Mg,Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic MgSr-HA Macro-Granules
2.2. The 3D Porous Scaffold of MgSr-HA
2.3. Characterizations of the Materials
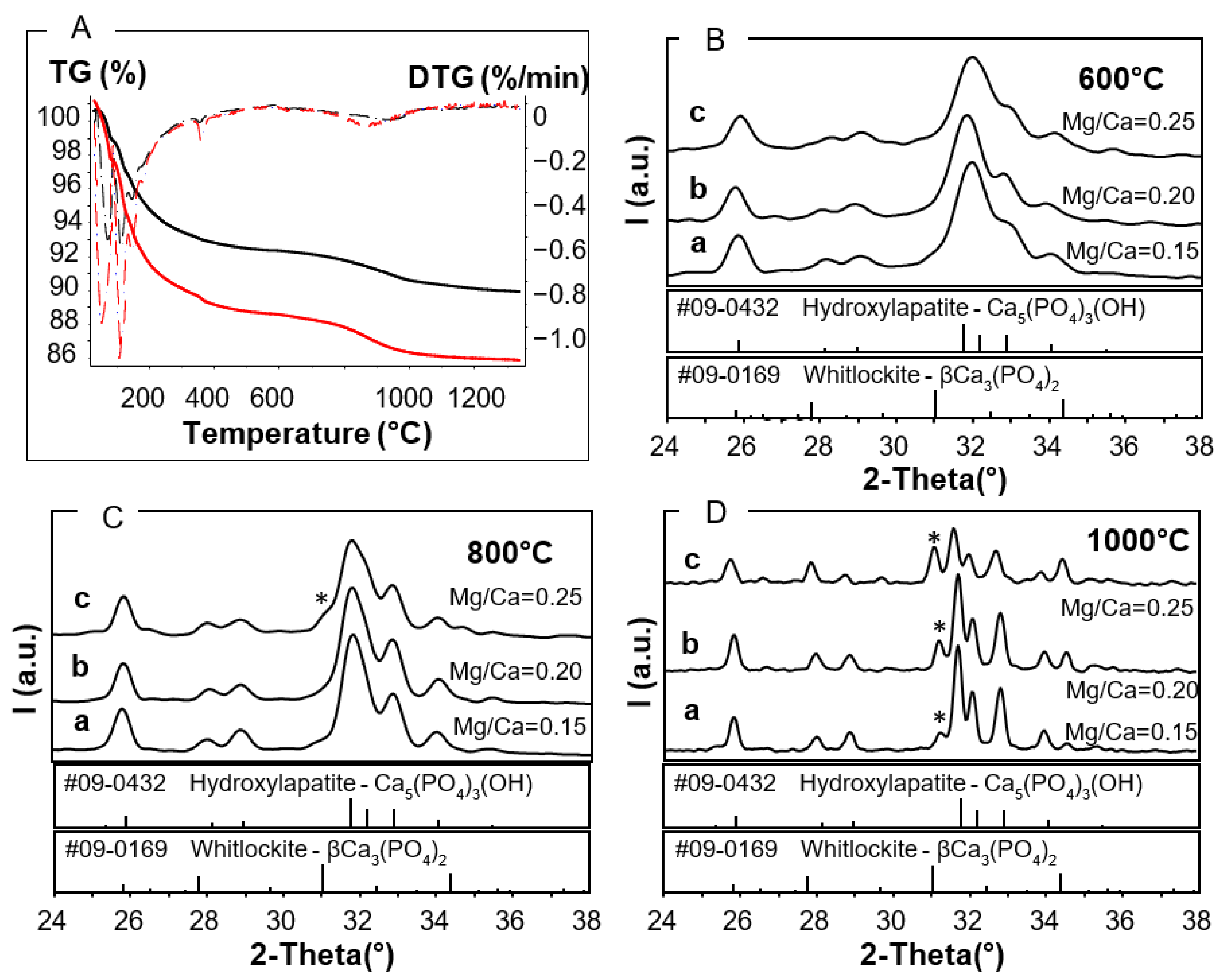
2.3.1. Thermal Stability Analysis
2.3.2. XRD Analysis
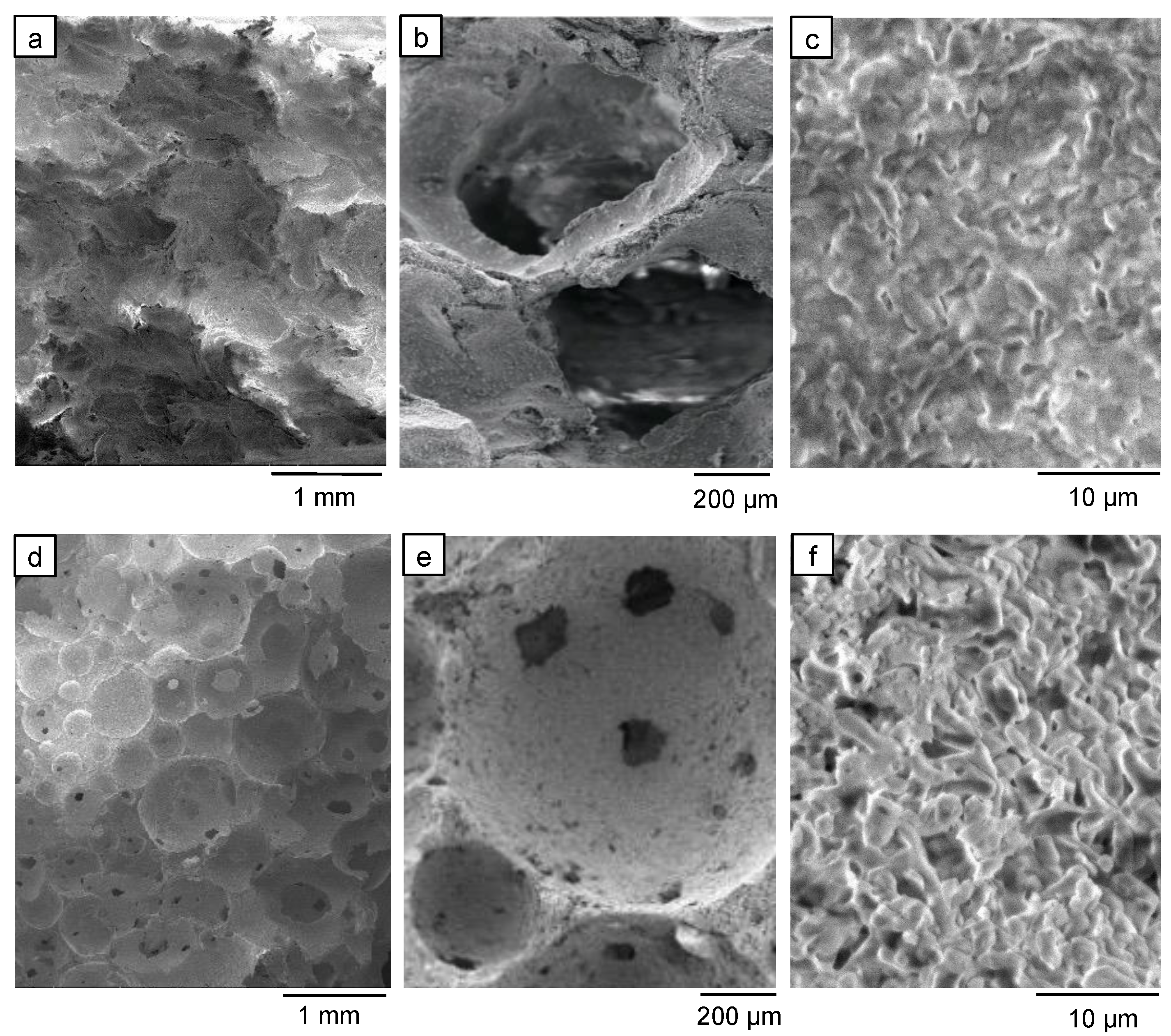
2.3.3. Macro- and Microstructural Compositional Evaluation
2.3.4. Mechanical Characterization
3. Results and Discussion
3.1. Thermal Stability of As-Prepared Mg,Sr-Cosubstituted HA
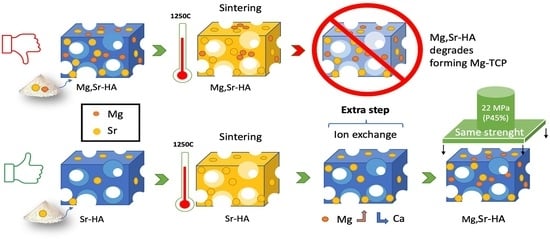
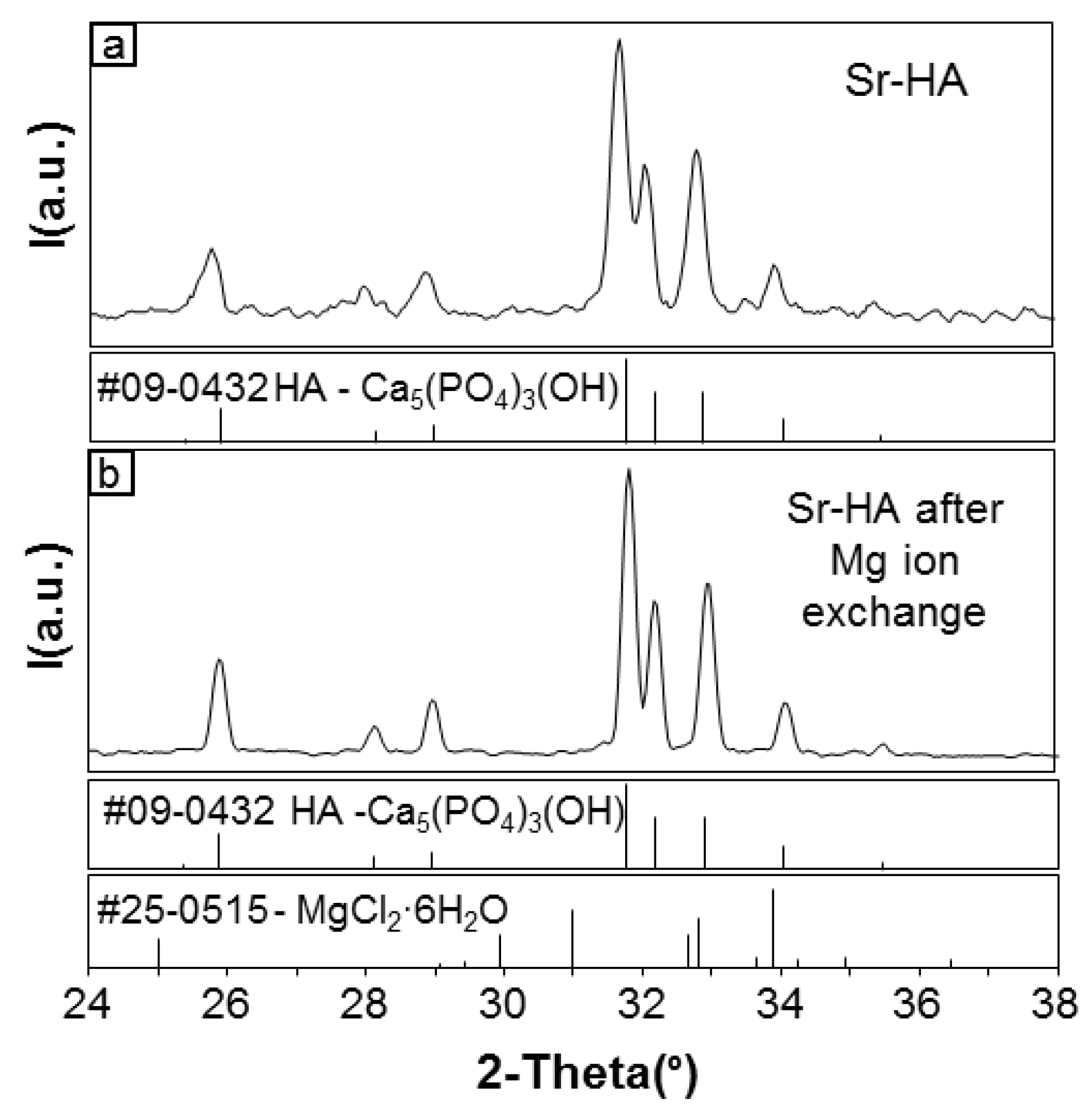
3.2. Ion Exchange of Mg in Sr-HA Samples: “Subsequent Mg Substitution”
3.2.1. Development of the Process
3.2.2. Effectiveness of the Process
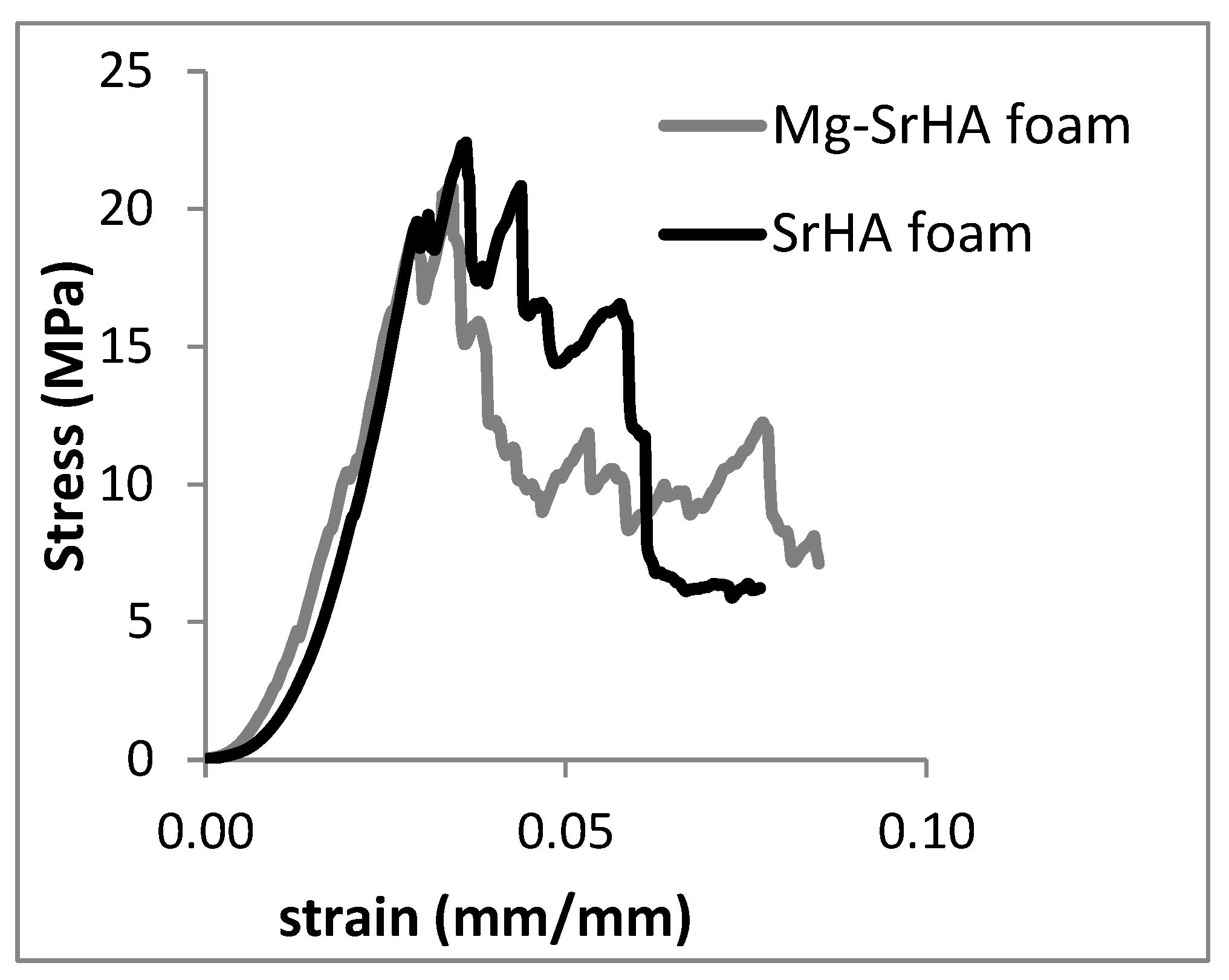
3.2.3. Evaluation of the Mechanical Properties of MgSr-HA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, M. Age variations in the properties of human tibial trabecular bone and cartilage. Acta Orthop. Scand. Suppl. 2000, 292, 1–45. [Google Scholar] [CrossRef]
- Chan, G.K.; Duque, G. Age-Related Bone Loss: Old Bone, New Facts. Gerontology 2002, 48, 62–71. [Google Scholar] [CrossRef]
