; Galdikas, A.; Pranevicius, L. Growth kinetics of sputtered amorphous carbon thin films composition studies and phenomenological model. Thin Solid Films 2000, 376, 56. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Libassi, A.; Tanner, B.K.; Stolojan, V.; Yuan, J.; Brown, L.M.; Rodil, S.E.; Robertson, B.K.A.J. Density SP3 fraction, and cross sectional structure of amorphous carbon films determined by X-ray reflectivity and electron energy-loss spectroscopy. Phys. Rev. B 2000, 62, 11089. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, A.; Ferrari, C.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbon. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef] [Green Version]
- Donnet, J.R.C.; Erdemir, A. (Eds.) Tribology of Diamond-Like C Films. Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2008; pp. 13–24. [Google Scholar]
- Tian, J.; Zhang, Q.; Zhou, Q.; Yoon, S.F.; Ahn, J.; Wang, S.G.; Li, J.Q.; Yang, A.D.J. Study of well adherent DLC films deposited on piezoelectric LiTO3 substrate. Appl. Surf. Sci. 2005, 239, 255. [Google Scholar] [CrossRef]
- Robertson, D.J. Deposition mechanisms for promoting SP3 bonding in diamond-like carbon. Relat. Mater. 1993, 3, 361. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Ashraf, M.N.; Timmermann, A.; Schmid, C.; Ensinger, W. The Influence of Preparation Conditions on the Structural Properties and Hardness of Diamond-Like C Films, Prepared by Plasma Source Ion Implantation. Coatings 2020, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Yamamoto, K. Mechanical and tribological properties of DLC films for sliding parts. Kobelco Technol. Rev. 2017, 35, 55–60. Available online: https://www.kobelco.co.jp/english/ktr/ktr_35.html (accessed on 1 December 2020).
- Písarík, P.; Jelínek, M.; Kocourek, T.; Remsa, J.; Zemek, J.; Lukeš, J.; Šepitka, J. Influence of diamond and graphite bonds on mechanical properties of DLC thin films. J. Phys. Conf. Ser. 2015, 594, 012008. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.A.; Woodford, J.B.; Chen, X.; Andersson, J.; Erdemir, A.; Fenske, G.R. Insights into ‘near frictionless carbon films’. Appl. Phys. 2004, 95, 7765. [Google Scholar]
- Sanchez-Lopez, J.C.; Erdemir, A.C.; Rojas, A.T. Friction-induced structural transformations of diamond like carbon coatings under various atmospheres. Surf. Coat. Technol. 2003, 163, 444. [Google Scholar] [CrossRef]
- Aurang, P.; Demircioglu, O.; Es, F.; Turan, R.; Unalan, H.E. ZnO Nanorods as Antireflective Coatings for Industrial-Scale Single-Crystalline Silicon Solar Cells. J. Am. Ceram. Soc. 2013, 96, 1253–1257. [Google Scholar] [CrossRef]
- Gao, G.T.; Mikulski, P.T.; Harrison, J.A. Molecular scale tribology of amorphous carbon coatings: Effects of film thickness, adhesion and long range interaction. J. Am. Chem. Soc. 2002, 124, 7202. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.T.; Mikulski, P.T.; Chateauneuf, G.M.; Harrison, J.A. The effects of film structure and surface hydrogen on the properties of amorphous carbon films. J. Phys. Chem. B 2003, 107, 11082. [Google Scholar] [CrossRef]
- Bilek, M.M.M.; Mckenzie, D.R.; Moeller, W. Use of low energy and high frequency PBII during thin film deposition to achieve relief of intrinsic stress and microstructural changes. Surf. Coat. Technol. 2004, 186, 21. [Google Scholar] [CrossRef]
- Bilek, M.M.; Verdon, M.; Ryves, L.; Oates, T.W.; Ha, C.T.; McKenzie, D.R. A model for stress generation and stress relief mechanisms applied to as deposited filtered cathodic vacuum arc amorphous carbon films. Thin Solid Films 2005, 482, 69. [Google Scholar] [CrossRef]
- Memming, R.; Tolle, H.J.; Wierenga, P.E. Properties of polymeric layers of hydrogenated amorphous carbon produced by a plasma-activated chemical vapor deposition process II: Tribological and mechanical properties. Thin Solid Films 1986, 143, 31. [Google Scholar] [CrossRef]
- Klages, P.; Memming, R. Microstructure and physical properties of metal-containing hydrogenated carbon films. Mater. Sci. Forum. 1990, 52, 609. [Google Scholar] [CrossRef]
- Available online: Http://Www.Ist.Fraunhofer.De/English/C-Products/Tab/Complete.Html (accessed on 1 December 2020).
