Phytochemical Analysis of Symphytum officinale Root Culture Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Objects
2.2. Extraction of Biologically Active Components
2.3. Determination of the Contents of Individual Phenolic Compounds by HPLC
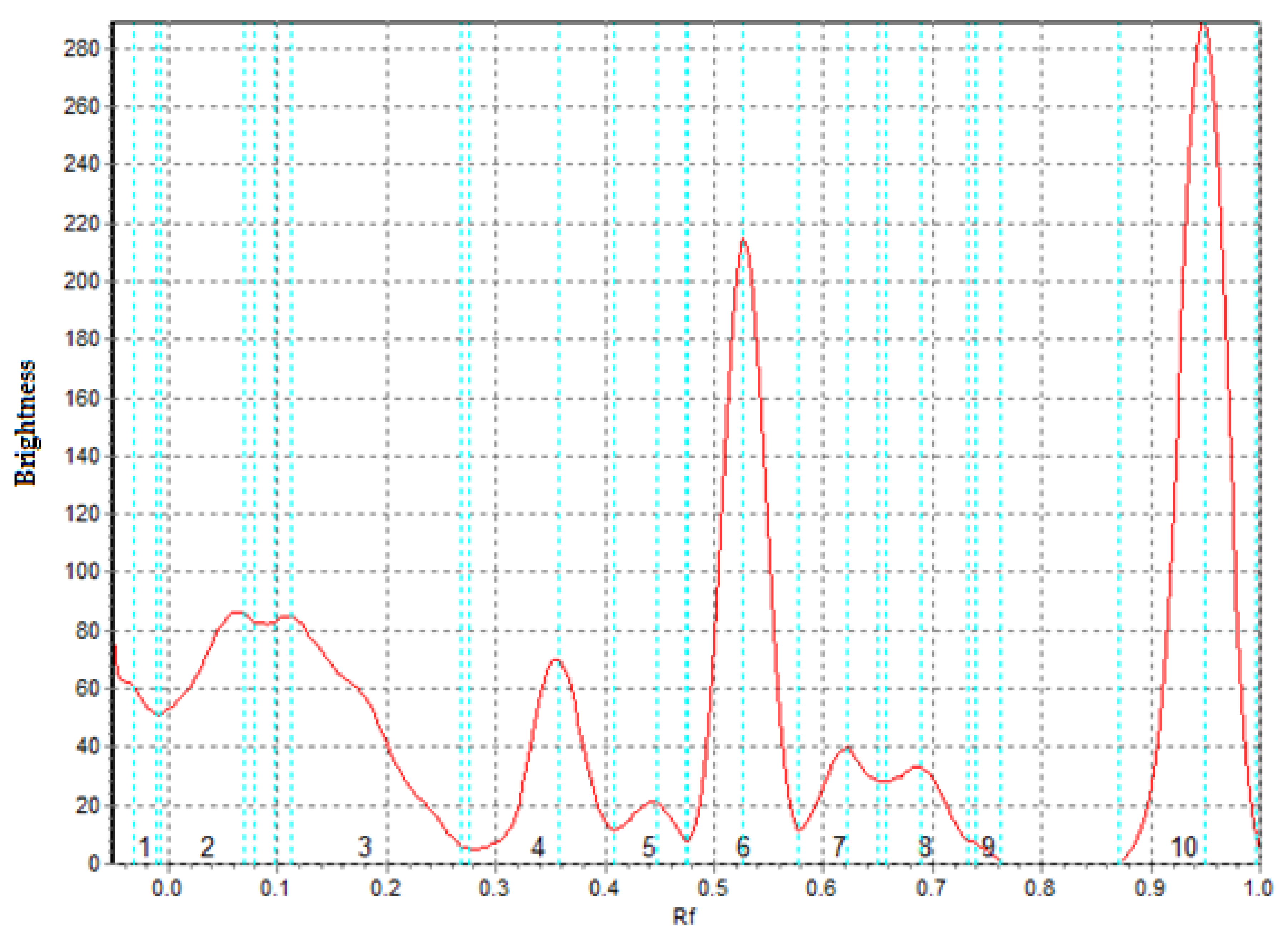
2.4. Determination of the Contents of Individual Phenolic Compounds by Layer Chromatography
2.5. Determination of Anti-Inflammatory Activity
2.5.1. Cellular Enzyme-Linked Immunoassay
2.5.2. Cyclooxygenase Assays
2.5.3. Immunofluorescence Microscopy
2.6. Determination of Antioxidant Activity by Amperometric Method
2.7. Determination of Antioxidant Activity by Spectrophotometric Method (DPPH-Method)
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Trifan, A.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; Kruk, A.; Marcourt, L.; Wolfender, J.-L.; Wolfram, E.; Esslinger, N.; Grubelnik, A.; et al. Symphytum officinale L.: Liquid-liquid chromatography isolation of caffeic acid oligomers and evaluation of their influence on pro-inflammatory cytokine release in LPS-stimulated neutrophils. J. Ethnopharmacol. 2020, 262, 113169. [Google Scholar] [CrossRef]
- Ulubelen, A.; Doğanca, S. Anadoline, a new senecio alkaloid from symphytum orientale. Tetrahedron Lett. 1970, 11, 2583–2585. [Google Scholar] [CrossRef]
- Frost, R.; MacPherson, H.; O’Meara, S. A critical scoping review of external uses of comfrey (Symphytum spp.). Complement. Ther. Med. 2013, 21, 724–745. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sinan, K.I.; Ak, G.; Angeloni, S.; Maggi, F.; Capriolib, G.; Kaplan, A.; Çakılcıoğlu, U.; Akan, H.; Jugreet, S.; et al. Preliminary investigation on chemical composition and bioactivity of differently obtained extracts from Symphytum aintabicum Hub.- Mor. &Wickens. Biochem. Syst. Ecol. 2021, 94, 104203. [Google Scholar] [CrossRef]
- Seigner, J.; Junker-Samek, M.; Plaza, A.; D’Urso, G.; Masullo, M.; Piacente, S.; Holper-Schichl, Y.M.; de Martin, R. A symphytum officinale root extract exerts anti-inflammatory properties by affecting two distinct steps of NF-κB signaling. Front. Pharmacol. 2019, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Vostinaru, O.; Conea, S.; Mogosan, C.; Toma, C.C.; Borza, C.C.; Vlase, L. Anti-inflammatory and antinociceptive effect of Symphytum officinale root. Rom. Biotechnol. Lett. 2018, 23, 14160–14167. [Google Scholar]
