Recent Advances in Myoelectric Control for Finger Prostheses for Multiple Finger Loss
Abstract
:1. Introduction
2. Methodology
3. Signals and Finger Motor Function
4. Cosmetic Gloves for Myoelectric Prosthetic Hand or Fingers
5. Osseointegrated Implant Retained Myoelectric Prosthesis
6. Discussion
7. Future Perspectives in Myoelectric Prosthesis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aydin, C.; Nemli, S.K.; Yilmaz, H. Esthetic, functional, and prosthetic outcomes with implant-retained finger prostheses. Prosthet. Orthot. Int. 2013, 37, 168–174. [Google Scholar] [CrossRef]
- Aydin, C.; Karakoca, S.; Yilmaz, H. Implant-retained digital prostheses with custom-designed attachments: A clinical report. J. Prosthet. Dent. 2007, 97, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Manurangsee, P.; Isariyawut, C.; Chatuthong, V.; Mekraksawanit, S. Osseointegrated finger prosthesis: An alternative method for finger reconstruction. J. Hand Surg. 2000, 25, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Thongpulsawasdi, N.; Amornvit, P.; Rokaya, D.; Keawcharoen, K. Adhesive vs Implant Retained Fingers Prosthesis: A Comparative Study on Esthetic and Functional Outcome. World Appl. Sci. J. 2014, 29, 1015–1019. [Google Scholar]
- Rokaya, D.; Kitisubkanchana, J.; Wonglamsam, A.; Santiwong, P.; Srithavaj, T.; Humagain, M. Nepalese Esthetic Dental (NED) Proportion in Nepalese Population. Kathmandu Univ. Med. J. 2015, 13, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aszmann, O.C.; Vujaklija, I.; Roche, A.D.; Salminger, S.; Herceg, M.; Sturma, A.; Hruby, L.A.; Pittermann, A.; Hofer, C.; Amsuess, S.; et al. Elective amputation and bionic substitution restore functional hand use after critical soft tissue injuries. Sci. Rep. 2016, 6, 34960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humagain, M.; Rokaya, D. Integrating Digital Technologies in Dentistry to Enhance the Clinical Success. Kathmandu Univ. Med. J. 2019, 17, 256–257. [Google Scholar]
- Chang, Y.S. Advances in technology are changing the future of medicine. Asia Pac. Allergy 2016, 6, 137–138. [Google Scholar] [CrossRef] [Green Version]
- Adam, N.R.; Wieder, R.; Ghosh, D. Data science, learning, and applications to biomedical and health sciences. Ann. N. Y. Acad. Sci. 2017, 1387, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Avetisyan, A.; Markaryan, M.; Rokaya, D.; Tovani-Palone, M.R.; Zafar, M.S.; Khurshid, Z.; Vardanyan, A.; Heboyan, A. Characteristics of Periodontal Tissues in Prosthetic Treatment with Fixed Dental Prostheses. Molecules 2021, 26, 1331. [Google Scholar] [CrossRef]
- Weir, R.; Grahn, E.; Duff, S. A new externally powered, myoelectrically controlled prosthesis for persons with partial-hand amputations at the metacarpals. J. Prosthet. Orthot. 2001, 13, 26–31. [Google Scholar] [CrossRef]
- Agnew, P.J. Functional effectiveness of a myo-electric prosthesis compared with a functional split-hook prosthesis: A single subject experiment. Prosthet. Orthot. Int. 1981, 5, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Pistohl, T.; Cipriani, C.; Jackson, A.; Nazarpour, K. Abstract and proportional myoelectric control for multi-fingered hand prostheses. Ann. Biomed. Eng. 2013, 41, 2687–2698. [Google Scholar] [CrossRef] [Green Version]