- Muhl, S.; Mendez, J. A review of preparation of carbon nitride films. Relat. Mater. 1999, 8, 1809–1830. [Google Scholar] [CrossRef]
- Oguri, K.; Arai, T. Tribological properties and characterization of diamond-like carbon coatings with silicon prepared by plasma-assisted chemical vapour deposition. Surf. Coat. Technol. 1991, 47, 710. [Google Scholar] [CrossRef]
- Zhang, S.; Bui, X.L.; Zeng, X.L.; Li, X.M. Towards high adherent and tough aC coatings. Thin Solid Films 2005, 482, 138. [Google Scholar] [CrossRef]
- Kaczorowski, W.; Świątek, H.; Łuczak, K.; Głuszek, M.; Cłapa, M. Impact of Plasma Pre-Treatment on the Tribological Properties of DLC Coatings on PDMS Substrates. Materials 2021, 14, 433. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Kang, J.; Ma, G.; Zhu, L.; Wang, H.; Fu, Z.; Huang, H.; Yue, W. Tribological Properties of Ti-Doped Diamond Like C Coatings Under Boundary Lubrication with ZDDP. J. Tribol. ASME. 2021, 143, 091901. [Google Scholar] [CrossRef]
- Fiaschi, G.; Rota, A.; Ballestrazzi, A.; Marchetti, D.; Vezzalini, E.; Sergio, V.A. Chemical, Mechanical, and Tribological Analysis of DLC Coatings Deposited by Magnetron Sputtering. Lubricant 2019, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Field, J.E. The Properties of Diamond; Academic Press Inc.: New York, NY, USA, 1979. [Google Scholar]
- Outka, A.; Hsu, W.L.; Boehme, D.R.; Yang, N.Y.C.; Ottesen, D.K.; Johnsen, H.A.; Headley, T.J.; Clift, W.M. Sandia Report Sand94–8219, Uc-404; Unlimited Release Printed February; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 1994. [Google Scholar]
- Hoffman, R.W. The Mechanical Properties of Thin Condensed Films. In Physics of Thin Films; Hass, G., Thun, R.E., Eds.; Academic: New York, NY, USA, 1966; p. 266. [Google Scholar]
- Chopra, K.L. Thin Film Phenomena; Mcgraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Field, J.E. Strength and Fracture Properties of Diamond. In The Properties of Diamond; Field, J.E., Ed.; Academic: New York, NY, USA, 1979; p. 281. [Google Scholar]
- Gray, E. (Ed.) American Institute of Physics Handbook, 3rd ed.; Mcgraw-Hill: New York, NY, USA, 1972. [Google Scholar]
- Campbell, S. Mechanical Properties of Thin Films. In Handbook of Thin Films Technology, Maissel, L.I.; Glang, R., Ed.; Mcgraw-Hill: New York, NY, USA, 1970; p. 12. [Google Scholar]
- Weast, R.C. (Ed.) Handbook of Chemistry and Physics, 70th ed.; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Holleck, H. Material selection for hard coatings. J. Vac. Sci. Technol. A 1986, 42661. [Google Scholar] [CrossRef]
- Gere, J.M.; Timoshenko, S.P. Mechanics of Materials, 3rd ed.; Pws-Kent: Boston, MA, USA, 1990. [Google Scholar]
- Jaccodine, R.J.; Schlegel, W.A. Measurement of strains at SiO2 interface. J. Appl. Phys. 1966, 372429. [Google Scholar] [CrossRef]
- Enke, K. Some new results on the fabrication of and the mechanical, electrical and optical properties of i-carbons layers. Thin Solid Films 1981, 80227. [Google Scholar] [CrossRef]
- Matuda, N.; Baba, S.; Kinbara, A. Internal stress, young;s modulus and adhesion energy of carbon films on glass substrate. Thin Solid Films 1981, 8, 1301. [Google Scholar]
- Zelez, J. Low stress carbon like carbon films. J. Vac. Sci. Technol. A 1983, 1, 305. [Google Scholar] [CrossRef]
- Gille, B.R. Buckling instability and adhesion of carbon layers. Thin Solids Films 1984, 120, 109. [Google Scholar] [CrossRef]
- Outka, D.A.; Hsu, W.L.; Phillips, K.; Boehme, D.R.; Yang, N.Y.; Ottesen, D.K.; Johnsen, H.A.; Clift, W.M.; Headley, T.J. Compilation of diamond like carbon properties for barriers and hard coatings. Sandia 1994, 10151476. [Google Scholar]
