Accessing Ancient Population Lifeways through the Study of Gastrointestinal Parasites: Paleoparasitology
Abstract
:1. Introduction
2. Parasites, Parasitism, and Paleoparasitology
3. From the Field to the Lab
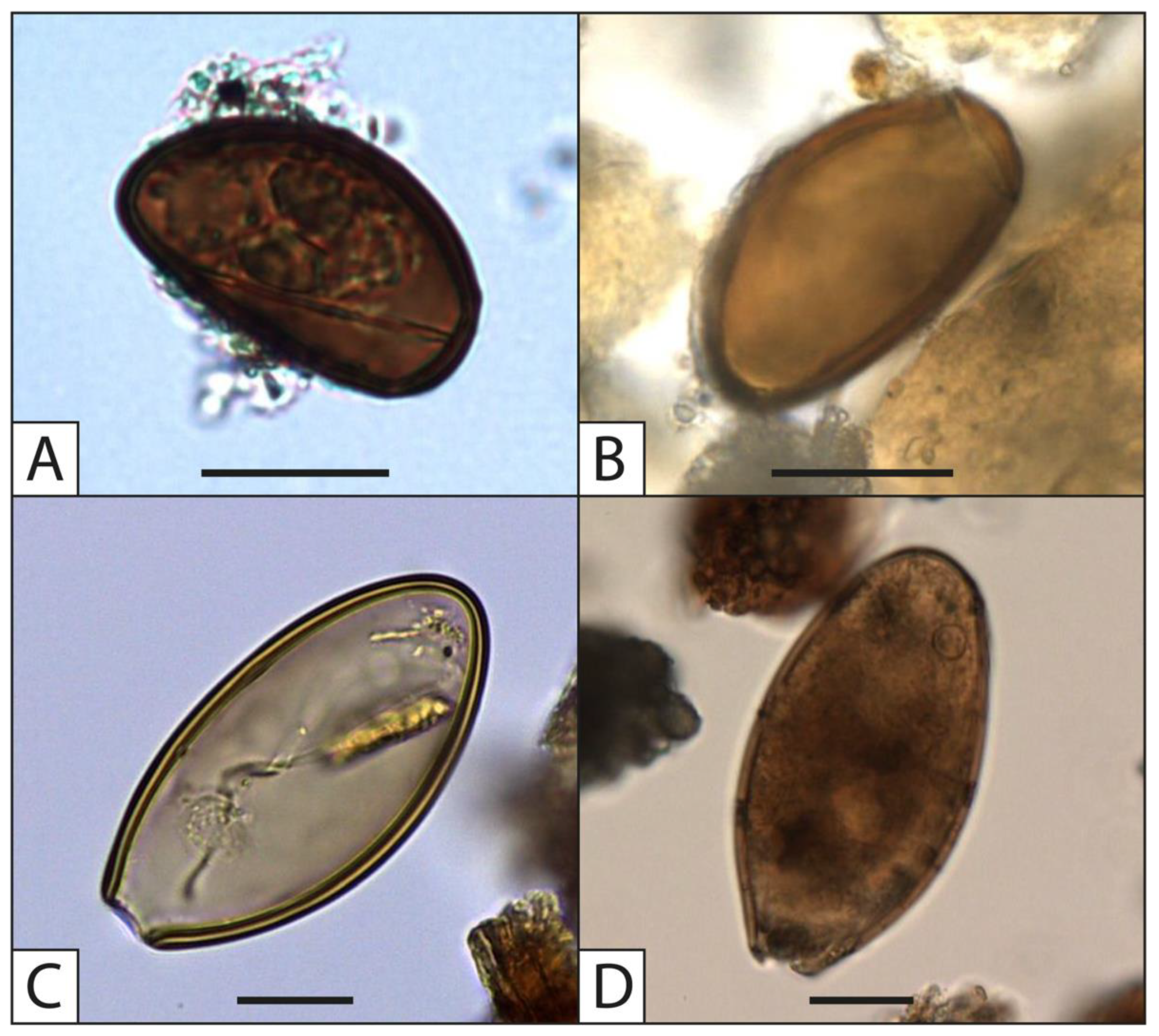
4. Recent Applications of Paleoparasitology
4.1. Multi-Scale Analysis in Roman Sites
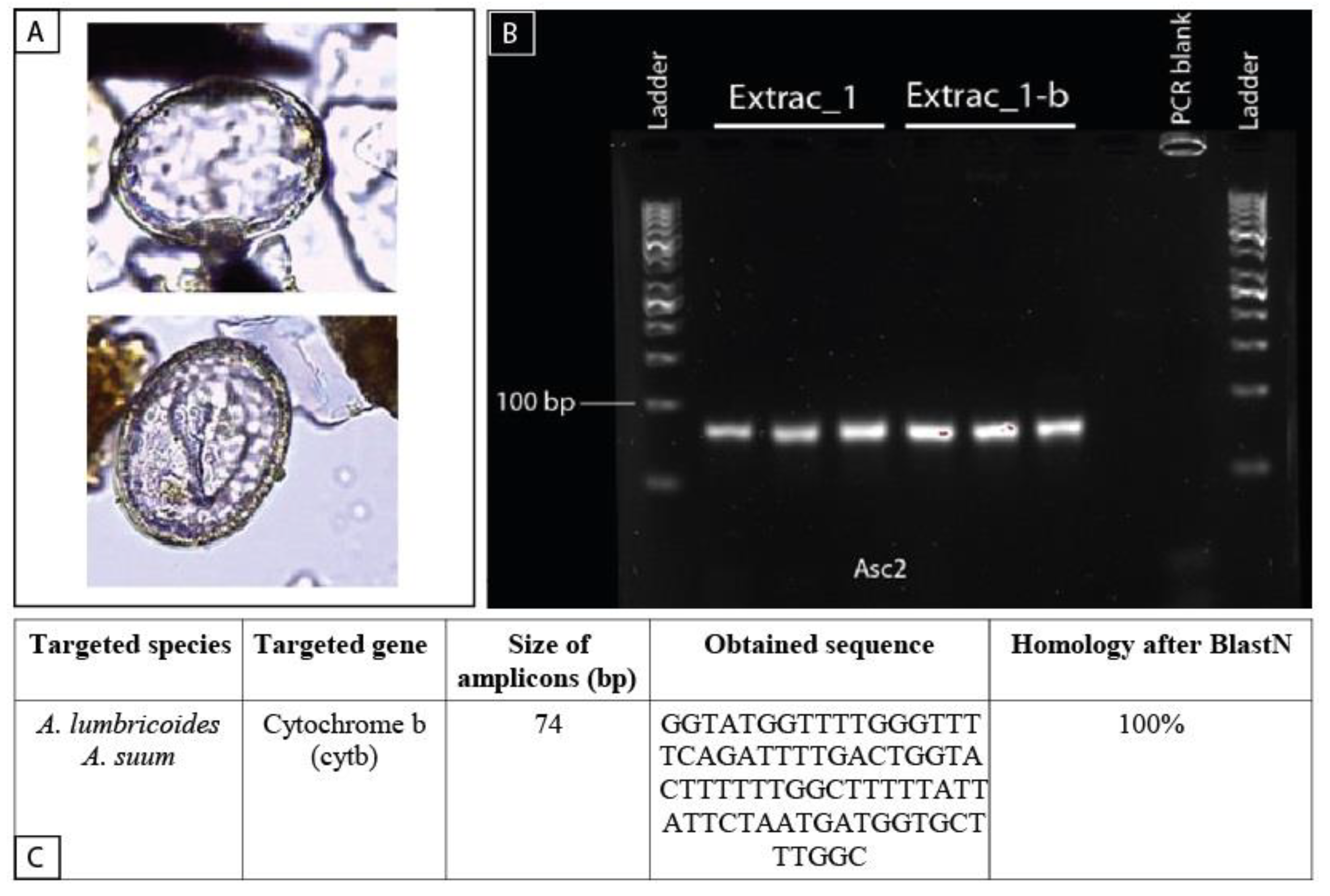
4.2. Contribution of Paleogenetics to Paleoparasitology
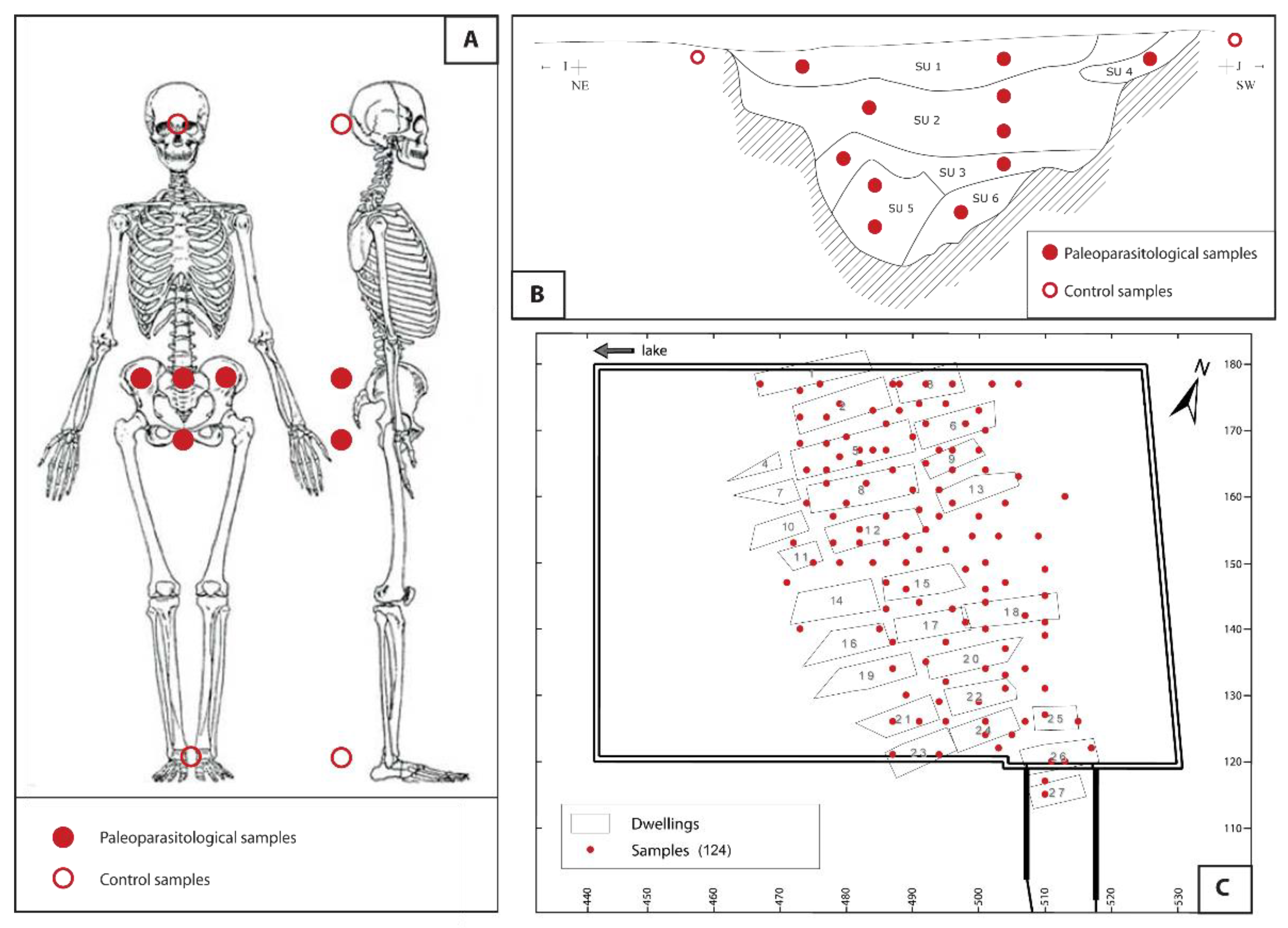
4.3. Using Statistical Treatment and Spatializing Paleoparasitological Data
4.4. Integration of Paleoparasitology in Paleoecological Reconstructions
4.5. Contribution to Our Knowledge of Parasite History
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouchet, F.; Guidon, N.; Dittmar, K.; Harter, S.; Ferreira, L.F.; Chaves, S.M.; Reinhard, K.; Araujo, A. Parasite Remains in Archaeological Sites. Mem. Inst. Oswaldo Cruz 2003, 98, 47–52. [Google Scholar] [CrossRef]
- Dittmar, K.; Araujo, A.; Reinhard, K.J. The study of parasites through time: Archaeoparasitology and Paleoparasitologu. In A Companion to Paleopathology; Grauer, A.L., Ed.; Wiley Blackwell: Oxford, UK, 2012; pp. 170–190. [Google Scholar]
- Le Bailly, M.; Araujo, A. Past Intestinal Parasites. In Paleomicrobiology of Humans; American Society of Microbiology: Washington, DC, USA, 2016; pp. 143–154. ISBN 978-1-55581-916-3. [Google Scholar]
- Reinhard, K.J.; Ferreira, L.F.; Bouchet, F.; Sianto, L.; Dutra, J.M.F.; Iniguez, A.; Leles, D.; Le Bailly, M.; Fugassa, M.; Pucu, E.; et al. Food, Parasites, and Epidemiological Transitions: A Broad Perspective. Int. J. Paleopathol. 2013, 3, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Dutour, O. Paleoparasitology and Paleopathology. Synergies for Reconstructing the Past of Human Infectious Diseases and Their Pathocenosis. Int. J. Paleopathol. 2013, 3, 145–149. [Google Scholar] [CrossRef]
- Ruffer, M.A. Note on the Presence of “Bilharzia Haematobia” in Egyptian Mummies of the Twentieth Dynasty. Br. Med. J. 1910, 16, 65. [Google Scholar]
