Toll-Like Receptors 1/2/4/6 and Nucleotide-Binding Oligomerization Domain-Like Receptor 2 Are Key Damage-Associated Molecular Patterns Sensors on Periodontal Resident Cells
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Periodontal Biopsies
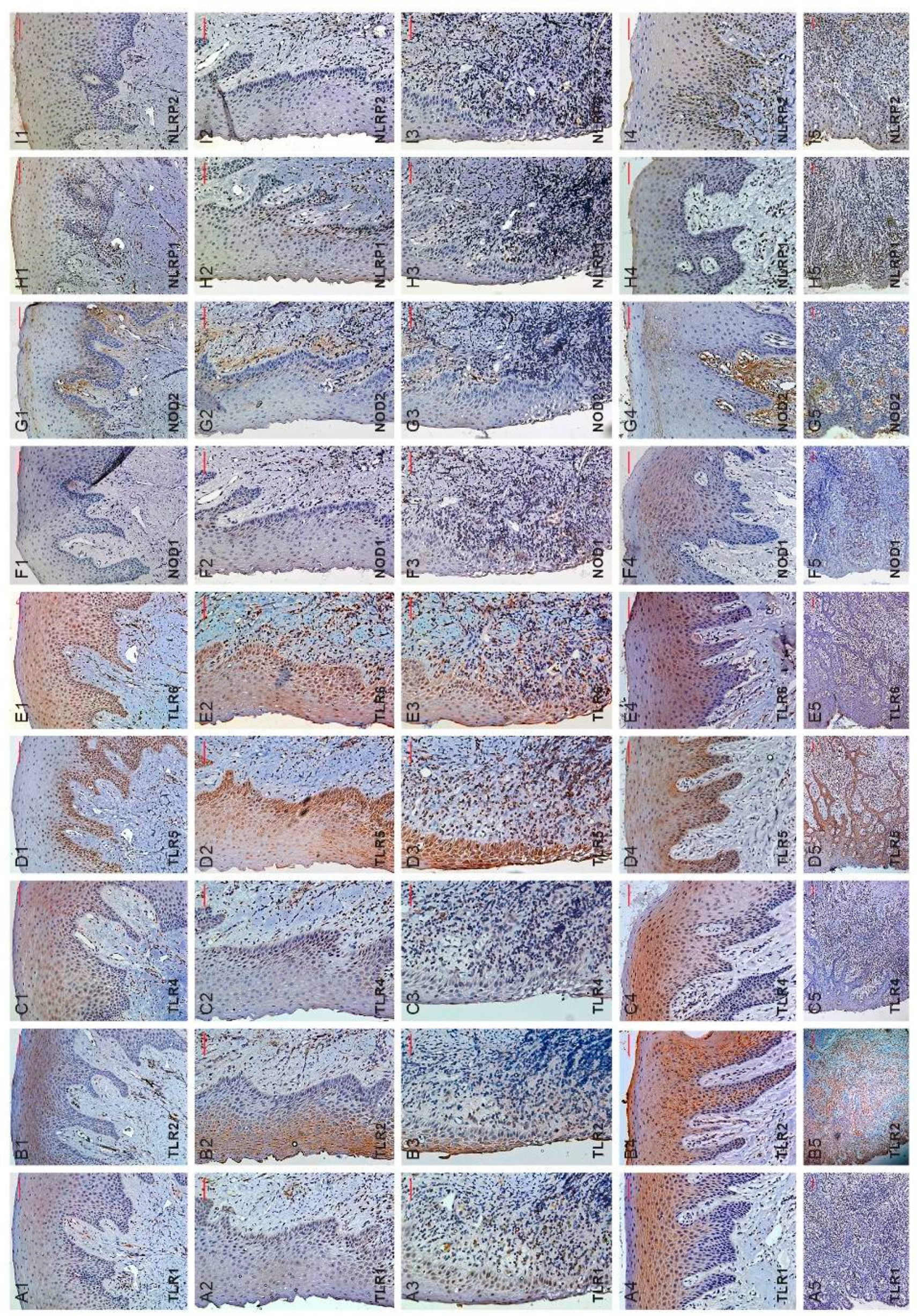
2.2. Immunohistochemistry
2.3. Cell Culture
2.4. MTT Assay
2.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative Reverse Transcription PCR (RT-Qpcr) Analysis
2.6. Western Blot
2.7. Statistical Analysis
3. Results
3.1. Selected TLRs and NLRs Localization in Healthy Gingival and Periodontitis Biopsies
3.2. Culture and Isolation of HGK and HGF
3.3. MTT Assay
3.4. Selected DAMP Sensors mRNA Detection in HGT, HGK, or HGF
3.5. Quantities of Selected DAMP Sensor Transcripts from HGK or HGF under Hypoxia or E. coli LPS Stimulation
3.6. Levels of Selected DAMP Sensor Proteins from HGK or HGF under Hypoxia and/or E. coli LPS Stimulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flemmig, T.F. Periodontitis. Ann. Periodontol. 1999, 4, 32–37. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.-T.A. The role of acquired immunity and periodontal disease progression. Crit. Rev. Oral Biol. Med. 2003, 14, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Erridge, C.; Pridmore, A.; Eley, A.; Stewart, J.; Poxton, I.R. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via toll-like receptor 2. J. Med. Microbiol. 2004, 53, 735–740. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Rhee, S.H.; Hwang, D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NFκB and expression of the inducible cyclooxygenase. J. Biol. Chem. 2000, 275, 34035–34040. [Google Scholar] [CrossRef] [Green Version]
- Esmon, C.T. Regulation of blood coagulation. Biochim. Biophys. Acta 2000, 1477, 349–360. [Google Scholar] [CrossRef]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Lamkanfi, M.; Núñez, G. Intracellular NOD-like receptors in host defense and disease. Immunity 2007, 27, 549–559. [Google Scholar] [CrossRef]
- Sugawara, Y.; Uehara, A.; Fujimoto, Y.; Kusumoto, S.; Fukase, K.; Shibata, K.; Sugawara, S.; Sasano, T.; Takada, H. Toll-like receptors, NOD1, and NOD2 in oral epithelial cells. J. Dent. Res. 2006, 85, 524–529. [Google Scholar] [CrossRef]
- Lien, E.; Sellati, T.J.; Yoshimura, A.; Flo, T.H.; Rawadi, G.; Finberg, R.W.; Carroll, J.D.; Espevik, T.; Ingalls, R.R.; Radolf, J.D.; et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 1999, 274, 33419–33425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhat, K.; Riekenberg, S.; Heine, H.; Debarry, J.; Lang, R.; Mages, J.; Buwitt-Beckmann, U.; Röschmann, K.; Jung, G.; Wiesmüller, K.H.; et al. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leuko. Biol. 2008, 83, 692–701. [Google Scholar] [CrossRef]
