Recent Applications of Deep Eutectic Solvents in Environmental Analysis
Abstract
1. Introduction
2. Recent Applications of DESs in Environmental Analysis
2.1. Analysis of Organic Compounds in Environmental Samples
2.2. Analysis of Inorganic Compounds in Environmental Samples
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Analytes | |
| EDC | endocrine disrupting compound |
| iHg | inorganic mercury |
| OPP | organophosphorus pesticide |
| PAE | phthalic acid ester |
| PAH | polycyclic aromatic hydrocarbon |
| DES | components |
| ChCl | choline chloride |
| DMU | N,N′-dimethyl urea |
| DTMAC | dodecyltrimethylammonium chloride |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| TBAB | tetrabutylammonium bromide |
| TBAC | tetrabutylammonium chloride |
| THTDPC | trihexyl(tetradecyl)phosphonium chloride |
| TOAC | trioctylammonium chloride |
| TOMAC | trioctylmethylammonium chloride |
| Extraction procedure | |
| AA | air-assisted |
| DLLME | dispersive liquid–liquid microextraction |
| ELLME | emulsification liquid–liquid microextraction |
| GAPI | Green Analytical Procedure Index |
| GLE | gas–liquid extraction |
| LLME | liquid–liquid microextraction |
| m-µ-dSPE | magnetic-micro-dispersive solid-phase extraction |
| SA | shaker-assisted |
| SAP | solidified aqueous phase |
| SFOD | solidified floating organic droplet |
| SLE | solid–liquid extraction |
| UA | ultrasound-assisted |
| UAE | ultrasound-assisted extraction |
| VA | vortex-assisted |
| Analytical technique | |
| µ-ECD | micro-electron capture detector |
| AAS | atomic absorption spectrometry |
| CPMAS/NMR | cross-polarisation magic-angle spinning/nuclear magnetic resonance |
| DAD | diode array detector |
| ETAAS | electrothermal atomic absorption spectrometry |
| FAAS | flame atomic absorption spectrometry |
| FD | fluorescence detector |
| GC | gas chromatography |
| HG | hydride generation |
| HPLC | high performance liquid chromatography |
| ICP | inductively coupled plasma |
| LOD | limit of detection |
| MS/MS | tandem mass spectrometry |
| MS | mass spectrometry |
| OES | optical emission spectrometry |
| PDA | photodiode array detector |
| QqQ | triple quadrupole |
| UHPLC | ultra-high performance liquid chromatography |
| UV | ultraviolet |
| VWD | variable wavelength detector |
| Reagents, sorbents and solvents | |
| ACN | acetonitrile |
| CRM | certified reference material |
| DES | deep eutectic solvent |
| GRAS | generally recognised as safe |
| HDES | hydrophobic deep eutectic solvent |
| IL | ionic liquid |
| m-NP | magnetic nanoparticle |
| MWCNT | multi-walled carbon nanotube |
| NaDES | natural deep eutectic solvent |
| SUPRAS | supramolecular solvent |
| TDES | ternary deep eutectic solvent |
| THF | tetrahydrofuran |
| β-CD | beta-cyclodextrin |
References
- Huang, T.; Tang, X.; Luo, K.; Wu, Y.; Hou, X.; Tang, S. An overview of graphene-based nanoadsorbent materials for environmental contaminants detection. TrAC Trends Anal. Chem. 2021, 139, 116255. [Google Scholar] [CrossRef]
- Chen, T.-L.; Kim, H.; Pan, S.-Y.; Tseng, P.-C.; Lin, Y.-P.; Chiang, P.-C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, N.; Passos, H.; Billard, I.; Papaiconomou, N.; Coutinho, J.A. Recovery of metals from waste electrical and electronic equipment (WEEE) using unconventional solvents based on ionic liquids. Crit. Rev. Environ. Sci. Technol. 2018, 48, 859–922. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Dehdashtian, S. Application of green solvents as sorbent modifiers in sorptive-based extraction techniques for extraction of environmental pollutants. TrAC Trends Anal. Chem. 2018, 109, 50–61. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Emerging green solvents and their applications during pesticide analysis in food and environmental samples. Talanta 2021, 223, 121507. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kang, S.; Baik, N.; Hwang, I.; Chung, D.S. Applications of deep eutectic solvents to quantitative analyses of pharmaceuticals and pesticides in various matrices: A brief review. Arch. Pharmacal. Res. 2020, 43, 900–919. [Google Scholar] [CrossRef] [PubMed]