- Salem, F.H.A.; Mohamed, K.S.; Mohamed, S.B.K.; Gehani, A.A.E. The development of body-powered prosthetic hand controlled by EMG signals using DSP processor with virtual prosthesis implementation. Conf. Pap. Eng. 2013, 598945. [Google Scholar] [CrossRef] [Green Version]
- Montagnani, F.; Controzzi, M.; Cipriani, C. Independent long fingers are not essential for a grasping hand. Sci. Rep. 2016, 6, 35545. [Google Scholar] [CrossRef] [Green Version]
- Khushaba, R.N.; Al-Timemy, A.; Kodagoda, S.; Nazarpour, K. Combined influence of forearm orientation and muscular contraction on EMG pattern recognition. Expert Syst. Appl. 2016, 61, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Touillet, A.; Peultier-Celli, L.; Nicol, C.; Jarrassé, N.; Loiret, I.; Martinet, N.; Paysant, J.; De Graaf, J.B. Characteristics of phantom upper limb mobility encourage phantom-mobility-based prosthesis control. Sci. Rep. 2018, 8, 15459. [Google Scholar] [CrossRef]
- Schieber, M.H.; Lang, C.E.; Reilly, K.T.; McNulty, P.; Sirigu, A. Selective activation of human finger muscles after stroke or amputation. Adv. Exp. Med. Biol. 2009, 629, 559–575. [Google Scholar]
- Wang, N.; Lao, K.; Zhang, X. Design and myoelectric control of an anthropomorphic prosthetic hand. J. Bionic Eng. 2017, 14, 47–59. [Google Scholar] [CrossRef]
- Atzori, M.; Cognolato, M.; Müller, H. Deep learning with convolutional neural networks applied to electromyography data: A resource for the classification of movements for prosthetic hands. Front. Neurorobot. 2016, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Chadwell, A.; Kenney, L.; Granat, M.H.; Thies, S.; Head, J.; Galpin, A.; Baker, R.; Kulkarni, J. Upper limb activity in myoelectric prosthesis users is biased towards the intact limb and appears unrelated to goal-directed task performance. Sci. Rep. 2018, 8, 11084. [Google Scholar] [CrossRef]
- Urbanchek, M.G.; Baghmanli, Z.; Moon, J.D.; Sugg, K.B.; Langhals, N.B.; Cederna, P.S. Quantification of regenerative peripheral interface signal transmission. Plast. Reconstr. Surg. 2012, 130, 55–56. [Google Scholar] [CrossRef] [Green Version]
- Chestek, C.A.; Gilja, V.; Nuyujukian, P.; Foster, J.D.; Fan, J.M.; Kaufman, M.T.; Churchland, M.M.; Rivera-Alvidrez, Z.; Cunningham, J.P.; Ryu, S.I.; et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural Eng. 2011, 8, 045005. [Google Scholar] [CrossRef] [Green Version]
- Kuiken, T.A.; Li, G.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 2009, 301, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Atzori, M.; Müller, H. Control capabilities of myoelectric robotic prostheses by hand amputees: A scientific research and market overview. Front. Neurosci. 2015, 9, 162. [Google Scholar] [CrossRef]
- Engdahl, S.M.; Christie, B.P.; Kelly, B.; Davis, A.; Chestek, C.A.; Gates, D.H. Surveying the interest of individuals with upper limb loss in novel prosthetic control techniques. J. NeuroEng. Rehabil. 2015, 12, 53. [Google Scholar] [CrossRef] [Green Version]
- Khushaba, R.N.; Kodagoda, S.; Takruri, M.; Dissanayake, G. Toward improved control of prosthetic fingers using surface electromyogram (EMG) signals. Exp. Syst. Appl. 2012, 39, 10731–10738. [Google Scholar] [CrossRef]