- Szidat, L. Uber Die Erhaltungsfähigkeit von Helmintheneiern in Vor- Und Frühgeschichtlichen Moorleichen. Zeitschrift für Parasitenkunde 1944, 13, 265–274. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Reinhard, K.J.; Araujo, A. Foundations of Paleoparasitology; Editora Fiocruz: Rio de Janeiro, Brazil, 2014; ISBN 978-85-7541-440-8. [Google Scholar]
- Slepchenko, S. Opisthorchis Felineus as the Basis for the Reconstruction of Migrations Using Archaeoparasitological Materials. J. Archaeol. Sci. Rep. 2020, 33, 102548. [Google Scholar] [CrossRef]
- Paknazhad, N.; Mowlavi, G.; Dupouy Camet, J.; Esmaeili Jelodar, M.; Mobedi, I.; Makki, M.; Kia, E.B.; Rezaeian, M.; Mohebali, M.; Sarlak, S.; et al. Paleoparasitological Evidence of Pinworm (Enterobius vermicularis) Infection in a Female Adolescent Residing in Ancient Tehran (Iran) 7000 Years Ago. Parasites Vectors 2016, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Søe, M.J.; Nejsum, P.; Seersholm, F.V.; Fredensborg, B.L.; Habraken, R.; Haase, K.; Hald, M.M.; Simonsen, R.; Højlund, F.; Blanke, L.; et al. Ancient DNA from Latrines in Northern Europe and the Middle East (500 BC–1700 AD) Reveals Past Parasites and Diet. PLoS ONE 2018, 13, e0195481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, H.-Y.; Cheng, C.J.; Huang, C.; Zhan, X.; Wong, W.K.; Mitchell, P.D. Discovery of Eurytrema Eggs in Sediment from a Colonial Period Latrine in Taiwan. Korean J. Parasitol. 2019, 57, 595–599. [Google Scholar] [CrossRef]
- Flammer, P.G.; Ryan, H.; Preston, S.G.; Warren, S.; Přichystalová, R.; Weiss, R.; Palmowski, V.; Boschert, S.; Fellgiebel, K.; Jasch-Boley, I.; et al. Epidemiological Insights from a Large-Scale Investigation of Intestinal Helminths in Medieval Europe. PLoS Negl. Trop. Dis. 2020, 14, e0008600. [Google Scholar] [CrossRef]
- Myskova, E.; Ditrich, O.; Sak, B.; Kvac, M.; Cymbalak, T. Detection of Ancient DNA of Encephalitozoon Intestinalis (Microsporidia) in Archaeological Material. J. Parasitol. 2014, 100, 356–359. [Google Scholar] [CrossRef]