- Salehi, B.; Sharopov, F.; Boyunegmez Tumer, T.; Ozleyen, A.; Rodríguez-Pérez, C.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum species: A comprehensive review on chemical composition, food applications and phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef] [Green Version]
- Sowa, I.; Paduch, R.; Strzemski, M.; Zielińska, S.; Rydzik-Strzemska, E.; Sawicki, J.; Kocjan, R.; Polkowski, J.; Matkowski, A.; Latalski, M. Proliferative and antioxidant activity of Symphytum officinale root extract. Nat. Prod. Res. 2018, 32, 605–609. [Google Scholar] [CrossRef]
- Shang, H.; Zhou, H.; Duan, M.; Li, R.; Wu, H.; Lou, Y. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from comfrey (Symphytum officinale L.) root. Int. J. Biol. Macromol. 2018, 112, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Opitz, S.E.W.; Josuran, R.; Grubelnik, A.; Esslinger, N.; Pete, S.; Wolfram, E. Is comfrey root more than toxic pyrrolizidine alkaloids? Salvianolic acids among antioxidant polyphenols in comfrey (Symphytum officinale L.) roots. Food Chem. Toxicol. 2018, 112, 178–187. [Google Scholar] [CrossRef]
- Selmar, D.; Wittke, C.; Beck-von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pollut. 2019, 248, 456–461. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Noorwala, M.; Mohammad, F.V.; Sener, B.; Gilan, A.-H.; Aftab, K. Symphytoxide A, A triterpenoid saponin from the roots of Symphytum officinale. Phytochemistry 1993, 32, 1003–1006. [Google Scholar] [CrossRef]
- Roeder, E.; Bourauel, T.; Neuberger, V. Symviridine, a new pyrrolizidine alkaloid from Symphytum species. Phytochemistry 1992, 31, 4041–4042. [Google Scholar] [CrossRef]
- Furuya, T.; Hikichi, M. Alkaloids and triterpenoids of Symphytum officinale. Phytochemistry 1971, 10, 2217–2220. [Google Scholar] [CrossRef]
- Avila, C.; Breakspear, I.; Hawrelakc, J.; Salmond, S.; Evans, S. A systematic review and quality assessment of case reports of adverse events for borage (Borago officinalis), coltsfoot (Tussilago farfara) and comfrey (Symphytum officinale). Fitoterapia 2020, 142, 104519. [Google Scholar] [CrossRef]
- Davydenko, O.; Ledovskykh, V. Carboxylic acids electrooxidation on shungite electrode. Вісник Національного Авіаційного Університету 2017, 70, 120–130. [Google Scholar] [CrossRef]
