Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus calamus L., Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. Microorganisms Studied
2.3. Antimicrobial Activity
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. Determination of Radical Scavenging Activity by DPPH Method
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antifungal Potential of Methanolic Extracts from Acorus calamus, Chlorella vulgaris, Lemna minuta and Scenedesmus dimorphus
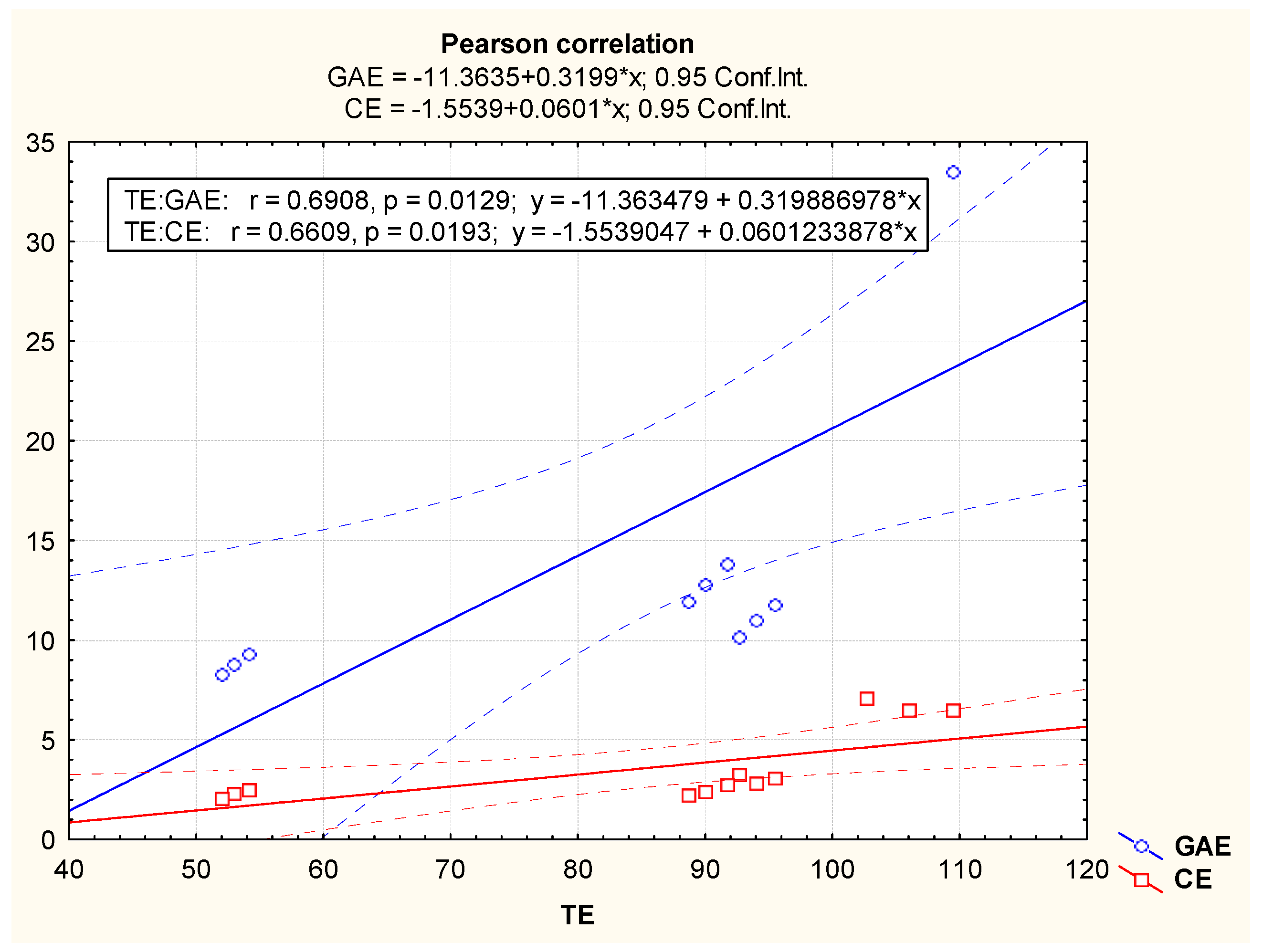
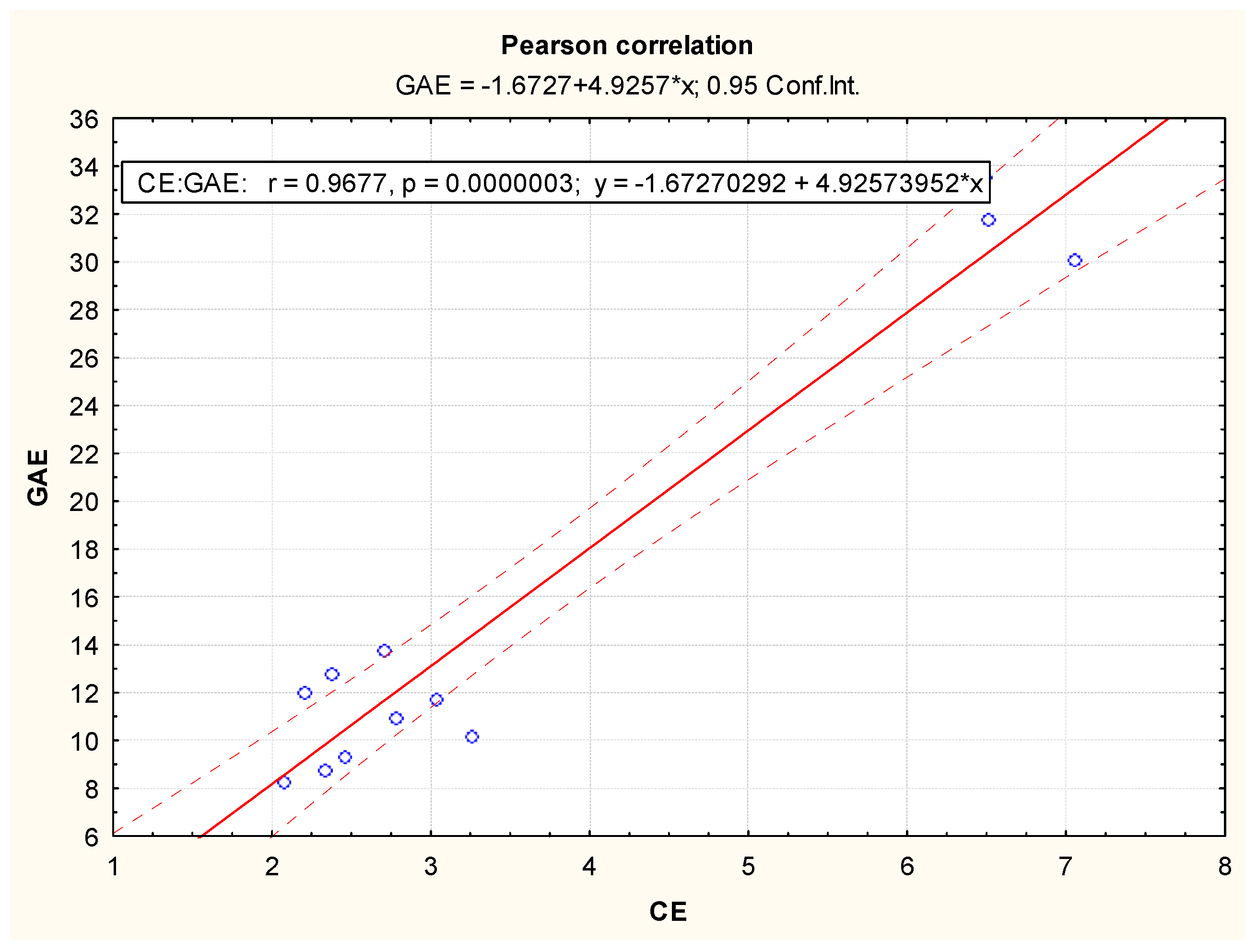
3.2. Antioxidant Potential of Methanolic Extracts from Acorus calamus, Chlorella vulgaris, Lemna minuta and Scenedesmus dimorphus
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rawal, P.; Adhikari, R.S.; Danu, K.; Tiwari, A. Antifungal activity of Acorus calamus against Fusarium oxysporum f. sp. lycopersici. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 710–715. [Google Scholar]
- Mahdavi, B.; Yaacob, W.A.; Din, L.B. Antioxidant and antimicrobial activity of the extracts from different parts of Etlingera sayapensis (Zingiberaceae). Sains Malays. 2017, 46, 1565–1571. [Google Scholar] [CrossRef]
- Devi, S.A.; Ganjewala, D. Antimicrobial activity of Acorus calamus (L.) rhizome and leaf extract. Acta Biol. Szeged. 2009, 53, 45–49. [Google Scholar]
- Bonifácio, B.V.; dos Santos Ramos, M.A.; da Silva, P.B.; Bauab, T.M. Antimicrobial activity of natural products against Helicobacter pylori: A review. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 54. [Google Scholar] [PubMed] [Green Version]