- Leuckart, R. Die Parasiten Des Menschen Und Die Virulence in Microparasites. Emerg. Infec. Dis. 1879, 2, 93–102. [Google Scholar]
- Brumpt, E. Précis de Parasitologie; Masson: Paris, France, 1913. [Google Scholar]
- Price, P.W. Evolutionary Biology of Parasites; Monographs in Population Biology; Princeton University Press: Princeton, NJ, USA, 1980; ISBN 978-0-691-08256-1. [Google Scholar]
- Timm, R.M.; Clauson, B.L. Coevolution: Mammalia. In 1988 McGraw-Hill Yearbook of Science & Technology; McGraw-Hill Book Company: New York, NY, USA, 1988; pp. 212–214. ISBN 978-0-07-046183-3. [Google Scholar]
- Poulin, R.; Morand, S. The Diversity of Parasites. Q. Rev. Biol. 2000, 75, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; Renaud, F.; Selosse, M.-A.; Thomas, F. Evolution des interactions entre espèces. In Biologie Évolutive; Lefèvre, T., Raymond, M., Thomas, F., Eds.; De Boeck: Bruxelles, Belgium, 2016; pp. 555–655. ISBN 978-2-8073-0296-9. [Google Scholar]
- Araujo, A.; Jansen, A.M.; Bouchet, F.; Reinhard, K.; Ferreira, L.F. Parasitism, the Diversity of Life, and Paleoparasitology. Mem. Inst. Oswaldo Cruz 2003, 98, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Combes, C.; Gavotte, L.; Moulia, C.; Sicard, M. Parasitisme: Ecologie et Evolution des Interactions Durables; Dunod: Paris, France, 2018; ISBN 978-2-10-076172-2. [Google Scholar]
- De Meeûs, T.; Prugnolle, F.; Agnew, P. Asexual Reproduction in Infectious Diseases. In Lost Sex; Schön, I., Martens, K., Dijk, P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 517–533. ISBN 978-90-481-2769-6. [Google Scholar]
- Dentzien-Dias, P.C.; Poinar, G., Jr.; de Figueiredo, A.E.Q.; Pacheco, A.C.L.; Horn, B.L.D.; Schultz, C.L. Tapeworm Eggs in a 270 Million-Year-Old Shark Coprolite. PLoS ONE 2013, 8, e55007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlier, P.; Bouchet, F.; Weil, R.; Bonnet, B. Schistosomiasis in the Mummified Viscera of Saint-Louis (1270 AD). Forensic Sci. Med. Pathol. 2016, 12, 113–114. [Google Scholar] [CrossRef]