- Liu, F.; Wan, S.Y.; Jiang, Z.; Li, S.F.Y.; Ong, E.S.; Osorio, J.C.C. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography–electrospray ionization mass spectrometry. Talanta 2009, 80, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Miles, E.A.; Calder, P.C. A review of the potential health benefits of pine nut oil and its characteristic fatty acid pinolenic acid. J. Funct. Foods 2016, 23, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Robien, W.; Kopp, B.; Schabl, D.; Schwarz, H. Carbon-13 NMR spectroscopy of cardenolides and bufadienolides. Prog. Nucl. Magn. Reson. Spectrosc. 1987, 19, 131–181. [Google Scholar] [CrossRef]
- Dresler, S.; Szymczak, G.; Wojcik, M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbakadze, V.V.; Kemertelidze, E.P.; Shashkov, A.S.; Usov, A.I. Structure of a new anti-complementary dihydroxycinnamate-derived polymer from Symphytum asperum (Boraginaceae). Mendeleev Commun. 2000, 10, 148–149. [Google Scholar] [CrossRef]
- Barbakadze, V.; Van Den Berg, A.J.J.; Beukelman, C.J.; Kemmink, J.; Van Ufford, H.C.Q. Poly[3-(3,4-dihydroxyphenyl)glyceric acid] from Symphytum officinale roots and its biological activity. Chem. Nat. Compd. 2009, 45, 6–10. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.-O.; Kim, G.-H.; Heo, H.J. Fucoidan-rich substances from Ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid β production/tau hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef] [Green Version]
- Seung, T.W.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Park, S.H.; Kwon, B.S.; Lee, C.J.; Kang, J.E.; Kim, D.O.; Lee, U.; et al. Ethyl acetate fraction from Hibiscus sabdariffa L. attenuates diabetes-associated cognitive impairment in mice. Food Res. Int. 2018, 105, 589–598. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Parihar, M.S. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem. Int. 2016, 95, 100–108. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. An overview of the neuroprotective potential of rosmarinic acid and its association with nanotechnology-based delivery systems: A novel approach to treating neurodegenerative disorders. Neurochem. Int. 2019, 122, 47–58. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, Y.; Yang, F.; Li, S.; He, X.; Gao, Y.; Zhang, G.; Ren, E.; Wang, Y.; Kang, X. Rosmarinic acid exerts a neuroprotective effect on spinal cord injury by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Toxicol. Appl. Pharmacol. 