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Quality assessment of environmental water by a simple and fast non-ionic hydrophobic natural deep eutectic solvent-based extraction procedure combined with liquid chromatography tandem mass spectrometry for the determination of plastic migrants. Anal. Bioanal. Chem. 2021, 413, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Karami, F.; Nouri, N. A green dispersive liquid-liquid microextraction based on deep eutectic solvents doped with β-cyclodextrin: Application for determination of tetracyclines in water samples. Microchem. J. 2021, 163, 105914. [Google Scholar] [CrossRef]
- Ma, W.; Row, K.H. pH-induced deep eutectic solvents based homogeneous liquid-liquid microextraction for the extraction of two antibiotics from environmental water. Microchem. J. 2021, 160, 105642. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, L.; Xia, H.; Peng, M.; Zhou, Y.; Xu, Z.; Peng, X. Dispersive liquid-liquid microextraction based on a new hydrophobic deep eutectic solvent for the determination of phenolic compounds in environmental water samples. J. Sep. Sci. 2021, 1510–1520. [Google Scholar] [CrossRef]
- Gissawong, N.; Mukdasai, S.; Boonchiangma, S.; Sansuk, S.; Srijaranai, S. A rapid and simple method for the removal of dyes and organophosphorus pesticides from water and soil samples using deep eutectic solvent embedded sponge. Chemosphere 2020, 260, 127590. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. A green air assisted-dispersive liquid-liquid microextraction based on solidification of a novel low viscous ternary deep eutectic solvent for the enrichment of endocrine disrupting compounds from water. J. Chromatogr. A 2020, 1629, 461498. [Google Scholar] [CrossRef]
- Li, K.; Jin, Y.; Jung, D.; Park, K.; Kim, H.; Lee, J. In situ formation of thymol-based hydrophobic deep eutectic solvents: Application to antibiotics analysis in surface water based on liquid-liquid microextraction followed by liquid chromatography. J. Chromatogr. A 2020, 1614, 460730. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Qian, H.; Qu, Y.; Zhang, S.; Lu, R.; Gao, H.; Zhou, W. Ultrasound-assisted dispersive liquid-liquid microextraction based on a hydrophobic deep eutectic solvent for the preconcentration of pyrethroid insecticides prior to determination by high-performance liquid chromatography. Microchem. J. 2019, 146, 614–621. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Zhang, H.; Xu, W. Hydrophobic deep eutectic solvent-based dispersive liquid–liquid microextraction for the simultaneous enantiomeric analysis of five β-agonists in the environmental samples. Electrophoresis 2019, 40, 2828–2836. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Raeissi, S. Deep eutectic solvents for CO2 capture from natural gas by energy and exergy analyses. J. Environ. Chem. Eng. 2019, 7, 103411. [Google Scholar] [CrossRef]
- Ge, D.; Zhang, Y.; Dai, Y.; Yang, S. Air-assisted dispersive liquid-liquid microextraction based on a new hydrophobic deep eutectic solvent for the preconcentration of benzophenone-type UV filters from aqueous samples. J. Sep. Sci. 2018, 41, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.M.; Shemirani, F.; Ghorbanian, S.A. Hydrophobic Deep Eutectic Solvents in Developing Microextraction Methods Based on Solidification of Floating Drop: Application to the Trace HPLC/FLD Determination of PAHs. Chromatographia 2018, 81, 1201–1211. [Google Scholar] [CrossRef]