- Nir, D. Intrinsic stress in diamond-like carbon films and its dependence on deposition parameters. Thin Solid Films 1987, 146, 27. [Google Scholar] [CrossRef]
- Kikuchi, A.; Baba, S.; Kinbara, A. Measurement of the adhesion of silver films to glass substrate. Thin Solid Films 1985, 343–349. [Google Scholar] [CrossRef]
- Electrochemical Society; Dielectrics, Insulation Division, Electrochemical Society. High Temperature Materials Division; High Temperature Materials Division. Proceedings of the… International Symposium on Diamond and Diamond-like Films. Electrochem. Soc. 1989, 12–89. [Google Scholar]
- Ham, M.; Lou, K.A. Diamond like carbon films grown by a large scale direct current plasma-chemical vapor deposition reactor: System design, film characteristics and applications. J. Vac. Sci. Technol. A 1990, 8, 2143. [Google Scholar] [CrossRef]
- Berry, B.S.; Pritchet, W.C.; Cuomo, J.J.; Guarnieri, C.R.; Whitehair, S.J. Internal stress and elasticity of synthetic diamond films. Appl. Phys. Lett. 1990, 57, 302. [Google Scholar] [CrossRef]
- Specht, E.D.; Clausing, R.E.; Heatherly, L. Measurement of crystalline strain and orientation in diamond films grown by chemical vapor deposition. J. Mater. Res. 1990, 5, 2351. [Google Scholar] [CrossRef]
- Clausing, R.E.; Heatherly, L.; Specht, E.D.; More, K.L.; Begun, G.M. Growth mechanism, film morphology, texture and stresses for three types of HFCVD diamond film growth. Carbon 1990, 28, 762. [Google Scholar] [CrossRef]
- Angus, J.C.; Koidl, P.; Domitz, A.S. C Thin Films. In Plasma Deposited Thin Films; Mort, J., Jansen, F., Eds.; CRC Press: Boca Raton, FL, USA, 1986; p. 89. [Google Scholar]
- Tsai, H.-C.; Bogy, D.B.J. Charecterization of diamond like carbon films and their applications as overcoats on thin film media for magnetic recording. Vac. Sci. Technol. A 1987, 5, 3287. [Google Scholar] [CrossRef]
- Nir, D. Stress relief forms diamond-like carbon thin films under internal compressive stress. Thin Solid Films 1984, 112–141. [Google Scholar] [CrossRef]
- Anttila, A.; Koskinen, J.; Lappalainen, R.; Hirvonen, J.P.; Stone, D.; Paszkiet, C. Comparison of diamond like coatings with C+ and various hydrocarbon ion beams. Appl. Phys. Lett. 1987, 50, 132. [Google Scholar] [CrossRef] [Green Version]
- Shrnivashan, N.; Bhaskar, L.K.; Kumar, R.; Bhargeti, S. Residual Stress Gradient and Relaxation Upon Fatigue Deformation of Diamond-Like C Coated Aluminum Alloy in Air and Methanol Environments. Mat. Design 2018, 160, 303–312. [Google Scholar] [CrossRef]
- Ager, J.W.; Veirs, D.K.; Rosenblatt, G.M. Spatially resolved Raman studies of diamond films grown by chemical vapor deposition. Phys. Rev. B 1991, 6491. [Google Scholar] [CrossRef]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Proc. Roy. Soc. 1909, 32, 172. [Google Scholar]
- Spear, K.E. Diamond ceramic coatings of the future. J. Am. Ceram. Soc. 1989, 72, 171. [Google Scholar] [CrossRef] [Green Version]
- Hull, T.; Colligon, J.S. Measurement of thin film adhesion. Vacuum 1987, 37, 327. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, L. Effect of sandblasting on adhesion strength of diamond coatings. Thin Solid Films 1997, 307, 21–28. [Google Scholar] [CrossRef]
- Halpern, J. Determination and significance of transition metal-alkyl bond dissociation energy. Acc. Chem. Res. 1982, 15, 238–244. [Google Scholar] [CrossRef]
- Rossini, F.D. Selected Values of Chemical Thermodynamic Properties, National Bureau of Standards Circular 500; US Government Printing Office: Washington, DC, USA, 1952. [Google Scholar]