- Goncalves, M.L.; Araujo, A.; Duarte, R.; da Silva, J.P.; Reinhard, K.; Bouchet, F.; Ferreira, L.F. Detection of Giardia Duodenalis Antigen in Coprolites Using a Commercially Available Enzyme-Linked Immunosorbent Assay. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 640–643. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, M.L.; da Silva, V.L.; de Andrade, C.M.; Reinhard, K.; da Rocha, G.C.; Le Bailly, M.; Bouchet, F.; Ferreira, L.F.; Araujo, A. Amoebiasis Distribution in the Past: First Steps Using an Immunoassay Technique. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Le Bailly, M.; Romon, T.; Kacki, S. New Evidence of Entamoeba Histolytica Infections in Pre-Columbian and Colonial Cemeteries in the Caribbean. J. Parasitol. 2014, 100, 684–686. [Google Scholar] [CrossRef]
- Le Bailly, M.; Maicher, C.; Dufour, B. Archaeological Occurrences and Historical Review of the Human Amoeba, Entamoeba Histolytica, over the Past 6000 Years. Infect. Genet. Evol. 2016, 42, 34–40. [Google Scholar] [CrossRef]
- Morrow, J.J.; Reinhard, K.J. Cryptosporidium Parvum Among Coprolites from La Cueva de Los Muertos Chiquitos (600–800 CE), Rio Zape Valley, Durango, Mexico. J. Parasitol. 2016, 102, 429–435. [Google Scholar] [CrossRef]
- Loreille, O.; Roumat, E.; Verneau, O.; Bouchet, F.; Hänni, C. Ancient DNA from Ascaris: Extraction Amplification and Sequences from Eggs Collected in Coprolites. Int. J. Parasitol. 2001, 31, 1101–1106. [Google Scholar] [CrossRef]
- Madden, M.; Salo, W.L.; Streitz, J.; Aufderheide, A.C.; Fornaciari, G.; Jaramillo, C.; Vallejo, G.A.; Yockteng, R.; Arriaza, B.; Cárdenas-Arroyo, F.; et al. Hybridization Screening of Very Short PCR Products for Paleoepidemiological Studies of Chagas’ Disease. BioTechniques 2001, 30, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Côté, N. Apports de La Paléogénétique à l’étude Des Helminthes Gastro-Intestinaux Anciens; Université de Bourgogne Franche-Comté: Besançon, France, 2015. [Google Scholar]
- Côté, N.; Daligault, J.; Pruvost, M.; Bennett, E.A.; Gorge, O.; Guimaraes, S.; Capelli, N.; Le Bailly, M.; Geigl, E.M.; Grange, T. A New High-Throughput Approach to Genotype Ancient Human Gastrointestinal Parasites. PLoS ONE 2016, 11, e0146230. [Google Scholar] [CrossRef] [Green Version]