2020, 397, 115014. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Li, S.; Yang, J. Rosmarinic acid protects rat hippocampal neurons from cerebral ischemia/reperfusion injury via the Akt/JNK3/caspase-3 signaling pathway. Brain Res. 2017, 1657, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.R.; Vieira, C.G.; de Souza, L.P.; Moysés, F.; Basso, C.; Papke, D.K.M.; Pires, T.R.; Siqueira, I.R.; Picada, J.N.; Pereira, P. Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice. Life Sci. 2015, 122, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Khamse, S.; Sadr, S.S.; Roghani, M.; Hasanzadeh, G.; Mohammadian, M. Rosmarinic acid exerts a neuroprotective effect in the kainate rat model of temporal lobe epilepsy: Underlying mechanisms. Pharm. Biol. 2015, 53, 1818–1825. [Google Scholar] [CrossRef]
- Lee, A.Y.; Wu, T.T.; Hwang, B.R.; Lee, J.; Lee, M.; Lee, S.; Cho, E.J. The neuro-protective effect of the methanolic extract of perilla frutescens var. japonica and rosmarinic acid against H2O2-induced oxidative stress in C6 glial cells. Biomol. Ther. 2016, 24, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.H.; Xue, F.; Xue, S.S.; Sang, H.F.; Liu, L.; Wang, Y.; Cai, M.; Zhang, Z.J.; Tan, Q.R.; Wang, H.N.; et al. Electroacupuncture pretreatment ameliorates PTSD-Like behaviors in rats by enhancing hippocampal neurogenesis via the Keap1/Nrf2 antioxidant signaling pathway. Front. Cell. Neurosci. 2019, 13, 275. [Google Scholar] [CrossRef]
- Ghaffari, H.; Venkataramana, M.; Jalali, G.B.; Chandra, N.S.; Nataraju, A.; Geetha, N.P.; Prakash, H.S. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci. 2014, 113, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Zhang, X.J.; Yang, Y.; Zhang, C.; Zhu, C.H.; Miao, J.Y.; Chen, R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen. Res. 2018, 13, 2119–2128. [Google Scholar] [PubMed]
- Wang, J.; Xu, H.; Jiang, H.; Du, X.; Sun, P.; Xie, J. Neurorescue effect of rosmarinic acid on 6-hydroxydopamine-lesioned nigral dopamine neurons in rat model of Parkinson’s disease. J. Mol. Neurosci. 2012, 47, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gok, D.K.; Hidisoglu, E.; Ocak, G.A.; Er, H.; Acun, A.D.; Yargıcoglu, P. Protective role of rosmarinic acid on amyloid beta 42-induced echoic memory decline: Implication of oxidative stress and cholinergic impairment. Neurochem. Int. 2018, 118, 1–13. [Google Scholar]