- Isaković, M.; Malešević, J.; Keller, T.; Kostić, M.; Štrbac, M. Optimization of semiautomated calibration algorithm of multichannel electrotactile feedback for myoelectric hand prosthesis. Appl. Bionics Biomech. 2019, 9298758. [Google Scholar] [CrossRef] [PubMed]
- Došen, S.; Cipriani, C.; Kostić, M.; Controzzi, M.; Carrozza, M.C.; Popović, D.B. Cognitive vision system for control of dexterous prosthetic hands: Experimental evaluation. J. NeuroEng. Rehabil. 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Light, C.M.; Chappell, P.H.; Hudgins, B.; Engelhart, K. Intelligent multifunction myoelectric control of hand prostheses. J. Med. Eng. Technol. 2002, 26, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cotton, D.P.J.; Cranny, A.; Chappell, P.H.; White, N.M.; Beeby, S.P. Control strategies for a multiple degree of freedom prosthetic hand. Meas. Control. 2007, 40, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Gijsberts, A.; Atzori, M.; Castellini, C.; Müller, H.; Caputo, B. Movement error rate for evaluation of machine learning methods for sEMG-based hand movement classification. IEEE Trans. Neural. Syst. Rehabil. Eng. 2014, 22, 735–744. [Google Scholar] [CrossRef]
- Mnih, V.; Kavukcuoglu, K.; Silver, D.; Rusu, A.A.; Veness, J.; Bellemare, M.G.; Graves, A.; Riedmiller, M.; Fidjeland, A.K.; Ostrovski, G.; et al. Human-level control through deep reinforcement learning. Nature 2015, 518, 529–533. [Google Scholar] [CrossRef]
- Lange, F. Lehrbuch der Orthopädie; Gustav Fischer: New York, NY, USA, 1922. [Google Scholar]
- Weiner, N. Cybernetics, or Control and Communication in the Animal and the Machine; Wiley: New York, NY, USA, 1948. [Google Scholar]
- Reiter, R. Eine neue Elektrokunsthand. Grenzgeb Med. 1968, 4, 133–135. [Google Scholar]
- Sherman, E.D. A Russian bioelectric-controlled prosthesis: Report of a research team from the Rehabilitation Institute of Montreal. Can. Med. Assoc. J. 1964, 91, 1268–1270. [Google Scholar]
- Scott, R.N.; Tucker, F.R. Surgical implications of myoelectric control. Clin. Orthop. Relat. Res. 1968, 61, 248–260. [Google Scholar] [CrossRef]
- Dunfield, V.A.; Scott, R.N. A surgically implanted myotelemetry unit. In Proceedings of the 3rd Canadian Medical and Biological Engineering Conference, Halifax, NS, Canada, 9–11 September 1970; p. 32. [Google Scholar]
- Herberts, P.; Petersen, I. Possibilities for control of powered devices by myoelectric signals. Scand. J. Rehabil. Med. 1970, 2, 164–170. [Google Scholar]
- Englehart, K.B.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef]
- Belter, J.T.; Segil, J.L.; Dollar, A.M.; Weir, R.F. Mechanical design and performance specifications of anthropomorphic prosthetic hands: A review. J. Rehabil. Res. Dev. 2013, 50, 599–618. [Google Scholar] [CrossRef]
- Touch Bionics. Available online: http://www.touchbionics.com/ (accessed on 25 March 2021).
- Vincent Hand. Available online: http://handprothese.de/vincent-hand/ (accessed on 25 March 2021).
- SensorHand Speed. Available online: http://www.ottobock.com/cps/rde/xchg/ob_com_en/hs.xsl/3652.html (accessed on 25 March 2021).
- RSL Steeper. Available online: http://rslsteeper.com/ (accessed on 25 March 2021).
- Michelangelo Prosthetic Hand. Available online: https://www.ottobockus.com/prosthetics/upper-limb-prosthetics/solution-overview/michelangelo-prosthetic-hand/ (accessed on 25 March 2021).