- Beklen, A.; Hukkanen, M.; Richardson, R.; Konttinen, Y.T. Immunohistochemical localization of Toll-like receptors 1–10 in periodontitis. Oral Microbiol. Immunol. 2008, 23, 425–431. [Google Scholar] [CrossRef]
- Zinkernagel, A.S.; Johnson, R.S.; Nizet, V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 2007, 85, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Schaible, B.; Schaffer, K.; Taylor, C.T. Hypoxia, innate immunity and infection in the lung. Respir. Physiol. Neurobiol. 2010, 174, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Hanioka, T.; Takaya, K.; Shizukuishi, S. Association of oxygen tension in human periodontal pockets with gingival inflammation. J. Periodontol. 1998, 69, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, T.; Tanaka, M.; Takaya, K.; Matsumori, Y.; Shizukuishi, S. Pocket oxygen tension in smokers and non-smokers with periodontal disease. J. Periodontol. 2000, 71, 550–554. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Leung, W.K. Role of the hypoxia-inducible factor in periodontal inflammation. In Hypoxia and Human Diseases; Zheng, J., Zhou, C., Eds.; IntechOpen Ltd.: Rijeka, Croatia, 2017; Chapter 15; pp. 285–302. [Google Scholar] [CrossRef] [Green Version]
- Greijer, A.; van der Groep, P.; Kemming, D.; Shvarts, A.; Semenza, G.L.; Meijer, G.A.; van de Wiel, M.A.; Belien, J.A.; van Diest, P.J.; van der Wall, E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J. Pathol. 2005, 206, 291–304. [Google Scholar] [CrossRef]
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Förster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 2003, 112, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.M.; Kolamunne, R.T.; Lock, F.E.; Matthews, J.B.; Chapple, I.L.; Griffiths, H.R. Oxygen tension modulates the cytokine response of oral epithelium to periodontal bacteria. J. Clin. Periodontol. 2010, 37, 1039–1048. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, Y.J.; Joung, S.M.; Lee, B.H.; Jung, Y.S.; Lee, J.Y. Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology 2010, 129, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Stridh, L.; Smith, P.L.; Naylor, A.S.; Wang, X.; Mallard, C. Regulation of toll-like receptor 1 and-2 in neonatal mice brains after hypoxia-ischemia. J. Neuroinflamm. 2011, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Kuhlicke, J.; Frick, J.S.; Morote-Garcia, J.C.; Rosenberger, P.; Eltzschig, H.K. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE 2007, 2, e1364. [Google Scholar] [CrossRef] [Green Version]
- Braza, F.; Brouard, S.; Chadban, S.; Goldstein, D.R. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat. Rev. Nephrol. 2016, 12, 281–290. [Google Scholar] [CrossRef]