- Rawlence, N.J.; Lowe, D.J.; Wood, J.R.; Young, J.M.; Churchman, G.J.; Huang, Y.; Cooper, A. Using Palaeoenvironmental DNA to Reconstruct Past Environments: Progress and Prospects. J. Quat. Sci. 2014, 29, 610–626. [Google Scholar] [CrossRef]
- Taberlet, P.; Bonin, A.; Zinger, L.; Coissac, E. Environmental DNA. Oxford University Press: Oxford, UK, 2018; Volume 1, ISBN 978-0-19-876722-0. [Google Scholar]
- Dufour, B.; Le Bailly, M. Testing New Parasite Egg Extraction Methods in Paleoparasitology and an Attempt at Quantification. Int. J. Paleopathol. 2013, 3, 199–203. [Google Scholar] [CrossRef]
- Reinhard, K. Reestablishing Rigor in Archaeological Parasitology. Int. J. Paleopathol. 2017, 19, 124–134. [Google Scholar] [CrossRef]
- Mulder, C. Pathogenic Helminths in the Past: Much Ado about Nothing. F1000Research 2017, 6, 852. [Google Scholar] [CrossRef] [Green Version]
- Dufour, B. Synthèse de Données et Nouvelle Contribution à L’étude des Parasites de L’époque Romaine, et Apports Méthodologiques de L’extraction des Marqueurs Au Traitement des Résultats; Université de Bourgogne Franche-Comté: Besançon, France, 2015. [Google Scholar]
- Mitchell, P.D. Human Parasites in the Roman World: Health Consequences of Conquering an Empire. Parasitology 2017, 144, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Gourevich, D. Pour Une Archéologie de la Médecine Romaine; De Boccard: Paris, France, 2011. [Google Scholar]
- Le Bailly, M.; Dufour, B.; Bouchet, F. La paléoparasitologie en période gallo-romaine. In Pour Une Archéologie de la Médecine Romaine; Gourevich, D., Ed.; De Boccard: Paris, France, 2011; pp. 67–68. [Google Scholar]
- Côté, N.M.-L.; Le Bailly, M. Palaeoparasitology and Palaeogenetics: Review and Perspectives for the Study of Ancient Human Parasites. Parasitology 2018, 145, 656–664. [Google Scholar] [CrossRef]