- Yabuki, Y.; Tanahashi, K.; Mouri, Y.; Murai, Y.; Togo, S.; Kato, R.; Jiang, Y.; Yokoi, H. Development of new cosmetic gloves for myoelectric prosthetic hand using superelastic rubber. Rob. Auton. Syst. 2019, 111, 31–43. [Google Scholar] [CrossRef]
- Sareen, A.; Singh, A.; Sinha, A.; Arya, A.; Arya, A.; Sapra, G.; Kumar, R.; Kumar, P.; Singh, D. Design and fabrication of prosthetic glove for hand rehabilitation. Mater. Today Proc. 2020, 28, 1612–1615. [Google Scholar] [CrossRef]
- Lundborg, G.; Brånemark, P.I.; Rosén, B. Osseointegrated thumb prostheses: A concept for fixation of digit prosthetic devices. J. Hand Surg. 1996, 21, 216–221. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. 1977, 16, 1–132. [Google Scholar]
- Amornvit, P.; Rokaya, D.; Keawcharoen, K.; Raucharernporn, S.; Thongpulsawasdi, N. One- vs two stage surgery technique for implant placement in finger prosthesis. J. Clin. Diagn. Res. 2013, 7, 1956–1968. [Google Scholar]
- Amornvit, P.; Rokaya, D.; Keawcharoen, K.; Raucharernporn, S.; Thongpulsawasdi, N.; Alam, M.K. One-Stage Surgery Technique for Implant Placement in Finger Prosthesis: A Clinical Note. Int. Med. J. 2015, 22, 309–312. [Google Scholar]
- Rahyussalim, J.; Marsetio, A.F.; Saleh, I.; Kurniawati, T.; Whulanza, Y. The needs of current implant technology in orthopaedic prosthesis biomaterials application to reduce prosthesis failure rate. J. Nanomater. 2016, 5386924. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, K.; Brånemark, R.; Gunterberg, B.; Rydevik, B. Osseointegrated trans-femoral amputation prostheses: Prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet. Orthot. Int. 2008, 32, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, R.; Brånemark, R.; Olmarker, K.; Rydevik, B.; Van