- Shaffer, P.T.B. Plenum Press Handbooks of High-Temperature Materials; Plenum: New York, NY, USA, 1964. [Google Scholar]
- Storms, K. Tile Refractory Carbides; Academic: New York, NY, USA, 1967. [Google Scholar]
- Schick, H.L. Thermodynamics of Certain Refractory Compounds; Academic: New York, NY, USA, 1966. [Google Scholar]
- Mirtich, M.J.; Nir, D.; Swec, D.; Banks, B. The use of intermediate layers to improve the adherence of diamond like carbon films on Zns and ZnSe. J. Vac. Sci. Technol. A 1986, 4, 2680. [Google Scholar] [CrossRef]
- Swec, D.M.; Mirtich, M.J.; Nir, D.; Banks, B.A. Comparison of protective coatings for infrared transmitting windows. J. Vac. Sci. Technol. A 1986, 4, 3030. [Google Scholar] [CrossRef]
- Anton, P.G.M.B.H. Characterization of Hard Coatings–Part, I. DLC Coatings; Anton Paar GmbH: Vienna, Austrian, 2021. [Google Scholar]
- Miyoshi, K.; Buckley, D.H. Adhesion and friction of single crystal diamond in contact with transition metal. Appl. Surf. Sci. 1980, 6161. [Google Scholar] [CrossRef]
- Shibuki, K.; Yagi, M.; Saijo, K.; Takatsu, S. Adhesion strength of diamond films on cemented carbide substrates. Surf. Coat. Technol. 1988, 3, 295. [Google Scholar] [CrossRef]
- Saijo, K.; Yagi, M.; Shibuki, K.; Takatsu, S. The improvement of the adhesion strength of diamond films. Surf. Coat. Technol. 1990, 43, 30. [Google Scholar] [CrossRef]
- Grill, A.; Meyerson, B.; Patel, A.V. Interface modifications for improving the adhesion of aC: H films to metals. J. Mater. Res. 1988, 3, 214. [Google Scholar] [CrossRef]
- Mccune, R.C. Stresses and Mechanical Properties. Mater. Res. Soc. 1989, 50, 261. [Google Scholar]
- Murakawa, M.; Takeuchi, S. Quantitative adhesion strength measurement of diamond coatings. Thin Solid Films 1989, 443–450. [Google Scholar] [CrossRef]
- Kuo, C.-T.; Yen, T.-Y.; Huang, A.T.-H. Adhesion and tribological properties of diamond films on various substrates. J. Mater. Res. 1990, 5, 2515–2523. [Google Scholar] [CrossRef] [Green Version]
- Galuska, A. A Copper glassy carbon adhesion: Improvement through 27 Al+ implantation. Surf. Coat. Technol. 1990, 975–985. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X.; Stritzker, A.B. Adhesion of hydrogenated amorphous carbon films on silicon substrates and its enhancement. Thin Solid Films 1991, 97, 57–66. [Google Scholar] [CrossRef]
- Hartnett, T.; Miller, R.; Montanari, D.; Willingham, C.; Tustison, R. Intermediate layers for the deposition of polycrystalline diamonds films. J. Vac. Sci. Technol. A 1990, 8, 2129. [Google Scholar] [CrossRef]
- Murakawa, M.; Watanabe, S. Tribological properties of cubics amorphous and hexagonal boron nitride films. Surf. Coat. Technol. 1990, 145. [Google Scholar] [CrossRef]
- Abermann, R.; Kramer, R.; Mäser, J. Structure and internal stress in ultra-thin silver films deposited on MgF2 and SiO substrate. Thin Solid Films 1978, 52, 215–229. [Google Scholar] [CrossRef]
- Thurner, G.; Abennaram, R. Internal stress and structure of ultra-high vacuum evaporated chromium and iron films and their dependence on substrate temperature and oxygen particle pressure during deposition. Thin Solid Films 1990, 192, 277–285. [Google Scholar] [CrossRef]
- Staudhammer, K.P.; Mutt, L.E. Atlas T_’Binaly Alloys; Marcel Dekker Inc.: New York, NY, USA, 1973. [Google Scholar]
- NBS. Selected Values of the Thermod. ’Namic Properties of Binal 3, Alloys; American Society for Metals: Metals Park, OH, USA, 1973. [Google Scholar]
- Metals Reference Book, 5th ed.; Butterworths: Boston, MA, USA, 1976.