- Kandanelli, R.; Thulluri, C.; Mangala, R.; Rao, P.V.; Gandham, S.; Velankar, H.R. A novel ternary combination of deep eutectic solvent-alcohol (DES-OL) system for synergistic and efficient delignification of biomass. Bioresour. Technol. 2018, 265, 573–576. [Google Scholar] [CrossRef]
- Zarei, A.R.; Nedaei, M.; Ghorbanian, S.A. Ferrofluid of magnetic clay and menthol based deep eutectic solvent: Application in directly suspended droplet microextraction for enrichment of some emerging contaminant explosives in water and soil samples. J. Chromatogr. A 2018, 1553, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.M.; Shemirani, F.; Ghorbanian, S.A. Deep eutectic solvent magnetic bucky gels in developing dispersive solid phase extraction: Application for ultra trace analysis of organochlorine pesticides by GC-micro ECD using a large-volume injection technique. Talanta 2017, 168, 73–81. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.; Marrucho, I. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilib. 2017, 448, 135–142. [Google Scholar] [CrossRef]
- Werner, J. Ligandless, deep eutectic solvent-based ultrasound-assisted dispersive liquid-liquid microextraction with solidification of the aqueous phase for preconcentration of lead, cadmium, cobalt and nickel in water samples. J. Sep. Sci. 2019, 43, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Craveiro, R.; Faria, P.; Silva, A.; Mateus, E.; Barreiros, S.; Paiva, A.; Ribeiro, A. Electrodialytic removal of tungsten and arsenic from secondary mine resources–Deep eutectic solvents enhancement. Sci. Total Environ. 2020, 710, 136364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, X.; Liang, P. Preconcentration of Copper and Lead Using Deep Eutectic Solvent Modified Magnetic Nanoparticles and Determination by Inductively Coupled Plasma Optical Emission Spectrometry. At. Spectrosc. 2020, 41, 36–42. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. Natural deep eutectic solvent-based ultrasound-assisted-microextraction for extraction, pre-concentration and analysis of methylmercury and total mercury in fish and environmental waters by spectrophotometry. Food Addit. Contam. Part A 2019, 36, 1079–1097. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. Innovative and practical deep eutectic solvent based vortex assisted microextraction procedure for separation and preconcentration of low levels of arsenic and antimony from sample matrix prior to analysis by hydride generation-atomic absorption spectrometry. Food Chem. 2019, 293, 378–386. [Google Scholar] [CrossRef]
- Söldner, A.; Zach, J.; König, B. Deep eutectic solvents as extraction media for metal salts and oxides exemplarily shown for phosphates from incinerated sewage sludge ash. Green Chem. 2018, 21, 321–328. [Google Scholar] [CrossRef]
- Erbas, Z.; Soylak, M.; Yilmaz, E.; Dogan, M. Deep eutectic solvent based liquid phase microextraction of nickel at trace level as its diethyldithiocarbamate chelate from environmental samples. Microchem. J. 2019, 145, 745–750. [Google Scholar] [CrossRef]
- Panhwar, A.; Tuzen, M.; Kazi, T.G. Use of Deep Eutectic Solvent-based Air-assisted Emulsification Liquid-liquid Microextraction of Palladium and Determination by Flame Atomic Absorption Spectrometry in Water and Environmental Samples. At. Spectrosc. 2019, 40, 227–232. [Google Scholar] [CrossRef]