Steenberghe, D.; Brånemark, P.I. Evaluation of the psychophysical detection threshold level for vibrotactile and pressure stimulation of prosthetic limbs using bone anchorage or soft tissue support. Prosthet. Orthot. Int. 2000, 24, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jönsson, S.; Caine-Winterberger, K.; Brånemark, R. Osseointegration amputation prostheses on the upper limbs: Methods, prosthetics and rehabilitation. Prosthet. Orthot. Int. 2011, 35, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Lundborg, G.; Waites, A.; Björkman, A.; Rosén, B.; Larsson, E.-M. Functional magnetic resonance imaging shows cortical activation on sensory stimulation of an osseointegrated prosthetic thumb. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2006, 40, 234–239. [Google Scholar] [CrossRef]
- Bates, T.J.; Fergason, J.R.; Pierrie, S.N. Technological Advances in Prosthesis Design and Rehabilitation Following Upper Extremity Limb Loss. Curr. Rev. Musculoskelet. Med. 2020, 13, 485–493. [Google Scholar] [CrossRef] [PubMed]
- e-OPRA Implant System for Lower Limb Amputees. Available online: https://clinicaltrials.gov/ct2/show/NCT03720171 (accessed on 12 March 2021).
- Ortiz-Catalan, M.; Hakansson, B.; Branemark, R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci. Transl. Med. 2014, 6, 257re6. [Google Scholar] [CrossRef] [PubMed]
- Mastinu, E.; Clemente, F.; Sassu, P.; Aszmann, O.; Brånemark, R.; Håkansson, B.; Controzzi, M.; Cipriani, C.; Ortiz-Catalan, M. Grip control and motor coordination with implanted and surface electrodes while grasping with an osseointegrated prosthetic hand. J. Neuroeng. Rehabil. 2019, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Roche, D.; Rehbaum, H.; Farina, D.; Aszmann, O.C. Prosthetic myoelectric control strategies: A clinical perspective. Curr. Surg. Rep. 2014, 2, 44. [Google Scholar] [CrossRef]