- Wang, J.; Wang, S.; Guo, H.; Li, Y.; Jiang, Z.; Gu, T.; Su, B.; Hou, W.; Zhong, H.; Cheng, D.; et al. Rosmarinic acid protects rats against post-stroke depression after transient focal cerebral ischemic injury through enhancing antioxidant response. Brain Res. 2021, 1757, 147336. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Zhang, L.; Wang, Q.; Yang, Z.; Liu, J.; Fenga, L. Caffeic acid reduces A53T α-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 2019, 150, 104538. [Google Scholar] [CrossRef]
- Habtemariam, S. Protective effects of caffeic acid and the Alzheimer’s brain: An update. Mini Rev. Med. Chem 2017, 17, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Saeeduddin, M.; Zeng, X. Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid. Carbohydr. Polym. 2017, 170, 206–216. [Google Scholar] [CrossRef]
- Ohkawara, T.; Takeda, H.; Nishihira, J. Protective effect of chlorogenic acid on the inflammatory damage of pancreas and lung in mice with l-arginine-induced pancreatitis. Life Sci. 2017, 190, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wang, G.; Wang, X.; Tang, J.; Yu, Q.; Zhang, X.; Wang, S. Neuro-protection of Chlorogenic acid against Al-induced apoptosis in PC12 cells via modulation of Al metabolism and Akt/GSK-3β pathway. J. Funct. Foods 2020, 70, 103984. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic acid and mental diseases: From chemistry to medicine. Curr. Neuropharmacol. 2017, 15, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yu, X.; Zeng, X.; Zhao, H.; Cao, J.; Jiang, W. Effects of chlorogenic acid against aluminium neurotoxicity in ICR mice through chelation and antioxidant actions. J. Funct. Foods 2018, 40, 365–376. [Google Scholar] [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.H.; Jung, Y.S.; Kim, J.M.; Nam, T.G.; Lee, S.-H.; Cho, H.S.; Song, M.C.; Heo, H.J.; Kim, D.-O. Neuroprotective effects of Actinidia eriantha cv. Bidan kiwifruit on amyloid beta-induced neuronal damages in PC-12 cells and ICR mice. J. Funct. Foods 2021, 79, 104398. [Google Scholar] [CrossRef]
- Lim, M.K.; Lee, S.; Kim, J.Y.; Jeong, J.; Han, E.H.; Lee, S.H.; Ryud, J.H.; Lee, J. Neuroprotective and anti-neuroinflammatory effects of ethanolic extract from leaves and stems of Aster glehni. J. Funct Foods 2021, 79, 104400. [Google Scholar] [CrossRef]