- Ezoddin, M.; Lamei, N.; Siami, F.; Abdi, K.; Karimi, M.A. Deep Eutectic Solvent Based Air Assisted Ligandless Emulsification Liquid–Liquid Microextraction for Preconcentration of Some Heavy Metals in Biological and Environmental Samples. Bull. Environ. Contam. Toxicol. 2018, 101, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ali, M.C.; Yang, Q.; Zhang, Z.; Bao, Z.; Su, B.; Xing, H.; Ren, Q. Hybrid Deep Eutectic Solvents with Flexible Hydrogen-Bonded Supramolecular Networks for Highly Efficient Uptake of NH3. ChemSusChem 2017, 10, 3368–3377. [Google Scholar] [CrossRef]
- Matong, J.M.; Nyaba, L.; Nomngongo, P.N. Determination of As, Cr, Mo, Sb, Se and V in agricultural soil samples by inductively coupled plasma optical emission spectrometry after simple and rapid solvent extraction using choline chloride-oxalic acid deep eutectic solvent. Ecotoxicol. Environ. Saf. 2017, 135, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Zounr, R.A.; Tuzen, M.; Khuhawar, M.Y. Ultrasound assisted deep eutectic solvent based on dispersive liquid liquid microextraction of arsenic speciation in water and environmental samples by electrothermal atomic absorption spectrometry. J. Mol. Liq. 2017, 242, 441–446. [Google Scholar] [CrossRef]
- Panhwar, A.H.; Tuzen, M.; Kazi, T.G. Ultrasonic assisted dispersive liquid-liquid microextraction method based on deep eutectic solvent for speciation, preconcentration and determination of selenium species (IV) and (VI) in water and food samples. Talanta 2017, 175, 352–358. [Google Scholar] [CrossRef]
- Karimi, M.; Shabani, A.M.H.; Dadfarnia, S. Deep eutectic solvent-mediated extraction for ligand-less preconcentration of lead and cadmium from environmental samples using magnetic nanoparticles. Microchim. Acta 2016, 183, 563–571. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Ultrasound assisted-deep eutectic solvent based on emulsification liquid phase microextraction combined with microsample injection flame atomic absorption spectrometry for valence speciation of chromium(III/VI) in environmental samples. Talanta 2016, 160, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Xu, X.; Wan, Y. DES-Fe3O4 composite for rapid extraction of residual plant growth regulators in edible vegetable oil. Chin. Chem. Lett. 2019, 30, 1182–1185. [Google Scholar] [CrossRef]

| Sample (Amount) | Analytes | DES (Molar Ratio; Amount) | Extraction Procedure | Analytical Technique | Recovery % | LODs | Comments | Reference |
|---|---|---|---|---|---|---|---|---|
| Treated wastewater, runoff, and pond water(10 mL) | 14 PAEs and one adipate | Menthol:thymol (1:2; 100 µL) | VA-LLME | UHPLC–QqQ-MS/MS | 70–127 | 0.013–0.425 μg/L | - Molar ratios of 2:1 and 1:2 were also studied. - Final extract was 20-fold diluted with ACN. - The method was applied to the analysis of real samples. | [11] |
| Well, rainforest, coastal sea, gardening and mineral water (10 mL) | 3 tetracyclines | Thymol:octanoic acid (1:1; 175 µL) | DLLME | HPLC–UV | 74–113 | 1.37–4.38 μg/L | - Nonanoic and decanoic acids were also tested as HBDs. - ChCl:ethylene glycol (1:2) DES was used as disperser (750 µL). - The extraction media was doped with β-CD (5 mg). - The method was applied to the analysis of real samples. - Greenness of the method was assessed using analytical eco-scale and GAPI approaches. | [12] |
| Feed, tap and wastewater (5 mL) | Levofloxacin and ciprofloxacin | Thymol:hexanoic acid (2:1; 100 µL) | Acid-base induced LLME | HPLC–UV | 95–111 | 0.018–0.027 mg/L | - Octanoic, decanoic and dodecanoic acids were also tested as HBDs. - Molar ratios of 1:1, 1:2 and 3:1 were also studied. - KOH (6 M,100 µL) and HCl (6 M, 150 µL) solutions were used to induce pH changes. - Final extract was 10-fold diluted with methanol. | [13] |