- Aufderheide, A.C.; Salo, W.; Madden, M.; Streitz, J.; Buikstra, J.; Guhl, F.; Arriaza, B.; Renier, C.; Wittmers, L.E., Jr.; Fornaciari, G.; et al. A 9,000-Year Record of Chagas’ Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2034–2039. [Google Scholar] [CrossRef] [Green Version]
- Guedes, L.; Borba, V.H.; Camacho, M.; Neto, J.; Dias, O.; Iñiguez, A.M. African Helminth Infection out of Africa: Paleoparasitological and Paleogenetic Investigations in Pretos Novos Cemetery, Rio de Janeiro, Brazil (1769–1830). Acta Trop. 2020, 205, 105399. [Google Scholar] [CrossRef] [PubMed]
- Roche, K.; Pacciani, E.; Bianucci, R.; Le Bailly, M. Assessing the Parasitic Burden in a Late Antique Florentine Emergency Burial Site. Korean J. Parasitol. 2019, 57, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Roche, K.; Capelli, N.; Pacciani, E.; Lelli, P.; Pallecchi, P.; Bianucci, R.; Le Bailly, M. Gastrointestinal Parasite Burden in 4th-5th c. CE Florence Highlighted by Microscopy and Paleogenetics. Infect. Genet. Evol. 2021, 90, 104713. [Google Scholar] [CrossRef]
- Saña, M.; Bogdanovic, I.; Navarrete, V. Taphonomic Evaluation of the Degree of Historical Representation of the Archaeological Bone Samples in Anaerobic versus Aerobic Environments: The Neolithic Site of La Draga (Banyoles, Spain). Quat. Int. 2014, 330, 72–87. [Google Scholar] [CrossRef]
- Tarrus, J.; Chinchilla, J.; Bosch, À. La Draga (Banyoles): Un site lacustre du Néolithique ancien cardial en Catalogne. bspf 1994, 91, 449–456. [Google Scholar] [CrossRef]
- Maicher, C.; Hoffmann, A.; Côté, N.M.; Palomo Pérez, A.; Saña Segui, M.; Le Bailly, M. Paleoparasitological Investigations on the Neolithic Lakeside Settlement of La Draga (Lake Banyoles, Spain). Holocene 2017, 095968361770223. [Google Scholar] [CrossRef]
- Revelles, J.; Burjachs, F.; Morera, N.; Barceló, J.A.; Berrocal, A.; López-Bultó, O.; Maicher, C.; Le Bailly, M.; Piqué, R.; Palomo, A.; et al. Use of Space and Site Formation Processes in a Neolithic Lakeside Settlement. Pollen and Non-Pollen Palynomorphs Spatial Analysis in La Draga (Banyoles, NE Iberia). J. Archaeol. Sci. 2017, 81, 101–115. [Google Scholar] [CrossRef]