- Kiryukhantsev-Korneev, P.; Sytchenko, A.; Sheveyko, A.; Moskovskikh, D.; Vorotylo, S. Two-Layer Nano composite TiC-Based Coatings Produced by a Combination of Pulsed Cathodic Arc Evaporation and Vacuum Electro-Spark Alloying. Materials 2020, 13, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitelaru, C.; Parau, A.C.; Constantin, L.R.; Kiss, A.E.; Vladescu, A.; Sobetkii, A.; Kubart, T. A Strategy for Alleviating Micro Arcing During HiPMS Deposition of DLC Coatings. Materials 2020, 13, 1038. [Google Scholar] [CrossRef] [Green Version]
- Meškinis, Š.; Vasiliauskas, A.; Andrulevičius, M.; Peckus, D.; Tamulevičius, S.; Viskontas, K. Diamond Like C Films Containing Si. Structure and Non-linear Optical Properties. Materials 2020, 13, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meškinis, Š.; Vasiliauskas, A.; Viskontas, K.; Andrulevičius, M.; Guobienė, A.; Tamulevičius, S. Hydrogen-Free Diamond Like C Films with Embedded Cu- Nanoparticles. Structure, Composition and Reverse Saturable Absorption Effect. Materials 2020, 13, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.T.; Hass, G.; Hunter, W.R. Infrared Reflectance of Silicon Oxide and Magnesium Fluoride Protected Aluminium Mirrors at Various Angles of Incidence From 8 µm to 12 µm. Appl. Opt. 1975, 14, 1247. [Google Scholar] [CrossRef]
- Lettington, A.H.; Ball, G.J. The Protection of Front Surfaced Aluminium Mirrors with Diamond-Like C Coatings for Use in the Infrared. RSRE Memo. 1981, 3295. [Google Scholar]
- Pellicori, S.F. Infrared Reflectance of a Variety of Mirrors at 480 Incidences. Appl. Opt. 1978, 17, 3335. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.T.; Hass, G. Aluminium Mirrors A1203 Protected with High Reflectance at Normal but Greatly Decreased Reflectance at Higher Angles of Incidence in the 8–12 µm Region. Appl. Opt. 1978, 17, 333. [Google Scholar] [CrossRef]
- Hahn, R.E.; Seraphin, B.O. Spectrally Selective Surfaces for Photo Thermal Energy Conversion. Phys. Thin Ftilms 1978, 10, 1. [Google Scholar]
- Drummeter, L.F.; Hass, G. Phys. Thin Films 1964, 2, 305.
- Seraphin, B.O. Chemical Vapour Deposition of Spectrally Selective Surfaces. J. Vac. Sci. Technol. 1979, 16, 193–196. [Google Scholar] [CrossRef]
- Janai, M.A.; Allred, D.D.; Booth, D.C.; Seraphin, B.O. Optical Properties and Structure of Amorphous Silicon Films Prepared by Cvd. Solar Energy Mater. 1979, 1, 11. [Google Scholar] [CrossRef]
- Ball, G.J.; Lettington, A.H. Diamond-Like C Coatings for the Photo Thermal Conversion of Solar Energy. RSRE Memo. 1983, 3617. [Google Scholar]
- Enke, K.; Dimigen, H.; Hubsch, H. Frictional Properties of Diamond-Like C Layers. Appl. Phys. Lett. 1980, 36, 291. [Google Scholar] [CrossRef]
- Bowden, F.P.; Young, J.E. Friction of Diamond, Graphite, and C and the Influence of Surface Films. Proc. R. Soc. Lond. A 1951, 208, 444. [Google Scholar]
- Kim, D.S.; Fischer, T.E.; Gallois, B. The Effects ofOand Humidity on Friction and Wear of Diamond-Like C Films. Surf. Coat. Technol. 1991, 49, 537. [Google Scholar] [CrossRef]
- Marchon, B.; Heiman, N.; Khan, M.R.; Lautie, A.; Ager, J.W.; Veirs, D.K. Raman And Resistivity Investigations of C Overcoats of Thin-Film Media. Correlations with Tibological Properties. J. Appl. Phys. 1991, 69, 5748. [Google Scholar] [CrossRef]
- Kim, S.B.; Wager, J.F. Diamond-Like C Films for Electroluminescent Applications. Surf. Coat. Technol. 1990, 43, 99. [Google Scholar] [CrossRef]