- Sales, M.D.C.; Costa, H.B.; Fernandes, P.M.B.; Ventura, J.A.; Meira, D.D. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickymaray, S.; Al Aboody, M.S. In vitro antioxidant and bactericidal efficacy of 15 common spices: Novel therapeutics for urinary tract infections? Medicina 2019, 55, 289. [Google Scholar] [CrossRef] [Green Version]
- Shreelaxmi, S.H.; Ramachandra, C.T.; Roopa, R.S.; Hanchinal, S.G. Antimicrobial activity of supercritical fluid extracted Acorus calamus oil against different microbes. J. Pharmacogn. Phytochem. 2018, 7, 2836–2840. [Google Scholar]
- Hasan, N.A.; Nawahwi, M.Z.; Malek, H.A. Antimicrobial activity of Nigella sativa seed extract. Sains Malays. 2013, 42, 143–147. [Google Scholar]
- Djarkasi, G.S.S.; Lalujan, L.E.; Nurali, E.; Sumual, M.F. Antioxidant activity of karimenga (Acorus calamus). AIP Conf. Proc. 2019, 2155, 020051. [Google Scholar]
- Li, K.S.; Wah, C.S. Antioxidant and antibacterial activity of Acorus calamus L leaf and rhizome extracts. J. Gizi Klinik Indones. 2017, 13, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Tadhani, M.; Patel, V.; Subhash, V. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J. Food Compos. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, R.; Choudhary, S. Antifungal and HPLC analysis of the crude extracts of Acorus calamus, Tinospora cordifolia and Celestrus paniculatus. J. Agric. Technol. 2010, 6, 149–158. [Google Scholar]
- Dissanayake, K.G.C.; Perera, B.T.; Perera, W.P.R.T. Potential of the pathogenic microorganisms mitigation using rhizome extract of Acorus calamus as a medicinal herb. World J. Pharm. Pharm. Sci. 2020, 9, 85–99. [Google Scholar]