- Bleicher, N.; Harb, C. Settlement and Social Organisation in the Late Fourth Millennium BC in Central Europe: The Waterlogged Site of Zurich-Parkhaus Opéra. Antiquity 2018, 92, 1210–1230. [Google Scholar] [CrossRef] [Green Version]
- Maicher, C.; Bleicher, N.; Le Bailly, M. Spatializing Data in Paleoparasitology: Application to the Study of the Neolithic Lakeside Settlement of Zürich-Parkhaus-Opéra, Switzerland. Holocene 2019, 29, 1198–1205. [Google Scholar] [CrossRef]
- Maicher, C. Evolution Des Relations Homme/Parasite/Environnement Au Néolithique: Approche Intégrée et Premiers Essais de Spatialisation Sur Les Sites Lacustres Européens; Bourgogne Franche-Comté: Besançon, France, 2019. [Google Scholar]
- Huber, R.; Schaeren, G. Zum Stand Der Pfahlbauforschung Im Kanton Zug. Tugium 2009, 25, 111–140. [Google Scholar]
- Steiner, B. Aspects of Archaeobotanical Methodology Applied to the Sediments of Archaeological Wetland Deposits. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2017. [Google Scholar]
- Jouffroy-Bapicot, I.; Vannière, B.; Iglesias, V.; Debret, M.; Delarras, J.-F. 2000 Years of Grazing History and the Making of the Cretan Mountain Landscape, Greece. PLoS ONE 2016, 11, e0156875. [Google Scholar] [CrossRef] [Green Version]
- van Geel, B.; Aptroot, A. Fossil Ascomycetes in Quaternary Deposits. Nova Hedwigia 2006, 82, 313–329. [Google Scholar] [CrossRef]
- Cugny, C.; Mazier, F.; Galop, D. Modern and Fossil Non-Pollen Palynomorphs from the Basque Mountains (Western Pyrenees, France): The Use of Coprophilous Fungi to Reconstruct Pastoral Activity. Veget. Hist. Archaeobot. 2010, 19, 391–408. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.L.; McLauchlan, K.K.; Skibbe, A.M.; Goring, S.; Zirbel, C.R.; Williams, J.W. Linking Abundances of the Dung Fungus Sporormiella to the Density of Bison: Implications for Assessing Grazing by Megaherbivores in Palaeorecords. J. Ecol. 2013, 101, 1125–1136. [Google Scholar] [CrossRef]