- Staiger, C. Comfrey root: From tradition to modern clinical trials. Wien. Med. Wochenschr. 2013, 163, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Nossa, G.D.L.; Talero, P.Y.V.; Rozo, N.W.E. Determination of polyphenols and antioxidant activity of polar extracts of comfrey (Symphytum officinale L). Rev. Cubana Plant. Med. 2016, 21, 125–132. [Google Scholar]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern trends in the in vitro production and use of callus, suspension cells and root cultures of medicinal plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef] [PubMed]


| Sample No. | Temperature, °C | Volume Fraction of Ethanol in the Extractant, % | OD | m, mg/100 mL |
|---|---|---|---|---|
| 1 | 30 | 30 | 0.0160 ± 0.0001 a | 58 ± 0.1 a |
| 2 | 30 | 40 | 0.0230 ± 0.0001 a | 60 ± 0.2 a |
| 3 | 30 | 50 | 0.0260 ± 0.0001 a | 75 ± 0.1 a |
| 4 | 30 | 60 | 0.3562 ± 0.0050 b | 78 ± 0.2 a |
| 5 | 30 | 70 | 0.2845 ± 0.0030 c | 73 ± 0.1 a |
| 6 | 40 | 30 | 0.2118 ± 0.0030 c | 72 ± 0.2 a |
| 7 | 40 | 40 | 0.2138 ± 0.0030 c | 160 ± 0.1 a |
| 8 | 40 | 50 | 0.2652 ± 0.0030 c | 180 ± 0.1 a |
| 9 | 40 | 60 | 0.1942 ± 0.0030 c | 240 ± 0.1 b |
| 10 | 40 | 70 | 0.3827 ± 0.0056 b | 235 ± 0.2 b |
| 11 | 50 | 30 | 0.2918 ± 0.0050 c | 230 ± 0.1 b |
| 12 | 50 | 40 | 0.3315 ± 0.0056 b | 390 ± 0.2 b |
| 13 | 50 | 50 | 0.2180 ± 0.0050 c | 370 ± 0.1 b |
| 14 | 50 | 60 | 0.4490 ± 0.0059 d | 330 ± 0.1 b |
| 15 | 50 | 70 | 0.3680 ± 0.0056 b | 332 ± 0.1 b |
| 16 | 60 | 30 | 0.1290 ± 0.0010 e | 250 ± 0.2 b |
| 17 | 60 | 40 | 0.0400 ± 0.0001 a | 330 ± 0.1 b |
| 18 | 60 | 50 | 0.1260 ± 0.0010 e | 620 ± 0.1 c |
| 19 | 60 | 60 | 0.2070 ± 0.0030 c | 210 ± 0.2 b |
| 20 | 60 | 70 | 0.2410 ± 0.0030 c | 490 ± 0.3 c |
| 21 | 70 | 30 | 0.2210 ± 0.0030 c | 370 ± 0.1 b |
| 22 | 70 | 40 | 0.3350 ± 0.0050 b | 260 ± 0.1 b |
| 23 | 70 | 50 | 0.3490 ± 0.0050 b | 510 ± 0.3 c |
| 24 | 70 | 60 | 0.2000 ± 0.0010 c | 320 ± 0.1 b |
| 25 | 70 | 70 | 0.0600 ± 0.0001 a | 509 ± 0.1 c |
| 26 | 30 | 30 | 0.0800 ± 0.0001 a | 454 ± 0.3 c |
| 27 | 30 | 40 | 0.0600 ± 0.0001 a | 316 ± 0.1 b |
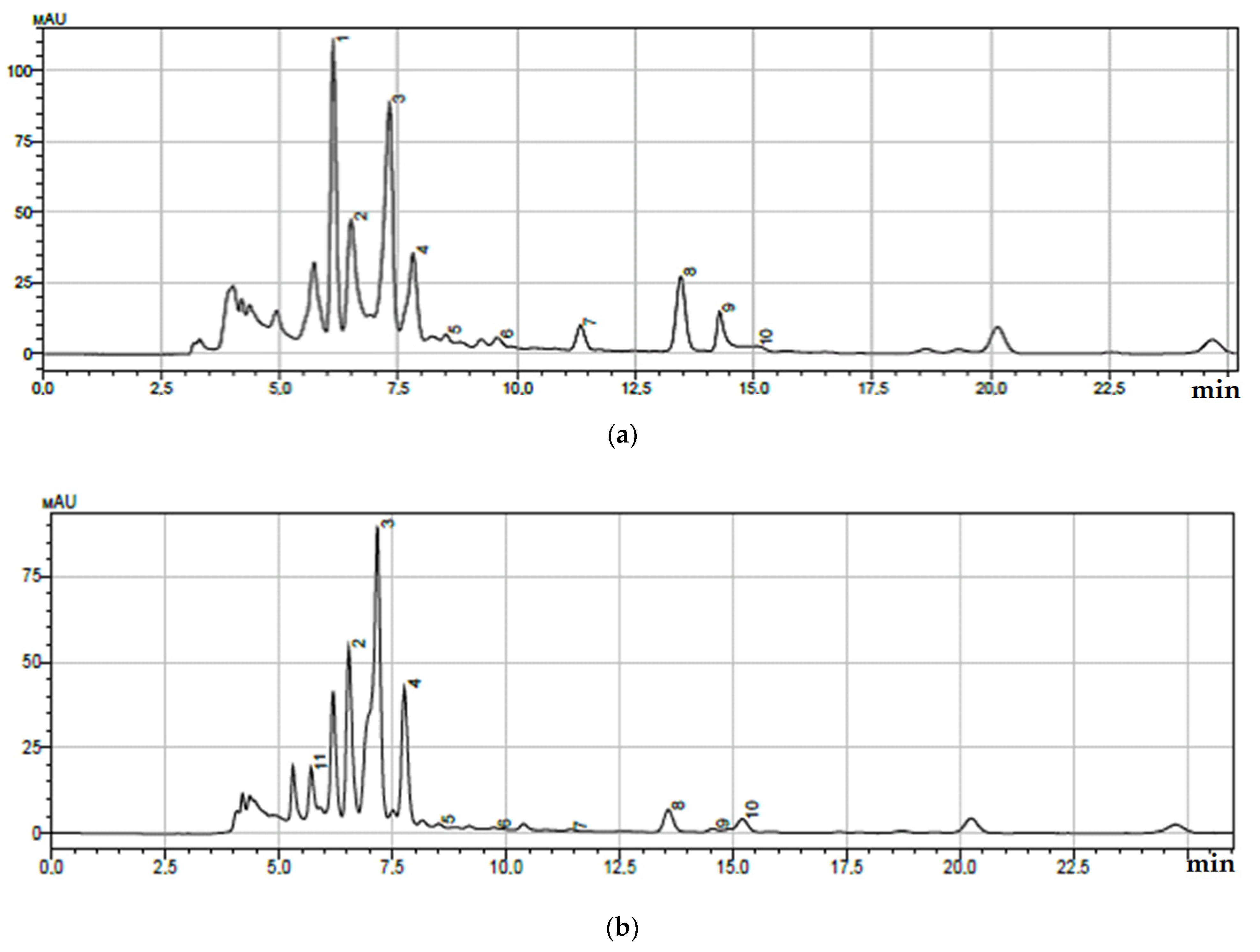
| Peak No. | Retention Time, min | Component | Quantitative Content *, μg/mL |
|---|---|---|---|
| 1 | 6.164 | m-methoxybenzoic acid | 30.05 ± 0.49 |
| 2 | 6.502 | 5-caffeoylquinic acid | 14.73 ± 0.72 |