- Kapoor, V.J.; Mirtich, M.J.; Banks, B.A. Diamond-Like C Films on Semiconductors for Insulated-Gate Technology. J. Vac. Sci. Technol. 1986, 4, 1013. [Google Scholar] [CrossRef]
- Rothschild, M.; Arnone, C.; Ehrich, D.J. Eximer-Laser Etching of Diamond and Hard C Films by Direct Writing and Optical Projection. J. Vac. Sci. Technol. 1986, 4, 310. [Google Scholar] [CrossRef]
- Marotta, E.; Bakhru, N.; Grill, A.; Patel, V.; Meyerson, B. Diamond-Like C as an Electrical Insulator of Copper Devices for Chip Cooling. Thin Solid Films 1991, 206, 188–191. [Google Scholar] [CrossRef]
- Jenkins, G.M. Biomedical Applications of Cs and Graphites. Clin. Phys. Physiol. Meas. 1980, 1, 171–194. [Google Scholar] [CrossRef]
- Jenkins, G.M.; De Carvallo, F.X. Biomedical Applications of carbon Fibre Reinforced Cin Implanted Prostheses. Carbon 1977, 15, 33–37. [Google Scholar] [CrossRef]
- Thomson, L.A.; Law, F.C.; Rushton, N.; Franks, J. Biocompatibility of Diamond-Like. Coat. Biomater. 1991, 12, 37. [Google Scholar] [CrossRef]
- Feng, W.; Jiaqi, L.; Jianlu, X. The Studies of Diamond-Like C Films as Biomaterials. Review. Colloid Surf. Sci. 2017, 2, 81–95. [Google Scholar]
- Narin, S.; Shuichi, W.; Nutthanun, M. Elements-Added Diamond-Like C Film for Biomedical Applications. Adv. Mater. Sci. Eng. Hindawi 2019, 2019, 6812092. [Google Scholar]
- Zhou, H.; Jiang, M.; Xin, Y.; Sun, G.; Long, S.; Bao, S.; Cao, X.; Ji, S.; Jin, P. Surface Deposition of Graphene Layer for Bioactivity Improvement of Biomedical 316 Stainless Steel. Mater. Lett. 2017, 192, 123–127. [Google Scholar] [CrossRef]
- Amanov, A.; Lee, S.W.; Pyun, Y.S. Low Friction and High Strength of 316L Stainless Steel Tubing for Biomedical Applications. Mater. Sci. Eng. C 2017, 71, 176–185. [Google Scholar] [CrossRef]
- Bociaga, D.; Kaminska, M.; Sobczyk-Guzenda, A.; Jastrzebski, K.; Swiatek, L.; Olejnik, A. Surface Properties and Biological Behaviour of Si-DLC Coatings Fabricated by A Multi-Target DC-RF Magnetron Sputtering Method for Medical Applications. Diam. Relat. Mater. 2016, 67, 41–50. [Google Scholar] [CrossRef]
- Moolsradoo, N.; Watanabe, S. Influence of Elements on The Corrosion Resistance of DLC Films. Adv. Mater. Sci. Eng. 2017, 6, 3571454. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.C.; Pong, W.F. Papakonstantinou, Iron, Nitrogen and Silicon Doped Diamond Like C (DLC) Thin Films. A Comparative Study. Thin Solid Films 2016, 610, 42–47. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Huang, W.; Wang, L.; He, H.; Liu, C. The Investigation of The Structures and Tribological Properties Of F-DLC Coatings Deposited on Ti-6Al-4V Alloys. Surf. Coat. Technol. 2017, 316, 22–29. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Wang, Y.; Liu, L.; Li, B.; Li, Y.; Zhao, X. A Study on Poly (N-Vinyl-2-Pyrrolidone) Covalently Bonded Niti Surface For Inhibiting Protein Adsorption. Progress in Natural Science. Mater. Int. 2016, 26, 584–589. [Google Scholar]
- Kumar, A.M.; Suresh, B.; Das, S.; Obot, I.B.; Adesina, A.Y.; Ramakrishna, S. Promising Bio-Composites of Polypyrrole And Chitosan. Surface Protective and in Vitro Biocompatibility Performance on 316L SS Implants. Carbohydr. Polym. 2017, 173, 121–130. [Google Scholar] [CrossRef] [PubMed]
- PremKumar, K.P.; Duraipandy, N.; Syamala, K.N.; Rajendran, N. Antibacterial Effects, Biocompatibility and Electrochemical Behavior of Zinc Incorporated Niobium Oxide Coating On 316L SS For Biomedical Applications. Appl. Surf. Sci. 2018, 427, 1166–1181. [Google Scholar]