- Ghasemi, Y.; Moradian, A.; Mohagheghzadeh, A.; Shokravi, S.; Morowvat, M.H. Antifungal and antibacterial activity of the microalgae collected from paddy fields of Iran: Characterization of antimicrobial activity of Chroococcus dispersus. J. Biol. Sci. 2007, 7, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Marrez, D.A.; Naguib, M.M.; Sultan, Y.Y.; Higazy, A.M. Antimicrobial and anticancer activities of Scenedesmus obliquus metabolites. Heliyon 2019, 5, e01401. [Google Scholar] [CrossRef] [Green Version]
- Vehapi, M.; Koçer, A.T.; Yilmaz, A.; Özçimen, D. Investigation of the antifungal effects of algal extracts on apple-infecting fungi. Arch. Microbiol. 2020, 202, 455–471. [Google Scholar] [CrossRef]
- Gülçin, I.; Kireçci, E.; Akkemik, E.; Topal, F.; Hisar, O. Antioxidant, antibacterial, and anticandidal activities of an aquatic plant: Duckweed (Lemna minor L. Lemnaceae). Turk. J. Biol. 2010, 34, 175–188. [Google Scholar]
- Velichkova, K.; Sirakov, I.; Rusenova, N.; Beev, G.; Denev, S.; Valcheva, N.; Dinev, T. In Vitro antimicrobial activity on Lemna minuta, Chlorella vulgaris and Spirulina sp. extracts. Fresenius Environ. Bull. 2018, 27, 5736–5741. [Google Scholar]
- Petrova-Tacheva, V.; Ivanov, V.; Atanasov, A. Lemna minor L. as a source of antioxidants. Trakia J. Sci. 2020, 18 (Suppl. 1), 157–162. [Google Scholar]
- Culture Collection of Algae and Protozoa. Available online: http://www.ccap.ac.uk/media/documents/BB_000.pdf (accessed on 20 April 2021).