- Iaquinta, M.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [Green Version]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Biochem. Pharmacol. 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 204173141877681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. BioMetals 2021. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Foresti, E.; Gregorini, R.; Ripamonti, A.; Roveri, N.; Shah, J.S. The role of magnesium on the structure of biological apatites. Calcif. Tissue Int. 1992, 50, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Dahl, S.G.; Allain, P.; Marie, P.J.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.D.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef]
- Singh, S.S.; Roy, A.; Lee, B.; Parekh, S.; Kumta, P.N. Murine osteoblastic and osteoclastic differentiation on strontium releasing hydroxyapatite forming cements. Mater. Sci. Eng. C 2016, 63, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Grynpas, M.D.; Hamilton, E.; Cheung, R.; Tsouderos, Y.; Deloffre, P.; Hott, M.; Marie, P.J. Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone 1996, 18, 253–259. [Google Scholar] [CrossRef]
- Cattani-Lorente, M.; Rizzoli, R.; Ammann, P. In vitro bone exposure to strontium improves bone material level properties. Acta Biomater. 2013, 9, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Fini, M.; Bigi, A. Osteopenic bone cell response to strontium-substituted hydroxyapatite. J. Mater. Sci. Mater. Med. 2011, 22, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Lavet, C.; Mabilleau, G.; Chappard, D.; Rizzoli, R.; Ammann, P. Strontium ranelate stimulates trabecular bone formation in a rat tibial bone defect healing process. Osteoporos. Int. 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef]
- Cazalbou, S.; Combes, C.; Rey, C. Biomimetic Approach for Strontium-Containing Ca-P Bioceramics with Enhanced Biological Activity. Key Eng. Mater. 2000, 192–195, 147–150. [Google Scholar] [CrossRef]
- Meininger, S.; Moseke, C.; Spatz, K.; März, E.; Blum, C.; Ewald, A.; Vorndran, E. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Mater. Sci. Eng. C 2019, 98, 1145–1158. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional Printed Mg-Doped β-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Scalera, F.; Palazzo, B.; Barca, A.; Gervaso, F. Sintering of magnesium-strontium doped hydroxyapatite nanocrystals: Towards the production of 3D biomimetic bone scaffolds. J. Biomed. Mater. Res. Part A 2020, 108, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Yedekçi, B.; Tezcaner, A.; Alshemary, A.Z.; Yılmaz, B.; Demir, T.; Evis, Z. Synthesis and sintering of B, Sr, Mg multi-doped hydroxyapatites: Structural, mechanical and biological characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104230. [Google Scholar] [CrossRef] [PubMed]