| Tap, lake and wastewater (3.2 mL) | 4 phenolic pesticides | α-Terpineol:octanoic acid (1:2; 100 µL) | UA-DLLME | HPLC–DAD | 82–99 | 0.15–0.38 µg/L | - Undecanol and nonanoic acid were also tested as HBDs. - Molar ratios of 1:1 and 2:1 were studied. - ACN was used as disperser (100 µL). - The method was applied to the analysis of real samples. | [14] |
| Waste and agricultural water (10 mL) Agricultural soil (1 g) | 5 OPPs and 2 dyes | TBAB:decanoic acid (1:3; 200 µL) | VA-LLME in sponge | HPLC–VWD UV–visible | 70–95 | - | - DES was used for pollutants’ removal. - Heptanoic, decanoic and 4-phenylbutyric acids were also tested as HBDs. - Molar ratios of 1:1 and 1:4 were also studied. - Greenness of the method was assessed using analytical eco-scale approach. | [15] |
| River and tap water (5 mL) | 5 EDCs | Nonanoic acid:decanoic acid:dodecanoic acid (1:1:1; 200 µL) | AA-SFOD-LLME | HPLC–PDA | 90–104 | 0.96–2.30 μg/L | - Other binary DESs and TDESs formed by octanoic, nonanoic, decanoic and dodecanoic acids combinations, at different molar ratios, were also investigated. - Six pulling in/pushing out cycles were performed. - Final extract was 2-fold diluted with ACN. - The method was applied to the analysis of real samples. - Greenness of the method was assessed using Raynie pictogram, analytical eco-scale and GAPI approaches. | [16] |
| Surface water (10 mL) | 4 fluoroquinolones | Thymol:heptanoic acid (2:1; 100 µL) | SA-LLME | HPLC–UV | 83–119 | 3 µg/L | - In situ formation of HDESs was carried out. - Other DESs formed by monoterpenes, fatty acids and benzoate ester combinations, at molar ratio of 1:1, were also investigated. - Molar ratios of 1:1, 1:2, 1:3 and 3:1 were also studied. - Final extract was 4-fold diluted with mobile phase. | [17] |
| River and lake water (8 mL) | 5 pesticides | THTDPC:dodecanoic acid (1:2; 30 mg) | UA-LLME | HPLC–UV | 81–110 | 0.30–0.60 μg/L. | - Octanoic and decanoic acids were also tested as HBDs. - Molar ratios of 1:1, 2:3, 2:5 and 1:3 were also studied. - Final extract was diluted with methanol (50 µL). | [18] |
| Surface and river water (10 mL) | 5 β-agonists | TBAC:octanoic acid (1:3; 500 µL) | VA-DLLME | UHPLC–QqQ-MS/MS | 57–91 | 0.4–0.8 µg/L | - TOMAC, TBAC and DTMAC as HBAs combined with decanoic, octanoic and lauric acids as HBDs were investigated at molar ratio of 1:2. - Molar ratios of 1:4 and 1:5 were also studied. - ACN was used as disperser (1 mL). - Final extract was 1.5-fold diluted with methanol. - The method was applied to the analysis of real samples. | [19] |
| Natural gas (-) | CO2 | ChCl-based DESs (-; -) | - | - | - | - | - DESs were used for CO2 capture. - Urea and glycerol were also tested as HBDs. - Energy and exergy analyses were carried out. | [20] |
| Swimming pool, river and wastewater (8 mL) | 6 benzophenone-type UV filters | Menthol:decanoic acid (1:1; 100 µL) | AA-LLME | HPLC–DAD | 89–106 | 0.05–0.20 µg/L | - TBAB, TBAC, and menthol as HBAs combined with octanoic, decanoic and dodecanoic acids as HBDs were investigated at different molar ratios. - Five pulling in/pushing out cycles were performed. - The method was applied to the analysis of real samples. | [21] |
| River, lagoon, lake and well water (20 mL) | 6 PAHs | TBAB:decanoic acid (1:2; 80 µL) | UA-SFOD-LLME | HPLC–FD | 83–117 | 0.7–6.6 ng/L | - Oleic, decanoic, octanoic, propionic, acrylic, acetic and butyric acids were also tested as HBDs. - Molar ratios of 1:1, 1:1.5 and 2:1 were also studied. - Final extract was 2.5-fold diluted with mobile phase. - The method was applied to the analysis of real samples. | [22] |
| Rice husk, rice straw and wheat straw (2.25 g) | Lignin | ChCl:oxalic acid:n-butanol (2:2:1; 15 mL) | Heating and stirring | Microscopy and CPMAS/NMR studies | 50 | - | - DES combined with n-butanol was used for biomass delignification. - Other alcohols were tested, including n-propanol and ethyl acetate. - Different DES-to-alcohol ratios were studied, including 2:1. 1:1 and 1:2. | [23] |