- Baker, A.G.; Cornelissen, P.; Bhagwat, S.A.; Vera, F.W.M.; Willis, K.J. Quantification of Population Sizes of Large Herbivores and Their Long-term Functional Role in Ecosystems Using Dung Fungal Spores. Methods Ecol. Evol. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Ejarque, A.; Miras, Y.; Riera, S. Pollen and Non-Pollen Palynomorph Indicators of Vegetation and Highland Grazing Activities Obtained from Modern Surface and Dung Datasets in the Eastern Pyrenees. Rev. Palaeobot. Palynol. 2011, 167, 123–139. [Google Scholar] [CrossRef]
- Roche, K.; Jouffroy-Bapicot, I.; Vannière, B.; Le Bailly, M. Ancient Parasites from a Peat Bog: New Insights into Animal Presence and Husbandry in Crete over the Past 2000 Years. Holocene 2020, 30, 1243–1253. [Google Scholar] [CrossRef]
- Le Bailly, M.; Bouchet, F. Ancient Dicrocoeliosis: Occurences, Distribution and Migration. Acta Trop. 2010, 115, 175–180. [Google Scholar] [CrossRef]
- Dufour, B.; Hugot, J.P.; Lepetz, S.; Le Bailly, M. The Horse Pinworm (Oxyuris Equi) in Archaeology during the Holocene: Review of Past Records and New Data. Infect. Genet. Evol. 2015, 33, 77–83. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Bailly, M.; Maicher, C.; Roche, K.; Dufour, B. Accessing Ancient Population Lifeways through the Study of Gastrointestinal Parasites: Paleoparasitology. Appl. Sci. 2021, 11, 4868. https://doi.org/10.3390/app11114868
Le Bailly M, Maicher C, Roche K, Dufour B. Accessing Ancient Population Lifeways through the Study of Gastrointestinal Parasites: Paleoparasitology. Applied Sciences. 2021; 11(11):4868. https://doi.org/10.3390/app11114868
Chicago/Turabian StyleLe Bailly, Matthieu, Céline Maicher, Kévin Roche, and Benjamin Dufour. 2021. "Accessing Ancient Population Lifeways through the Study of Gastrointestinal Parasites: Paleoparasitology" Applied Sciences 11, no. 11: 4868. https://doi.org/10.3390/app11114868
APA StyleLe Bailly, M., Maicher, C., Roche, K., & Dufour, B. (2021). Accessing Ancient Population Lifeways through the Study of Gastrointestinal Parasites: Paleoparasitology. Applied Sciences, 11(11), 4868. https://doi.org/10.3390/app11114868