- Wachesk, C.C.; Trava-Airoldi, V.J.; Da-Silva, N.S.; Lobo, A.O.; Marciano, F.R. The Influence of Titanium Dioxide on Diamond-Like C Biocompatibility for Dental Applications. J. Nanomater. 2016, 7, 8194516. [Google Scholar] [CrossRef] [Green Version]
- Gotzmann, G.; Beckmann, J.; Wetzel, C.; Scholz, B.; Herrmann, U.; Neunzehn, J. Electron-Beam Modification of DLC Coatings for Biomedical Applications. Surf. Coat. Technol. 2017, 311, 248–256. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Szymanski, W.; Jedrzejczak, A.; Jastrzebska, A.; Olejnik, A.; Jastrzebski, K. Mechanical Properties, Chemical Analysis and Evaluation of Antimicrobial Response of Si-DLC Coatings Fabricated on AISI 316 LVM Substrate by A Multi-Target DC-RF Magnetron Sputtering Method for Potential Biomedical Applications. Appl. Surf. Sci. 2017, 417, 23–33. [Google Scholar] [CrossRef]
- Schäfer, L.; Gäbler, J.; Mulcahy, S.; Brand, J.; Hieke, A.; Wittorf, R. Tribological Applications of Amorphous C and Crystalline Diamond Coatings. In Proceedings of the 43rd Svc Ann. Techn. Conf., Denver, KL, USA, 3 April 2000. [Google Scholar]
- Hlin, M.; Hedenqvist, P. ‘Me-C. H Coatings in Motor Vehicles’. Wear 2001, 249, 302–309. [Google Scholar]
- Brand, J.; Beckmann, C.; Filfil, T.M.A.T. Reduzierung Von Reibungsverlusten Im Ventiltrieb Durch Besichtungen; Vdi Report, Vdi: Du Sseldorf, Germany, 1999; Volume 1472, pp. 299–312. [Google Scholar]
- Muthuraja, A.; Naik, S.; Rajak, D.K.; Pruncu, C.I. Experimental investigation on chromium-diamond like carbon (Cr-DLC) coating through plasma enhanced chemical vapour deposition (PECVD) on the nozzle needle surface. Diam. Relat. Mater. 2019, 100, 107588. [Google Scholar] [CrossRef]
- Grigoriev, S.; Volosova, M.; Fyodorov, S.; Lyakhovetskiy, M.; Seleznev, A. DLC-coating Application to Improve the Durabilityof Ceramic Tools. J. Mater. Eng. Perform. 2019, 28, 4415–4426. [Google Scholar] [CrossRef]
- Podgornik, S.J.; Hogmark, S. Dlc Coating of Boundary Lubricated Components. Advantages of Coating One of the Contact Surfaces Rather Than Both or None. Tribol. Int. 2003, 36, 843–849. [Google Scholar] [CrossRef]
- Persson, K.; Gahlin, R. Tribological Performance of a Dlc Coating in Combination with Water-Based Lubricants. Tribol. Int. 2003, 36, 851–855. [Google Scholar] [CrossRef]
- Kolawole, F.O.; Kolawole, S.K.; Varela, L.B.; Owa, A.F.; Ramirez, M.A.; Tschiptschin, A.P. Diamond-Like C (DLC) Coatings for Automobile Applications. In Engineering Applications of Diamond; IntechOpen: London, OH, USA, 2020. [Google Scholar]
| Kevlar Polyethylene PolyCate (Lexan) Poly (Styrenec Arylate) Silicon (Si) | 304 Stainless steel 440 Stainless steel Iron Tantalum Aluminum (Al) |
| Materials | Linear Coefficient of Thermal Expansion (at 20 °C) × 10−60C−1 | Modulus of Elasticity × 1011 (Pa) | Density (g cm−3) | Reference |
|---|---|---|---|---|
| Al | 23 | 0.706 | 2.702 | [91,92,93] |
| Al2O3 | 5.3 | 4.0 | 3.965 | [91,93,94] |
| Chromium | 5.0 | 2.79 | 7.20 | [91,92,93] |
| Cu | 16.7 | 1.298 | 8.92 | [91,92,93] |
| Diamond | 1.0 | 9.1 | 3.51 | [91,93,94] |
| Germanium | 5.7 | ----- | 5.35 | [91,92,93] |
| Glass | 4–9 | 0.48–0.83 | ----- | [91,95] |
| Graphite | 7.8 | ----- | 2.25 | [91,92,93] |
| Fe | 11.8 | 1.523–2.114 | 7.86 | [91,92,93] |
| Molybdenum | 5.0 | 3.248 | 10.2 | [91,92,93] |
| Ni | 12.8 | 1.995–2.192 | 8.9 | [91,92,93] |
| Platinum | 8.9 | 1.67 | 21.45 | [91,92,93] |
| Si | 2.5 | 6.6 | 2.33 | [91,93,96] |
| SiC | 3.7 | 4.8 | 3.217 | [91,93,94] |