- Hajimehdipoor, H.; Kondori, B.M.; Amin, G.R.; Adib, N.; Rastegar, H.; Shekarchi, M. Development of a validated HPLC method for the simultaneous determination of flavonoids in Cuscuta chinensis Lam. by ultra-violet detection. DARU J. Pharm. Sci. 2012, 20, 57. [Google Scholar] [CrossRef] [Green Version]
- Tzanova, M.; Grozeva, N.; Gerdzhikova, M.; Argirova, M.; Pavlov, D.; Terzieva, S. Flavonoid content and antioxidant activity of Betonica bulgarica Degen et Neič. Bulg. Chem. Commun. 2018, 50, 90–97. [Google Scholar]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.K.; Ayadi, M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2015, 5, 52–57. [Google Scholar]
- Mtaki, K.; Kyewalyanga, M.S.; Mtolera, M.S.P. Assessment of antioxidant contents and free radical-scavenging capacity of Chlorella vulgaris cultivated in low cost media. Appl. Sci. 2020, 10, 8611. [Google Scholar] [CrossRef]
- Bulut, O.; Akin, D.; Sönmez, Ģ.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic compounds, carotenoids, and antioxidant capacities of a thermo-tolerant Scenedesmus sp. (Chlorophyta) extracted with different solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Balendres, M.A.O.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the Philippines: A review. Foods 2019, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Shapiro, O.H.; Ziv, C.; Barda, O.; Zakin, V.; Sionov, E. Synergistic inhibition of mycotoxigenic fungi and mycotoxin production by combination of pomegranate peel extract and azole fungicide. Front. Microbiol. 2019, 10, 1919. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, R.; Joshi, V. Antimicrobial activity of rhizome extract of Acorus calamus against different microorganisms. Octa J. Biosci. 2014, 2, 59–63. [Google Scholar]
- Abedin, R.M.A.; Taha, H.M. Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis. GJBB 2008, 3, 22–31. [Google Scholar]
- Dantas, D.M.M.; Costa, R.M.P.B.; Carneiro-da-Cunha, M.G.; Galvez, A.O.; Drummond, A.R.; Bezerra, R.S. Bioproduction, antimicrobial and antioxidant activities of compounds from Chlorella vulgaris. Res. Rev. J. Bot. Sci. 2015, 4, 12–18. [Google Scholar]
- Vehapi, M.; Yilmaz, A.; Özçimen, D. Antifungal activities of Chlorella vulgaris and Chlorella minutissima microalgae cultivated in bold basal medium, wastewater and tree extract water against Aspergillus niger and Fusarium oxysporum. Rom. Biotechnol. Lett. 2018, 1, 1–8. [Google Scholar]
- Zielinski, D.; Fraczyk, J.; Debowsky, M.; Zielinski, M.; Kaminski, Z.J.; Kregiel, D.; Jacob, C.; Kolesinska, B. Biological activity of hydrophilic extract of Chlorella vulgaris grown on post-fermentation leachate from a biogas plant supplied with stillage and maize silage. Molecules 2020, 25, 1790. [Google Scholar] [CrossRef] [Green Version]
- Abbassy, M.A.; Marei, G.I.K.; Rabia, S.M.H. Antimicrobial activity of some plant and algal extracts. Int. J. Plant Soil Sci. 2014, 3, 1366–1373. [Google Scholar] [CrossRef]
- Effiong, B.N.; Sanni, A. Antifungal properties and phytochemical screening of crude extract of Lemna pauciscostata (Helgelm) against fish feed spoilage fungi. Life Sci. J. 2009, 6, 19–22. [Google Scholar]