| Well and surface water and soil (10 mL) | 11 explosives (nitrogen compounds) | Menthol:decanoic acid (1:2; 50 µL) | m-µ-dSPE | HPLC–UV | 88–104 | 0.22–0.91 µg/L | - DES was combined with montmorillonite clay@Fe3O4 (50 mg) to form a ferrofluid. - The extraction capacity of the nanocomposite, DES and ferrofluid was evaluated separately. - Seventeen carboxylic acids were tested as HBDs. - Molar ratios of 2:1 and 1:1 were studied in some cases. - ACN was used as back-extraction solvent (500 µL). - The method was applied to the analysis of real samples. | [24] |
| Farm, rural, lake and river water (50 mL) | 18 OPPs | ChCl:urea (1:2, 50 µL) | m-µ-dSPE | GC–µ-ECD | 80–115 | 0.04–0.27 ng/L | - DES was combined with MWCNTs@Fe3O4 (5 mg) to form a ferrofluid. - DES has a dual role: carrier and stabiliser for MWCNTs. - Phenol, acetic acid and glycerol were also tested as HBDs. - Molar ratios of 1:1, 1:1.5 and 2:1 were also studied. ACN was used as back-extraction solvent (150 µL). - The method was applied to the analysis of real samples. | [25] |
| Sample (Amount) | Analytes | DES (Molar Ratio; Amount) | Extraction Procedure | Analytical Technique | Recovery % | LODs | Comments | Reference |
|---|---|---|---|---|---|---|---|---|
| River, lake, and well water (15 mL) | Pb(II), Cd(II), Co(II), Ni(II) | THTDPC:thiosalicylic acid (1:2; 30 µL) | UA-SAP–LLME | HPLC–UV | 91–102 | 0.05–0.13 µg/L | - DES has a dual role: extractant and complexing agent. - 4,4′-thiobisbenzenethiol was also tested as HBD. - Molar ratios of 1:1 and 1:3 were also studied. - Final extract was 3-fold diluted with ACN. - Accuracy was evaluated using CRMs. - The method was applied to the analysis of real samples. | [27] |
| Mining residues (39 g) | W, As | ChCl:malonic acid (1:2; 5 mL) ChCl:oxalic acid (1:1; 5 mL) | Electrodialytic removal | ICP–OES | 22–35 | - | - ChCl:lactic acid (1:2) and propionic acid:urea (2:1) were also tested. - Liquid-to-solid ratio of 9/1 was used. - Conventional SLE, with electrical current, and electrodyalitic process with and without DES was compared. - Extraction times of 4–10 days were employed. | [28] |
| Lake and tap water (50 mL) Sediments and soil (0.05 g) | Cu(II), Pb(II) | ChCl:urea (1:2; -) | m-µ-dSPE | ICP–OES | 94–102 | 0.29–0.51 µg/L | - DES@Fe3O4 m-NPs (30 mg) was used as adsorbent. - Ethylene glycol, oxalic acid, and glycerol were also tested as HBDs at different molar ratios. - Molar ratios of 1:1.5 and 1:3 were also studied. - 1 M HNO3 was used as back-extraction solvent (500 µL). - A selectivity study was carried out using potential interfering ions. - Accuracy was evaluated using CRMs. - The method was applied to the analysis of real samples. | [29] |
| Lake, well, dam, dental and industrial wastewater (60 mL) | Hg, iHg, CH3Hg | Betaine:sorbitol (1:3; 600 µL) | UA-ELLME | UV/visible | 92–99 | 0.25–0.92 µg/L | - Different sample pre-treatments were carried out. - ChCl as HBA and glycerol, sorbitol, glucose, lactic acid, and sucrose as HBDs were also tested at different molar ratios. - Molar ratios of 1:1, 2:1, 1:2, 2:3, and 3:2 were also studied. - NaDES contained 10% water (v/v). - 3-(dimethylamino)-7-(methylamino)phenothiazin-5-ium chloride was used as complexing agent (300 µL, 1 mM). - ACN was used as emulsifier (375 µL). - Final extract was diluted with acidic methanol (0.1 M HCl) up to 0.5 mL. - A selectivity study for CH3Hg was carried out using potential interfering ions. - Fish samples were also evaluated. - The method was applied to the analysis of real samples. | [30] |