- Geethanjali, P. Myoelectric control of prosthetic hands: State-of-the-art review. Med. Devices 2016, 9, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Igual, C.; Pardo, L.A., Jr.; Hahne, J.M.; Igual, J. Myoelectric Control for Upper Limb Prostheses. Electronics 2019, 8, 1244. [Google Scholar] [CrossRef]
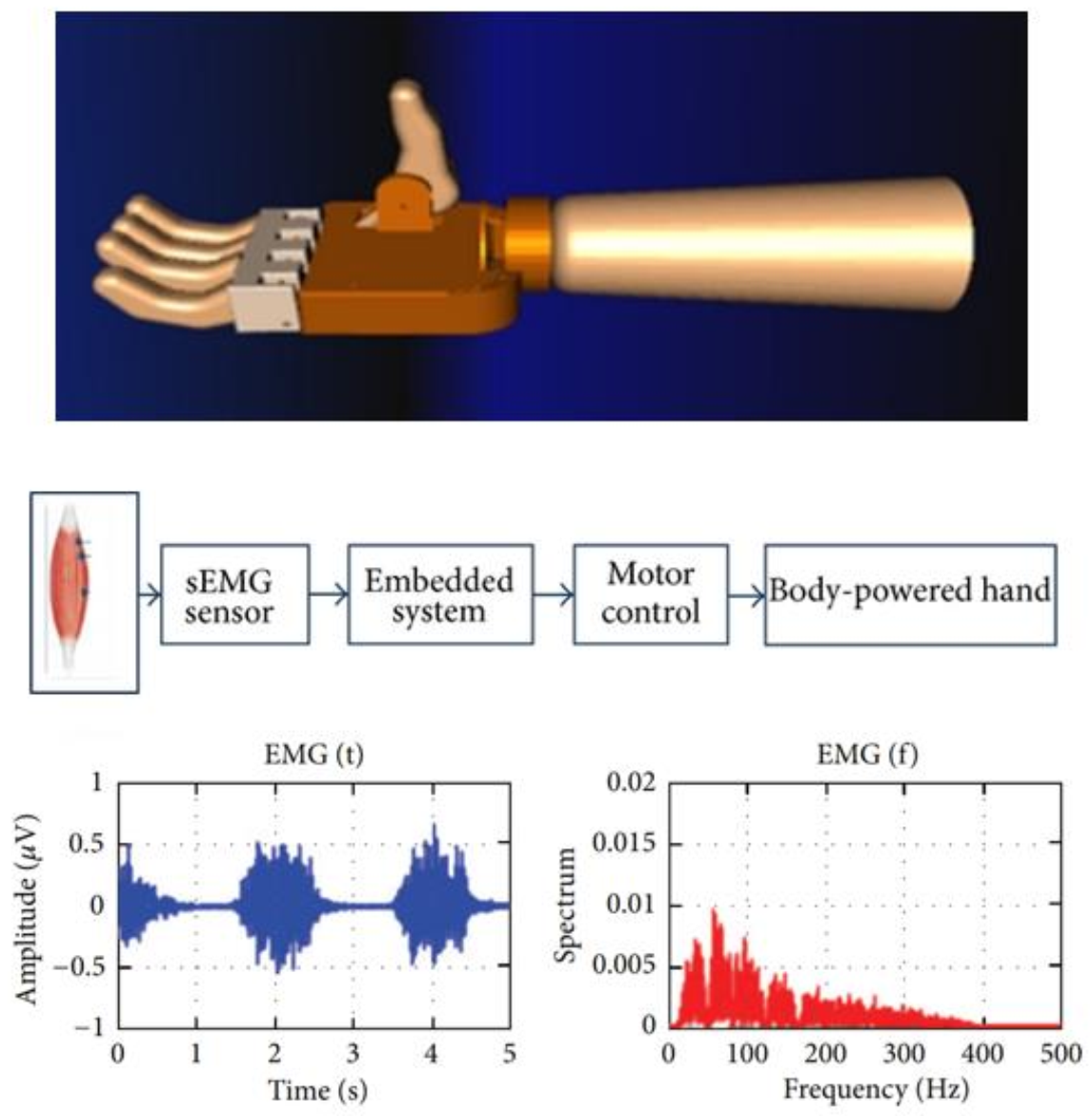
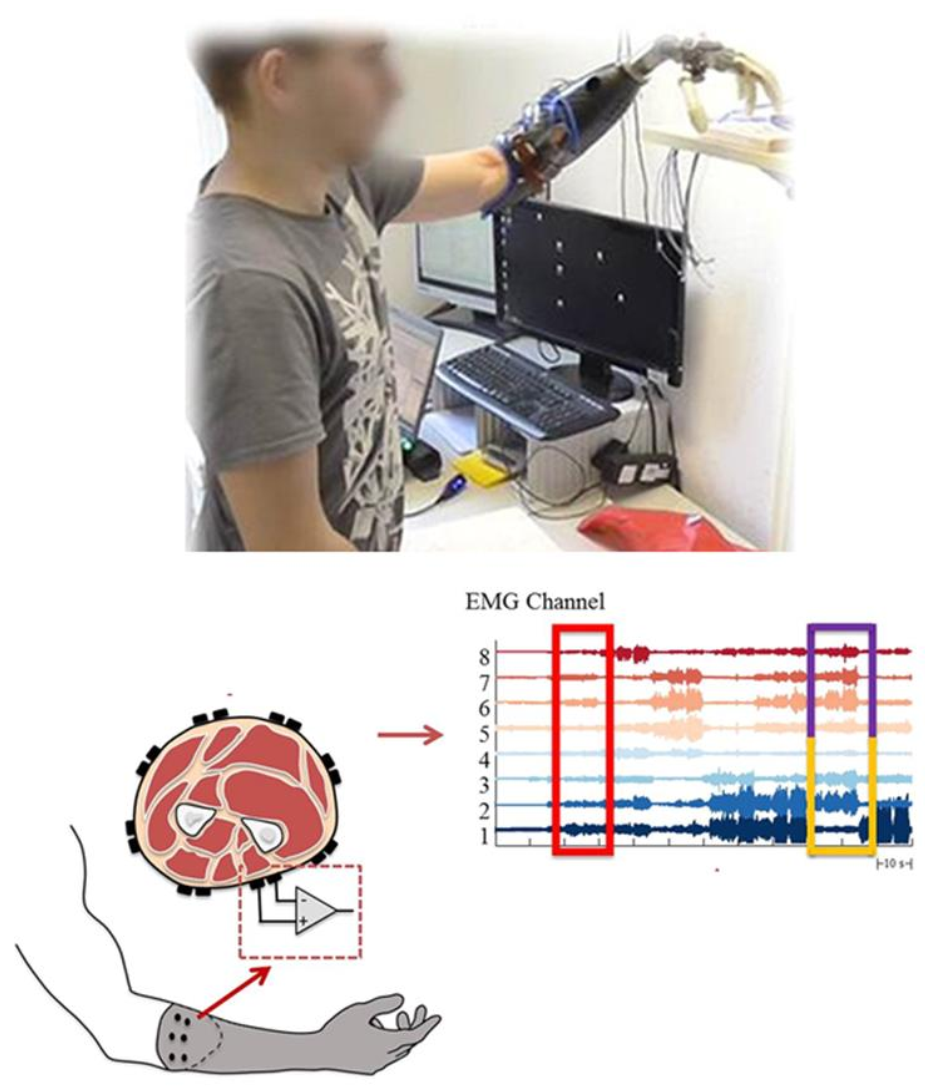
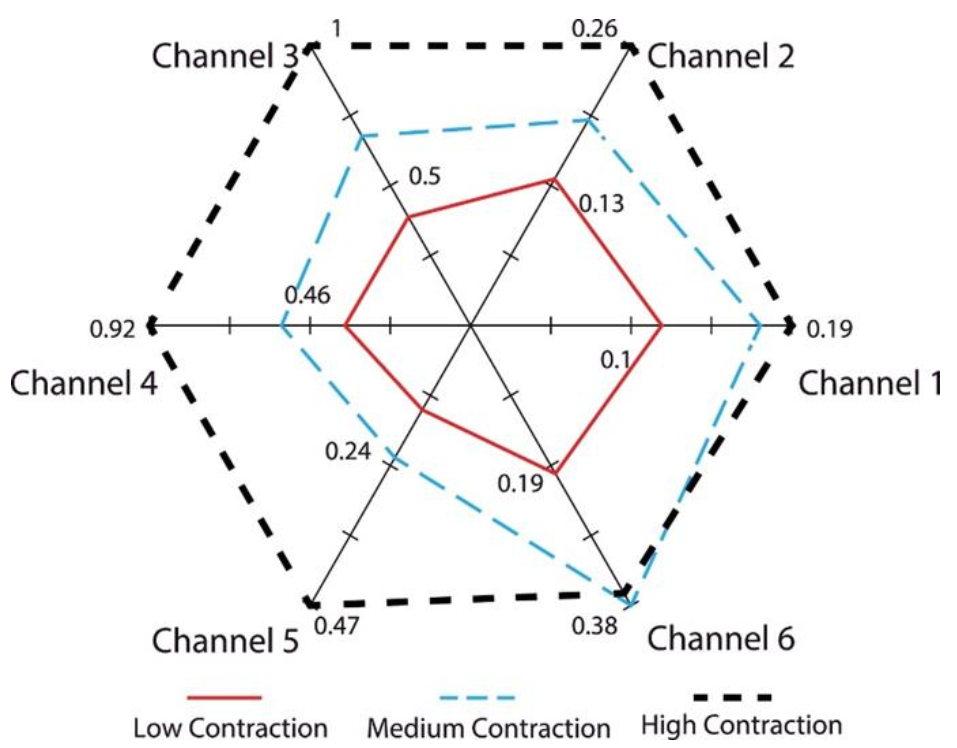

| Year | Developments | Study |
|---|---|---|
| 1922 | Passive hooks and shoulder harness were designed by Weimar. | [34] |
| 1948 | The concept of the prosthesis ideally should replace not only mechanical function but also cutaneous and kinesthetic sensation. | [35] |
| 1948 | Myoelectric control was first implemented by Reinhold Reiter, a physics student at Munich University and patent application. | [36] |
| 1968 | The first commercial myoelectric hands were available from the middle of the 1960s. | [37] |
| 1968 | The concept of natural appearance during function “dynamic cosmesis” which contributes to the complexity of design both in terms of segmental trajectories and in terms of mechanical noise is highlighted. | [38] |
| 1970 onwards | Various advancements in the Myoelectric control prostheses through research and training systems. Availability of advanced commercial limb prostheses, such as the i-Limb from Touch Bionics, BeBionic from RSL Steeper, and Michelangelo by Ottobock. | [11,14,19,20,21,24,39,40,41,42] |
| Developed Year | Name | Producer | Size | Number of Joints | Degree of Freedom | Joint Coupling Method | Study |
|---|---|---|---|---|---|---|---|