- Sekhon, A.S.; Padhye, A.A.; Garg, A.K. In vitro sensitivity of Penicillium marneffei and Pythium insidiosum to various antifungal agents. Eur. J. Epidemiol. 1992, 8, 427–432. [Google Scholar] [CrossRef]
- Lalitha, P.; Shapiro, B.L.; Srinivasan, M. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch. Ophthalmol. 2007, 125, 789–793. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Cuenca-Estrella, M.; Monzón, A.; Mellado, E.; Rodríguez-Tudela, J.L. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J. Antimicrob. Chemother. 2008, 61, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Alastruey-Izquierdo, A.; Cuesta, I.; Ros, L.; Mellado, E.; Rodríguez-Tudela, J.L. Antifungal susceptibility profile of clinical Alternaria spp. identified by molecular methods. J. Antimicrob. Chemother. 2011, 66, 2585–2587. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, H.; Inuzuka, H.; Hori, N.; Takahashi, N.; Ishida, K.; Mochizuki, K.; Ohkusu, K.; Muraosa, Y.; Watanabe, A.; Kamei, K. Inhibitory effects of antimicrobial agents against Fusarium species. Med. Mycol. 2015, 53, 603–611. [Google Scholar] [CrossRef] [Green Version]
- Dimitrova-Dyulgerova, I.; Merdzhanov, P.; Todorov, K.; Seymenska, D.; Stoyanov, P.; Mladenov, R.; Stoyanova, A. Essential oils composition of Betonica officinalis L. and Stachys sylvatica L. (Lamiaceae) from Bulgaria. Comptes Rendus Acad. Bulg. Sci. 2015, 68, 991–998. [Google Scholar]
- Dinev, T.G.; Rusenova, N.V.; Tzanova, M.T.; Grozeva, N.H.; Gerdzhikova, M.A.; Stoyanov, P.S.; Mladenova, T.R.; Beev, G.G. Antimicrobial potential of methanolic extracts from Betonica bulgarica Degen et Neič. (Lamiaceae). Ecol. Balk. 2020, 12, 165–174. [Google Scholar]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Tanase, C.; Coşarcă, S.; Muntean, D.-L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [Green Version]
- El-Chaghaby, G.A.; Rashad, S.; Abdel-Kader, S.F.; Rawash, E.-S.A.; Moneem, M.A. Assessment of phytochemical components, proximate composition and antioxidant properties of Scenedesmus obliquus, Chlorella vulgaris and Spirulina platensis algae extracts. Egypt. J. Aquat. Biol. Fish. 2019, 23, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Karim, O.H.; Gheda, S.F.; Ismail, G.A.; Abo-Shady, A.M. Phytochemical screening and antioxidant activity of Chlorella vulgaris. Sci. JBAS 2020, 41, 76–86. [Google Scholar]
- Bhuvana, P.; Anuradha, V.; Syed, A.M.; Suganya, V.; Sangeetha, P. In vitro antioxidant activity of methanolic extracts of Chlorella vulgaris. IJAR 2017, 5, 1465–1474. [Google Scholar]
- Gürlek, C.; Yarkent, Ç.; Köse, A.; Tuğcu, B.; Gebeloğlu, I.K.; Öncel, S.Ş.; Elibol, M. Screening of antioxidant and cytotoxic activities of several microalgal extracts with pharmaceutical potential. Health Technol. 2020, 10, 111–117. [Google Scholar] [CrossRef]
- Funde, S.G. Phytochemicals evaluation, anticancer, antioxidant and antimicrobial activity of Acorus calamus different solvent extracts. J. Chem. Pharm. Res. 2015, 7, 495–504. [Google Scholar]
- Parki, A.; Chaubey, P.; Prakash, O.; Kumar, R.; Pant, A.K. Chemical composition and antioxidant activity of Acorus calamus L. accessions from different altitudes of Uttarakhand Himalayas. J. Herb. Drug. 2019, 9, 171–178. [Google Scholar]