| SiO2 | 11.2 | ----- | 2.64 | [91,92,93] |
| NaCl | 42.3 | ----- | 2.165 | [91,92,93] |
| Stainless Steel | 15.9 | 2.153 | ----- | [91,92,93] |
| Ti | 8.6 | 4.7 | 4.5 | [91,92,93] |
| Tic | 6.2 | ----- | 4.93 | [91,92,93] |
| Tungsten | 4.5 | 4.11 | 19.35 | [91,92,93] |
| Uranium | 14.1 | ----- | 19.05 | [91,92,93] |
| Film Stress (Pa) (C) = compressive (T) = tensile | Film Composition | Deposition Process | Reference |
|---|---|---|---|
| 5. To 7 × 109 (C) | DLC on glass | Glow discharge | [97] |
| 5 × 108 (C) | DLC on glass | E-beam heated C rod | [98] |
| 7.5× 108 (C) To 9.6 × 108 (T) | DLC on glass | Bias sputtered and plasma deposited | [99] |
| 3. To 4 × 109 (C) | DLC on quartz glass and Si | Ion source | [100] |
| 0.5 To 3. × 109 (C) | DlCon Si | Ion source | [101,102] |
| 1.6 × 109 (C) | DLC on glass | RF magnetron sputter | [103] |
| ~ 109 (C) | DlC on ZnS and ZnSe | Ion source | [104] |
| 1.2 × 109 (C) | DLC on Si | DC plasma reactor | [105] |
| 9.4 To 139 × 106 (T) | Diamond on Si | Microwave plasma | [106] |
| 2.1 To 4.7 × 109 (C) | Diamond on Si (100) | Filament | [107,108] |
| Compound | H°f (25 °C) (kcal mol−1) | H°f (25 °C) (kcal mol−1) | Linear Coeff. of Thermal Expansion (at 25 °C) × 10−60C−1 | Reference |
|---|---|---|---|---|
| Al4C3 | −30.9 | −29 | ----- | [120] |
| CoC3 | 9.5 | 7.1 | ----- | [120] |
| Cr3C2 | −21 | −21.2 | 8.0 | [120,121] |
| Cr3C2 | −9 to −29 | −11 to −31 | ----- | [122] |
| Fe3C | 5.0 | 3.5 | ----- | [120] |
| MoC | 34.4 | ----- | 5.95 | [121] |
| Mo3C2 | −14 | −14.1 | ----- | [123] |
| Mo2C | −11.5 | −12.5 | 5.48 | [121,123] |
| Mo2C | ----- | ----- | 7.8 to 9.3 | [94] |
| Ni3C | 11.0 | ----- | ----- | [120] |
| SiC | −16.5 | −15.9 | 4.63 | [121,123] |
| TaC | −34.6 | −34.6 | 8.2 | [121,123] |
| TaC | −32.3 to −53 | −35 to −56 | 7.1 | [94,122] |
| Ta2C | −47 | −47 | ----- | [123] |
| TiC | −43.8 | −43.8 | 6.52 | [121,123] |
| TiC | −44.1 | −45.8 | 8.0 to 8.6 | [94,122] |
| UC | −21.1 | ----- | 9.47 | [121,122] |
| UC2 | 21 to 30 | ----- | 6.32 | [121,122] |
| U2C3 | −54 | ----- | 6.26 | [120,121] |
| U2C3 | −49 | ----- | ----- | [122] |
| WC | −9.09 | −8.5 | 4.9 | [120,121,122] |
| WC | −8.4 | ----- | 3.8 to 3.9 | [94,123] |
| W2C | −6.3 to −9.7 | ----- | ----- | [122] |
| Adhesion Strength (Pa) | Type of Film Process | Deposition | Measurement Technique | Reference |
|---|---|---|---|---|
| 1 to 3 × 109 (0.1 to 0.3 J m−2) | DLC on glass | e-beam heated C rod | Film buckling analysis | [98] |
| 4 to 7 × 1010 4 to 7 J m−2) | DLC on glass | Electron and ion beam source | Film buckling analysis | [100] |
| > 2. to 2.8 × 107 (failure occurred in substrate) | DLC on ZnS (ZnSe with 200 to 1000 Ge or Si interlayer) | Iron source | Sebastation pull test | [124,125] |
| 2 × 109 | DLC on glass | RF magnetron sputtering | Scratch test | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. https://doi.org/10.3390/app11104445
Rajak DK, Kumar A, Behera A, Menezes PL. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Applied Sciences. 2021; 11(10):4445. https://doi.org/10.3390/app11104445
Chicago/Turabian StyleRajak, Dipen Kumar, Ashwini Kumar, Ajit Behera, and Pradeep L. Menezes. 2021. "Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications" Applied Sciences 11, no. 10: 4445. https://doi.org/10.3390/app11104445
APA StyleRajak, D. K., Kumar, A., Behera, A., & Menezes, P. L. (2021). Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Applied Sciences, 11(10), 4445. https://doi.org/10.3390/app11104445