- Li, J.-P.; Chen, Y.; Ng, C.H.C.; Fung, M.L.; Xu, A.; Cheng, B.; Tsao, S.W.; Leung, W.K. Differential expression of Toll-like receptor 4 in healthy and diseased human gingiva. J. Periodontal Res. 2014, 49, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-P.; Li, F.Y.L.; Xu, A.; Cheng, B.; Tsao, S.W.; Fung, M.L.; Leung, W.K. Lipopolysaccharide and hypoxia-induced HIF-1 activation in human gingival fibroblasts. J. Periodontol. 2012, 83, 816–824. [Google Scholar] [CrossRef]
- Leung, W.K.; Wu, Q.; Hannam, P.M.; McBride, B.C.; Uitto, V.-J. Treponema denticola may stimulate both epithelial proliferation and apoptosis through MAP kinase signal pathways. J. Periodontal Res. 2002, 37, 445–455. [Google Scholar] [CrossRef]
- Ng, K.-T.; Li, J.-P.; Ng, K.M.; Tipoe, G.L.; Leung, W.K.; Fung, M.L. Expression of hypoxia-inducible factor-1α in human periodontal tissue. J Periodontol. 2011, 82, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kawai, T.; Taubman, M.A. Toll-like receptor signaling in B cell-mediated RANKL-dependent periodontitis bone resorption. In Interface Oral Health Science 2011, Proceedings of the 4th International Symposium for Interface Oral Health Science; Sasaki, K., Suzuki, O., Takahashi, N., Eds.; Springer: New York, NY, USA, 2012; pp. 373–375. [Google Scholar] [CrossRef]
- Uehara, A.; Sugawara, Y.; Kurata, S.; Fujimoto, Y.; Fukase, K.; Kusumoto, S.; Satta, Y.; Sasano, T.; Sugawara, S.; Takada, H. Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells. Cell Microbiol. 2005, 7, 675–686. [Google Scholar] [CrossRef]
- Aral, K.; Milward, M.R.; Kapila, Y.; Berdeli, A.; Cooper, P.R. Inflammasomes and their regulation in periodontal disease: A review. J. Periodontal Res. 2020, 55, 473–487. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Flynn, M.G.; Campbell, W.W.; Stewart, L.K.; Timmerman, K.L. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med. Sci. Sports Exerc. 2004, 36, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- De Creus, A.; Abe, M.; Lau, A.H.; Hackstein, H.; Raimondi, G.; Thomson, A.W. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J. Immunol. 2005, 174, 2037–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, S.K.; O’Carroll, C.E.; Wells, C.A.; Carmody, R.J. Toll-like receptors drive specific patterns of tolerance and training on restimulation of macrophages. Front. Immunol. 2018, 9, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Cook, J.A. Molecular mechanisms of endotoxin tolerance. J. Endotoxin Res. 2004, 10, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Ohta, K.; Nishi, H.; Shigeishi, H.; Tobiume, K.; Takechi, M.; Kamata, N. Interleukin-8 and CXCL10 expression in oral keratinocytes and fibroblasts via Toll-like receptors. Microbiol. Immunol. 2013, 57, 198–206. [Google Scholar] [CrossRef]