- Chaubey, P.; Archana; Prakash, O.; Rai, K.; Kumar, R.; Pant, A.K. In vitro antioxidant activity and total phenolic content of rhizome extracts from Acorus calamus Linn. Asian J. Chem. 2017, 29, 2357–2360. [Google Scholar] [CrossRef]
- Devi, S.A.; Ganjewala, D. Antioxidant activities of methanolic extracts of sweet-flag (Acorus calamus) leaves and rhizomes. J. Herbs Spices Med. Plants 2011, 17, 1–11. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Kozłowska, S.; Szostak-Wegierek, D. Flavonoids—Food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Coulombier, N.; Nicolau, E.; Déan, L.L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of light intensity on antioxidant activity of tropical microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- Zayova, E.; Stancheva, I.; Geneva, M.; Petrova, M.; Dimitrova, L. Antioxidant activity of in vitro propagated Stevia rebaudiana Bertoni plants of different origins. Turk. J. Biol. 2013, 37, 106–113. [Google Scholar]

| Plant Extract | Diameter of Inhibition Zones (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A. flavus | A. parasiticus | A. niger | A. carbonarius | A. ochraceus | F. graminearum | F. oxysporum | P. chrysogenum | Alt. alternata | |
| A. calamus | 10.0 ± 0 ab | - ** | 9.3 ± 0.3 ab | 7.8 ± 0.3 a | 8.7 ± 0.3 ab | 9.2 ± 0.3 ab | 10.3 ± 0.6 ab | 7.7 ± 0.3 a | 8.2 ± 0.3 a |
| C. vulgaris | - ** | - ** | 9.2 ± 0.3 ab | - ** | - ** | - ** | - ** | 8.3 ± 0.3 a | 7.0 ± 0 a |
| S. dimorphus | - ** | - ** | 8.2 ± 0.6 a | - ** | - ** | - ** | - ** | 8.0 ± 0 a | - ** |
| L. minuta | - ** | - ** | - ** | - ** | 7.0 ± 0 a | - ** | - ** | 7.0 ± 0 a | 7.7 ± 0.3 a |
| Amphotericin B | 11.5 ± 0.3 ac | 11.0 ± 0 ac | 9.0 ± 0 ab | 13.8 ± 0.3 ac | - ** | - ** | - ** | - ** | 11.5 ± 0.3 ac |
| Methanol | 6.0 ± 0 a | 6.0 ± 0 a | 6.0 ± 0 a | 6.0 ± 0 a | 6.0 ± 0 a | 6.0 ± 0 a | 6.0 ± 0 a | 6.0 ± 0 a | 6.0 ± 0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinev, T.; Tzanova, M.; Velichkova, K.; Dermendzhieva, D.; Beev, G. Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus calamus L., Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing. Appl. Sci. 2021, 11, 4745. https://doi.org/10.3390/app11114745
Dinev T, Tzanova M, Velichkova K, Dermendzhieva D, Beev G. Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus calamus L., Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing. Applied Sciences. 2021; 11(11):4745. https://doi.org/10.3390/app11114745
Chicago/Turabian StyleDinev, Toncho, Milena Tzanova, Katya Velichkova, Diyana Dermendzhieva, and Georgi Beev. 2021. "Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus calamus L., Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing" Applied Sciences 11, no. 11: 4745. https://doi.org/10.3390/app11114745
APA StyleDinev, T., Tzanova, M., Velichkova, K., Dermendzhieva, D., & Beev, G. (2021). Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus calamus L., Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing. Applied Sciences, 11(11), 4745. https://doi.org/10.3390/app11114745