| Well, tap, river, and wastewater (125 mL) | As(III/V), Sb(III/V) | ChCl:oxalic acid (1:3; 700 µL) | VA-ELLME | HG–AAS | 94–96 | 7.5–15.6 ng/L | - TBAC and TOAC as HBAs and phenol, glycerol, and decanoic acid as HBDs were also tested at different molar ratios. - Molar ratios of 1:1, 1:2, 1:4, and 2:1 were also studied. - Dithizone (600 µL) was used as complexing agent. - THF was used as emulsifier (300 µL). - Final extract was diluted with acidic ethanol up to 2.5 mL. - A selectivity study was carried out using potential interfering ions. - Bottled water, honey, and rice were also evaluated. - The method was applied to the analysis of real samples. | [31] |
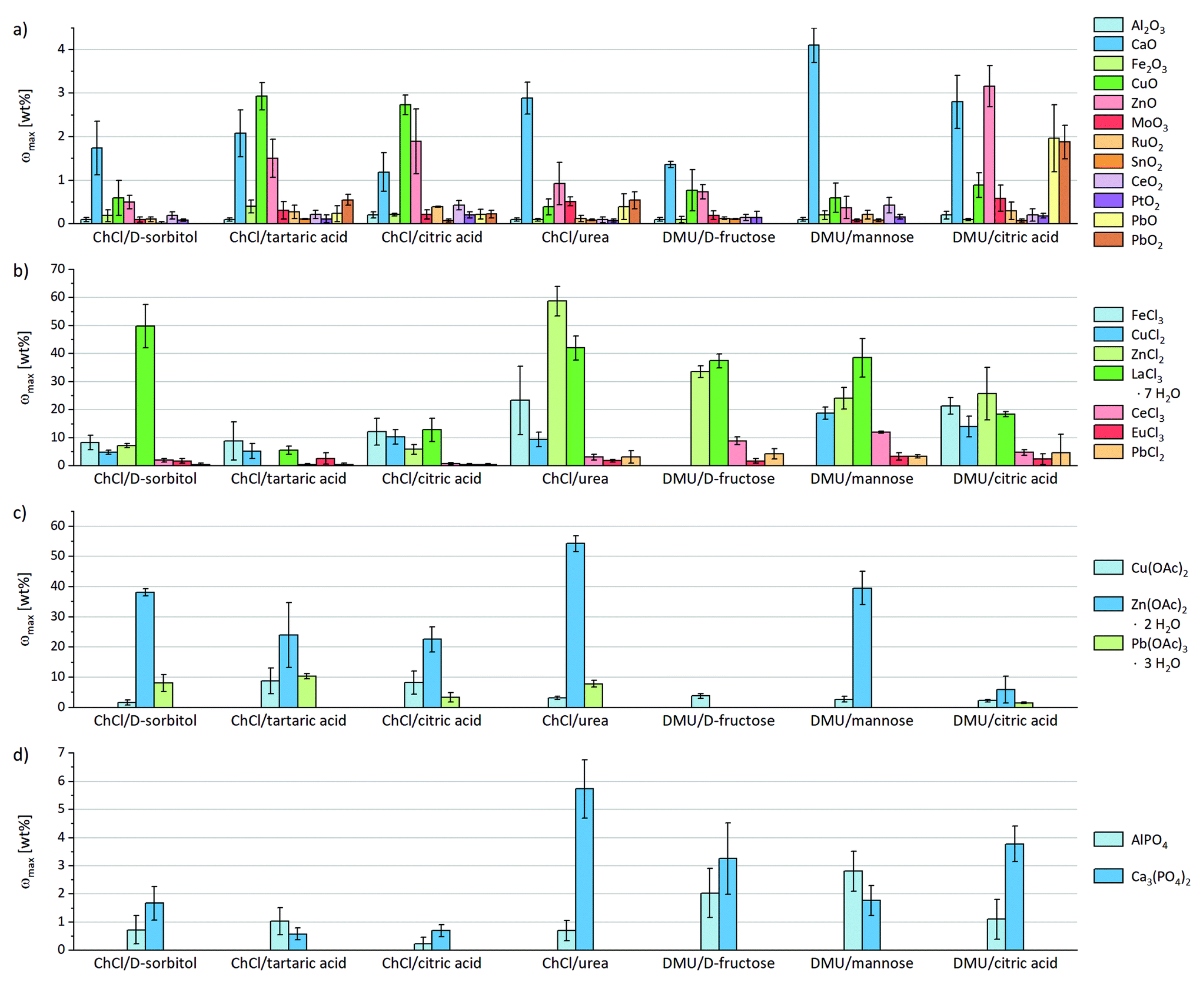
| Incinerated sewage sludge ash (0.1 g) | AlPO4 and Ca3(PO4)2 | ChCl and DMU-based DESs (-; 5 g) | SLE | ICP–OES ICP–MS | 1–47 | - | - d-sorbitol, tartaric and citric acid, urea, d-fructose, and mannose were tested as HBDs. - Solubility experiments of 12 metal oxides, 7 chlorides, 3 acetates, 2 phosphates, CaSO4, and CaCO3 in 7 different DESs were carried out. - Heavy metals were also measured by ICP–MS, AAS, or cold-vapor atomic spectrometry. | [32] |
| Sea, well, and wastewater (30 mL) | Ni(II) | TBAC:decanoic acid (1:3; 100 µL) | UA-DLLME | FAAS | 91–99 | 0.13 µg/L | - Molar ratios of 1:2 and 1:4 were also studied. - Sodium diethyldithiocarbamate was used as complexing agent (200 µL, 0.15% w/v). - THF was used as disperser (250 µL). - Final extract was 2-fold diluted with HNO3 conc. - A selectivity study was carried out using potential interfering ions. - Accuracy was evaluated using CRMs. - Mineral water samples were also evaluated. - The method was applied to the analysis of real samples. | [33] |
| Tap, river, and sea water (35 mL) Dust and catalytic converter (1.0 g) | Pd(II) | ChCl:phenol (1:4; 500 µL) | AA-ELLME | FAAS | 99 | 1.2 µg/L | - Urea and oxalic acid were also tested as HBDs. - Molar ratios of 1:1, 1:2, and 1:3 were also studied. - 2-hydroxy-3-methoxy benzaldehyde thiosemicarbazone was used as complexing agent (400 µL, 0.1% w/v). - THF was used as emulsifier (800 µL). - Eight pulling in/pushing out cycles were performed. - A selectivity study was carried out using potential interfering ions. - Accuracy was evaluated using a CRM. - Mineral water samples were also evaluated. - The method was applied to the analysis of real samples. | [34] |
| Tap water (20 mL) | Cd, Ni, Pb, Cu | ChCl:5,6,7,8-tetrahydro-5,5,8,8-tetramethylnaphthalen-2-ol (1:2; 50 µL) | AA-ELLME | FAAS | 99–106 | 0.31–0.99 µg/L | - Molar ratios of 1:1 and 1:3 were also studied. - A mixture of DES:triethylamine (1:1, v/v) was used as extraction solvent (100 µL). - THF was used as emulsifier (100 µL). - Five pulling in/pushing out cycles were performed. - A selectivity study was carried out using potential interfering ions. - Accuracy was evaluated using a CRM. - Bottled water, black tea, and urine samples were also evaluated. | [35] |
| NH3 gas (-) | NH3 | ChCl:resorcinol:glycerol (1:5:5; 10 g) | GLE | Gravimetry | - | - | - Different HBDs were tested to form binary DESs and TDESs. - A maximum mass solubility of 0.13 g/g at 40 °C was achieved. - A selectivity study of NH3 to CO2 was carried out. | [36] |
| Soil (100 mg) | As, Cr, Mo, Sb, Se, V | ChCl:oxalic acid (1:2; 10 mL) | UAE | ICP–OES | 99–102 | 0.009–0.1 mg/kg | - Molar ratios of 1:1, 1:1.5, and 2:1 were also studied. - 2 M HNO3 was used as co-extractant (5 mL). - Final extract was diluted with water up to 25 mL. - Optimisation of the method was carried out using a CRM. - Accuracy was evaluated using a CRM. - The method was compared with a conventional acid digestion method. - The method was applied to the analysis of real clay-loamy soil, slit loam, and loamy sand samples. | [37] |