- Simiantonaki, N.; Kurzik-Dumke, U.; Karyofylli, G.; Jayasinghe, C.; Michel-Schmidt, R.; Kirkpatrick, C.J. Reduced expression of TLR4 is associated with the metastatic status of human colorectal cancer. Int. J. Mol. Med. 2007, 20, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, H.; Fukudome, K.; Takao, S.; Tsuneyoshi, N.; Ohta, S.; Nagai, Y.; Ihara, H.; Miyake, K.; Ikeda, Y.; Kimoto, M. Reduced surface expression of TLR4 by a V254I point mutation accounts for the low lipopolysaccharide responder phenotype of BALB/c B cells. J. Immunol. 2013, 190, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Shah, G.; Patel, B.; Chorawala, M. Toll like receptors: An overview. Int. J. Pharmacol. Toxicol. 2014, 2, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Lee, H.; Berg, A.H.; Lisanti, M.P.; Shapiro, L.; Scherer, P.E. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J. Biol. Chem. 2000, 275, 24255–24263. [Google Scholar] [CrossRef] [Green Version]
- Yokota, S.-I.; Ohnishi, T.; Muroi, M.; Tanamoto, K.; Fujii, N.; Amano, K. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol. Med. Microbiol. 2007, 51, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Beklen, A.; Sorsa, T.; Konttinen, Y. Toll-like receptors 2 and 5 in human gingival epithelial cells co-operate with T-cell cytokine interleukin-17. Oral Microbiol. Immunol. 2009, 24, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.; Jiao, Y.; Schaff, R.A.; Hao, J.; Morelli, T.; Kinney, J.S.; Gerow, E.; Sheridan, R.; Rodrigues, V.; Paster, B.J.; et al. TLR4, NOD1 and NOD2 mediate immune recognition of putative newly identified periodontal pathogens. Mol. Oral Microbiol. 2016, 31, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Shibata, K. Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases. Mol. Oral Microbiol. 2018, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Ertlschweiger, S.; Moritz, A.; Bantleon, H.P.; Rausch-Fan, X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta Odontol. Scand. 2014, 72, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Barksby, H.; Nile, C.J.; Jaedicke, K.M.; Taylor, J.J.; Preshaw, P.M. Differential expression of immunoregulatory genes in monocytes in response to Porphyromonas gingivalis and Escherichia coli lipopolysaccharide. Clin. Exp. Immunol. 2009, 156, 479–487. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, Q.; An, R.; Wang, J.; Song, X.; Shen, Y.; Wang, M.; Xu, H. Comparison of microbiomes in ulcerative and normal mucosa of recurrent aphthous stomatitis (RAS)-affected patients. BMC Oral Health 2020, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Gölz, L.; Memmert, S.; Rath-Deschner, B.; Jäger, A.; Appel, T.; Baumgarten, G.; Götz, W.; Frede, S. LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediat. Inflamm. 2014, 2014, 986264. [Google Scholar] [CrossRef] [Green Version]
- Gölz, L.; Memmert, S.; Rath-Deschner, B.; Jäger, A.; Appel, T.; Baumgarten, G.; Götz, W.; Frede, S. Hypoxia and P. gingivalis synergistically induce HIF-1 and NF-κB activation in PDL cells and periodontal diseases. Mediat. Inflamm. 2015, 2015, 438085. [Google Scholar] [CrossRef] [Green Version]