| 3 | 7.300 | caffeic acid | 25.40 ± 0.60 |
| 4 | 7.811 | 3-caffeoylquinic acid | 12.57 ± 0.62 |
| 5 | 8.528 | rosmarinic acid | 2.03 ± 0.05 |
| 6 | 9.620 | 1-caffeoylquinic acid | 3.12 ± 0.20 |
| 7 | 11.350 | coumarin | 7.32 ± 0.41 |
| 8 | 13.426 | lithospermic acid | 11.27 ± 0.52 |
| 9 | 14.270 | salicylic acid | 9.25 ± 0.50 |
| 10 | 15.030 | m-hydroxybenzoic acid | 1.97 ± 0.20 |
| Peak No. | Retention Time, min | Component | Quantitative Content *, μg/mL |
|---|---|---|---|
| 2 | 6.500 | 5-caffeoylquinic acid | 6.50 ± 0.29 |
| 3 | 7.305 | caffeic acid | 35.70 ± 0.49 |
| 4 | 7.811 | 3-caffeoylquinic acid | 25.10 ± 0.27 |
| 5 | 8.528 | rosmarinic acid | 45.70 ± 0.36 |
| 6 | 9.620 | 1-caffeoylquinic acid | 17.30 ± 0.78 |
| 7 | 11.350 | coumarin | 2.45 ± 0.20 |
| 8 | 13.426 | lithospermic acid | 9.32 ± 0.32 |
| 9 | 14.270 | salicylic acid | 1.54 ± 0.20 |
| 10 | 15.030 | m-hydroxybenzoic acid | 8.92 ± 0.29 |
| 11 | 5.700 | 3,4-dimethoxybenzoic acid | 7.35 ± 0.36 |
| Samples | Concentration, μg/mL | Anti-Inflammatory Activity, % |
|---|---|---|
| I | 10.0 ± 0.5 a | 60 ± 2 a |
| 20.0 ± 0.7 b | 75 ± 3 b | |
| 30.0 ± 0.8 c | 75 ± 3 b | |
| II | 10.0 ± 0.6 a | 63 ± 2 a |
| 20.0 ± 0.8 b | 75 ± 3 b | |
| 30.0 ± 0.8 c | 75 ± 2 b |
| Sample | Antioxidant Activity, mg AA/g |
|---|---|
| I | 113.6 ± 6.56 |
| II | 112.8 ± 6.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, V.; Dolganyuk, V.; Sukhikh, A.; Babich, O.; Ivanova, S.; Prosekov, A.; Dyshlyuk, L. Phytochemical Analysis of Symphytum officinale Root Culture Extract. Appl. Sci. 2021, 11, 4478. https://doi.org/10.3390/app11104478
Le V, Dolganyuk V, Sukhikh A, Babich O, Ivanova S, Prosekov A, Dyshlyuk L. Phytochemical Analysis of Symphytum officinale Root Culture Extract. Applied Sciences. 2021; 11(10):4478. https://doi.org/10.3390/app11104478
Chicago/Turabian StyleLe, Violeta, Vyacheslav Dolganyuk, Andrey Sukhikh, Olga Babich, Svetlana Ivanova, Alexander Prosekov, and Lyubov Dyshlyuk. 2021. "Phytochemical Analysis of Symphytum officinale Root Culture Extract" Applied Sciences 11, no. 10: 4478. https://doi.org/10.3390/app11104478
APA StyleLe, V., Dolganyuk, V., Sukhikh, A., Babich, O., Ivanova, S., Prosekov, A., & Dyshlyuk, L. (2021). Phytochemical Analysis of Symphytum officinale Root Culture Extract. Applied Sciences, 11(10), 4478. https://doi.org/10.3390/app11104478