| Lake, mineral, tap and river water (25 mL) Sediments and soil (500 mg) | As(III/V) | ChCl:phenol (1:3; 1000 µL) | UA-ELLME | ETAAS | 96–99 | 10 ng/L | - Solid samples were previously acid digested. - Molar ratios of 1:1 and 1:2 were also studied. - Sodium diethyldithiocarbamate was used as complexing agent (500 µL, 0.1% w/v). - THF was used as emulsifier (500 µL). - Final extract was diluted with acidic ethanol up to 1 mL. - A selectivity study was carried out using potential interfering ions. - Accuracy was evaluated using CRMs. - Edible mushrooms, green and black tea, rice, and cigarette samples were also evaluated. - The method was applied to the analysis of real samples. | [38] |
| Tap water (25 mL) | Se, Se(IV/VI) | ChCl:phenol (1:3; 500 µL) | UA-ELLME | ETAAS | 96–99 | 4.61 ng/L | - TOAC and TBAC as HBAs and decanoic acid as HBD were also tested at molar ratio 1:2. - Molar ratios of 1:1 and 1:2 were also studied. - 3,3′-diaminobenzidine was used as complexing agent (400 µL, 4 mM). - THF was used as emulsifier (500 µL). - Final extract was diluted with acidic ethanol up to 500 µL. - A selectivity study was carried out using potential interfering ions. - Accuracy was evaluated using a CRM. - Mineral water and iced tea, as well as different food samples were also evaluated. | [39] |
| Soil (0.2 g) River, tap, well, and sea water (60 mL) | Pb(II), Cd(II) | ChCl:urea (1:2.5; 200 µL) | m-µ-dSPE | FAAS | 95–105 | 0.1–0.4 µg/L | - Soil samples were previously acid digested. - Fe3O4 m-NPs (20 mg) were separately added to the sample. - Ethylene glycol and oxalic acid were also tested as HBDs at molar ratio 1:2. - Other molar ratios of ChCl:urea were tested. - Al2O3 and TiO2 were also evaluated in combination with DES. - 1 M HNO3 was used as back-extraction solvent (600 µL). - A selectivity study was carried out using potential interfering ions. - Hair samples were also evaluated. - The method was applied to the analysis of real samples. | [40] |
| Tap, lake, and factory wastewater (10 mL) | Cr, Cr(III/VI) | ChCl:phenol (1:3; 450 µL) | UA-ELLME | FAAS | 93–108 | 5.5 µg/L | - TBAC and TOMAC as HBAs and decanoic acid as HBD were also tested at molar ratio 1:2. - Molar ratios of 1:2 and 1:4 were also studied. - Sodium diethyldithiocarbamate was used as complexing agent (400 µL, 0.125% w/v). - THF was used as emulsifier (450 µL). - Final extract was diluted with ethanol up to 750 µL. - A selectivity study was carried out using potential interfering ions. - Accuracy was evaluated using CRMs. - The method was applied to the analysis of real samples. | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ramos, R.; Santana-Mayor, Á.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Recent Applications of Deep Eutectic Solvents in Environmental Analysis. Appl. Sci. 2021, 11, 4779. https://doi.org/10.3390/app11114779
Rodríguez-Ramos R, Santana-Mayor Á, Socas-Rodríguez B, Rodríguez-Delgado MÁ. Recent Applications of Deep Eutectic Solvents in Environmental Analysis. Applied Sciences. 2021; 11(11):4779. https://doi.org/10.3390/app11114779
Chicago/Turabian StyleRodríguez-Ramos, Ruth, Álvaro Santana-Mayor, Bárbara Socas-Rodríguez, and Miguel Ángel Rodríguez-Delgado. 2021. "Recent Applications of Deep Eutectic Solvents in Environmental Analysis" Applied Sciences 11, no. 11: 4779. https://doi.org/10.3390/app11114779
APA StyleRodríguez-Ramos, R., Santana-Mayor, Á., Socas-Rodríguez, B., & Rodríguez-Delgado, M. Á. (2021). Recent Applications of Deep Eutectic Solvents in Environmental Analysis. Applied Sciences, 11(11), 4779. https://doi.org/10.3390/app11114779