| Experiment Group | n | Age Range (Years) | Gender (F/M) |
|---|---|---|---|
| Immuno-histochemistry | |||
| Heathy | 13 | 18–27 | 5/8 |
| Periodontitis | 17 | 38–46 | 8/9 |
| RT-PCR1 | |||
| HGT | 16 | 14–25 | 9/7 |
| HGK/HGF explant | 27 | 16–29 | 19/7 |
| MTT assay1 | 9 | 19–24 | 4/5 |
| Selected DAMP sensor transcripts (RT-qPCR) or protein detection1 | |||
| Control (18% O2, nil E. coli LPS) | 13 | 16–25 | 8/5 |
| Hypoxia only (1% O2, nil E. coli LPS) | 13 | 16–25 | 8/5 |
| 2 µg/mL E. coli LPS only (18% O2) | 13 | 16–25 | 8/5 |
| Hypoxia (1% O2) and 2 µg/mL E. coli LPS challenge2 | 13 | 16–25 | 8/5 |
| Gene | Primer Sequence (5′–3′) | Amplicon2 Size (bp) |
|---|---|---|
| TLR1 3,4 | F: CAGGATAACAAAGGCATATTGG R: GGATAGGTCTTTAGGAACG | 238 |
| TLR2 3 | F: GGTAGTTGTGGGTTGAAGC R: AAATCAGTATCTCGCAGTTCC | 727 |
| TLR2 4 | F: AGTTCTCCCAGTGTTTGGT R: CCAGTTGAAAGCAGTGAAAGAG | 132 |
| TLR3 3 | F: CAGTCATCCAACAGAATCATGAG R: GATGGAGTTCAGTCAAATTCGT | 405 |
| TLR3 4 | F: CTTCCCTGATGAAATGTCTG R: ATGATTCTGTTGGATGACTG | 70 |
| TLR4 3,4 | F: TTATCCAGGTGTGAAATCCA R: GATTTGTCTCCACAGCCA | 159 |
| TLR5 3 | F: GCATCCAGGGAAGATGTC R: GATCCTCGTTGTCCTAGC | 341 |
| TLR5 4 | F: AGTCCTTTCTCCTGATTCACC R: TCCCATGATCCTCGTTGTC | 164 |
| TLR6 3 | F: TTCTTGGGATTGAGTGCTA R: GTTTCTATGTGGTTGAGGG | 335 |
| TLR6 4 | F: GAGATCTTGAATTTGGACTC R: GGTTCTTTGTCTTTGGTC | 92 |
| TLR7 3,4 | F: CAAGAAAGTTGATGCTATTGGG R: CTGTCAAATGCTTGTCTGTG | 277 |
| TLR8 3,4 | F: GATTTCCCACCTACCCTCTG R: TCCCAGACTCACAATACTCTTCC | 284 |
| TLR9 3,4 | F: ACTATGCAAATGGCCTTTGAC R: AGGATGTTGGTATGGCTGAG | 686 |
| TLR10 3,4 | F: AACAACCCAAGAACAACTC R: CCACATTTACGCCTATCC | 428 |
| NOD1 3,4 | F: GGCTTATCCAGAATCAGATCAC R: GGTTTCCATTTAGGCAAATCTC | 98 |
| NOD2 3,4 | F: CGTCATGCTAGAAGAACTC R: GTTATTGGACAACTTCAGGA | 117 |
| NLRP1 3,4 | F: CTTGTACCGAGTTCACTTCC R: CTCAGCCTTGATGTCCAG | 183 |
| NLRP2 3,4 | F: GATGTCTGTGGTTGTGGG R: TGTCAAGGTTTCAAACAGCA | 151 |
| GAPDH 3 | F: CAACTTTGGTATCGTGGAAGGA R: AAGGTGGAGGAGTGGGTGTCG | 387 [26] |
| β-actin 4 | F: AAGATCAAGATCATTGCTCCT R: GGGTGTAACGCAACTAAGTC | 182 [27] |
| Health (n = 13) | Periodontitis (n = 17) | |||||||
|---|---|---|---|---|---|---|---|---|
| Epithelium | Connective Tissue | Epithelium | Connective Tissue | |||||
| OE | OSE | JE | OE | PE | ||||
| TLR1 | 16.8 ± 5.2 | 13.7 ± 5.4 | 17.9 ± 6.4 | 19.5 ± 6.7 | 57.9 ± 14.6 ** | 21.4 ± 7.1 | 59.3 ± 10.3 ** | |
| TLR2 | 36.5 ± 9.7 | 44.6 ± 18.4 | 40.7 ± 8.6 | 27.4 ± 8.4 | 65.4 ± 11.2 ** | 62.7 ± 11.0 * | 78.1 ± 12.6 ** | |
| TLR4 | 41.5 ± 12.6 | 12.9 ± 3.3 | 15.6 ± 6.1 | 28.3 ± 8.5 | 40.2 ± 11.6 | 30.3 ± 8.2 * | 50.3 ± 7.9 ** | |
| TLR5 | 29.3 ± 5.8 | 41.7 ± 20.1 | 72.6 ± 11.2 | 34.2 ± 7.3 | 50.8 ± 8.5 ** | 69.4 ± 15.7 | 60.8 ± 11.6 ** | |
| TLR6 | 63.7 ± 15.9 | 55.8 ± 18.4 | 41.8 ± 12.4 | 39.8 ± 9.2 | 59.7 ± 8.2 | 51.4 ± 9.2 | 64.9 ± 13.4 ** | |