| 2009 | i-Limb | Touch Bionics | 180–18-mm-long, 80–75-mm-wide, 35–41-mm-thick | 11 | 6 | Tendron linking MCP to PIP | [43] |
| 2010 | Vincent Hand | Vincent Systems | - | 11 | 6 | Linkage spanning MCP to PIP | [44] |
| 2010 | i-Limb Pulse | Touch Bionics | 180–182-mm-long, 80–75-mm-wide, 35–45-mm-thick | 11 | 6 | Tendron linking MCP to PIP | [43] |
| 2011 | SensorHand | Otto Bock | Glove sizes | 2 | 1 | DC Motor Worm Gear | [45] |
| 2011 | BeBionic | RSL Steeper | 198-mm-long, 90-mm-wide, 50-mm-thick | 11 | 6 | DC Motor Worm Gear | [46] |
| 2011 | BeBionic V2 | RSL Steeper | 190–200-mm-long, 84–92-mm-wide, 50-mm-thick | 11 | 6 | DC Motor Lead Screw | [46] |
| 2012 | Michelangelo | Otto Bock | - | 6 | 2 | - | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srimaneepong, V.; Heboyan, A.; Syed, A.U.Y.; Trinh, H.A.; Amornvit, P.; Rokaya, D. Recent Advances in Myoelectric Control for Finger Prostheses for Multiple Finger Loss. Appl. Sci. 2021, 11, 4464. https://doi.org/10.3390/app11104464
Srimaneepong V, Heboyan A, Syed AUY, Trinh HA, Amornvit P, Rokaya D. Recent Advances in Myoelectric Control for Finger Prostheses for Multiple Finger Loss. Applied Sciences. 2021; 11(10):4464. https://doi.org/10.3390/app11104464
Chicago/Turabian StyleSrimaneepong, Viritpon, Artak Heboyan, Azeem Ul Yaqin Syed, Hai Anh Trinh, Pokpong Amornvit, and Dinesh Rokaya. 2021. "Recent Advances in Myoelectric Control for Finger Prostheses for Multiple Finger Loss" Applied Sciences 11, no. 10: 4464. https://doi.org/10.3390/app11104464
APA StyleSrimaneepong, V., Heboyan, A., Syed, A. U. Y., Trinh, H. A., Amornvit, P., & Rokaya, D. (2021). Recent Advances in Myoelectric Control for Finger Prostheses for Multiple Finger Loss. Applied Sciences, 11(10), 4464. https://doi.org/10.3390/app11104464