| NOD1 | 15.2 ± 6.1 | 13.1 ± 4.6 | 18.7 ± 4.6 | 26.8 ± 5.5 | 41.5 ± 10.6 ** | 43.5 ± 9.4 ** | 68.1 ± 12.0 ** | |
| NOD2 | 22.0 ± 9.6 | 14.6 ± 5.1 | 15.4 ± 4.7 | 28.6 ± 4.6 | 25.7 ± 8.9 | 39.1 ± 10.2 * | 58.9 ± 11.2 ** | |
| NLRP1 | 17.8 ± 7.6 | 17.5 ± 6.3 | 17.3 ± 8.9 | 31.1 ± 5.7 | 21.8 ± 7.9 | 30.3 ± 8.1 | 61.4 ± 15.3 ** | |
| NLRP2 | 20.3 ± 9.1 | 14.1 ± 5.7 | 11.3 ± 5.8 | 29.0 ± 11.5 | 29.2 ± 6.2 | 24.3 ± 7.5 | 55.3 ± 14.5 ** | |
| HGK | HGF | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxygen Conc. | p-Value | LPS | p-Value | Oxygen Conc. | p-Value | LPS | p-Value | ||||||
| 18% | 1% | Nil | 2 µg/mL | 18% | 1% | Nil | 2 µg/mL | ||||||
| TLR1 | 1 | 0.140 | 0.002 | 1 | 1.416 | 0.658 | 1 | 1.481 | 0.005 | 1 | 2.390 | 0.030 | |
| TLR2 | 1 | 2.049 | 0.002 | 1 | 2.281 | 0.002 | 1 | 5.059 | 0.002 | 1 | 3.083 | 0.002 | |
| TLR3 | 1 | 0.286 | 0.003 | 1 | 0.575 | 0.780 | 1 | 0.739 | 0.002 | 1 | 0.723 | 0.070 | |
| TLR4 2 | 0.000 | 0.000 | 1.000 | 1 | 1.316 | 0.0655 | 1 | 1.955 | 0.010 | 1 | 0.776 | 0.113 | |
| TLR5 | 1 | 0.881 | 0.060 | 1 | 0.833 | 0.980 | 1 | 0.759 | 0.010 | 1 | 0.760 | 0.230 | |
| TLR6 | 1 | 0.109 | 0.002 | 1 | 1.046 | 0.900 | 1 | 0.751 | 0.239 | 1 | 3.281 | 0.002 | |
| NOD1 | 1 | 0.758 | 0.006 | 1 | 0.885 | 0.065 | 1 | 1.325 | 0.008 | 1 | 0.858 | 0.200 | |
| NOD2 | 1 | 4.596 | 0.002 | 1 | 3.978 | 0.003 | 1 | 0.816 | 0.063 | 1 | 6.336 | 0.002 | |
| NLRP1 | 1 | 1.819 | 0.002 | 1 | 1.115 | 0.650 | 1 | 1.729 | 0.002 | 1 | 0.947 | 0.380 | |
| NLRP2 3 | 1 | 0.309 | 0.002 | 1 | 1.231 | 0.082 | 0.000 | 0.000 | 0.593 | 1 | 0.953 | 0.080 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, X.X.; Ng, C.H.C.; Tsao, S.W.; Leung, W.K. Toll-Like Receptors 1/2/4/6 and Nucleotide-Binding Oligomerization Domain-Like Receptor 2 Are Key Damage-Associated Molecular Patterns Sensors on Periodontal Resident Cells. Appl. Sci. 2021, 11, 4724. https://doi.org/10.3390/app11114724
Chen Y, Wang XX, Ng CHC, Tsao SW, Leung WK. Toll-Like Receptors 1/2/4/6 and Nucleotide-Binding Oligomerization Domain-Like Receptor 2 Are Key Damage-Associated Molecular Patterns Sensors on Periodontal Resident Cells. Applied Sciences. 2021; 11(11):4724. https://doi.org/10.3390/app11114724
Chicago/Turabian StyleChen, Yu, Xiao Xiao Wang, Corrie H. C. Ng, Sai Wah Tsao, and Wai Keung Leung. 2021. "Toll-Like Receptors 1/2/4/6 and Nucleotide-Binding Oligomerization Domain-Like Receptor 2 Are Key Damage-Associated Molecular Patterns Sensors on Periodontal Resident Cells" Applied Sciences 11, no. 11: 4724. https://doi.org/10.3390/app11114724
APA StyleChen, Y., Wang, X. X., Ng, C. H. C., Tsao, S. W., & Leung, W. K. (2021). Toll-Like Receptors 1/2/4/6 and Nucleotide-Binding Oligomerization Domain-Like Receptor 2 Are Key Damage-Associated Molecular Patterns Sensors on Periodontal Resident Cells. Applied Sciences, 11(11), 4724. https://doi.org/10.3390/app11114724