- Arkin, V.H.; Narendrakumar, U.; Madhyastha, H.; Manjubala, I. Characterization and In Vitro Evaluations of Injectable Calcium Phosphate Cement Doped with Magnesium and Strontium. ACS Omega 2021, 6, 2477–2486. [Google Scholar] [CrossRef]
- Drouet, C.; Carayon, M.-T.; Combes, C.; Rey, C. Surface enrichment of biomimetic apatites with biologically-active ions Mg2+ and Sr2+: A preamble to the activation of bone repair materials. Mater. Sci. Eng. C 2008, 28, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.R.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium- and strontium-co-substituted hydroxyapatite: The effects of doped-ions on the structure and chemico-physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Pina, S.; Torres, P.M.C.; Ferreira, J.M.F. Synthesis and structural characterization of strontium- and magnesium-co-substituted β-tricalcium phosphate. Acta Biomater. 2010, 6, 571–576. [Google Scholar] [CrossRef]
- Landi, E.; Uggeri, J.; Medri, V.; Guizzardi, S. Sr, Mg cosubstituted HA porous macro-granules: Potentialities as resorbable bone filler with antiosteoporotic functions. J. Biomed. Mater. Res. Part A 2013, 101A, 2481–2490. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, R.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Huijing, B.; Li, X.; Huo, Q.; et al. Synthesis, characterization and biological evaluation of strontium/magnesium-co-substituted hydroxyapatite. J. Biomater. Appl. 2016, 31, 140–151. [Google Scholar] [CrossRef]
- Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021. [Google Scholar] [CrossRef]
- Santos, G.G.; Nunes, V.L.C.; Marinho, S.M.O.C.; Santos, S.R.A.; Rossi, A.M.; Miguel, F.B. Biological behavior of magnesium-substituted hydroxyapatite during bone repair. Brazilian J. Biol. 2021, 81, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, S.; Bowen, C.; Rautray, T. Dual response of osteoblast activity and antibacterial properties of polarized strontium substituted hydroxyapatite—Barium strontium titanate composites with controlled strontium substitution. J. Biomed. Mater. Res. Part A 2021, jbm.a.37195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, S.; Zhao, W.; Yang, L.; Yuan, B.; Ioan, V.S.; Iulian, A.V.; Yang, X.; Zhu, X.; Zhang, X. A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects. Theranostics 2020, 10, 1572–1589. [Google Scholar] [CrossRef]
- Fadeev, I.V.; Shvorneva, L.I.; Barinov, S.M.; Orlovskii, V.P. Synthesis and Structure of Magnesium-Substituted Hydroxyapatite. Inorg. Mater. 2003, 39, 947–950. [Google Scholar] [CrossRef]
- Bigi, A.; Marchetti, F.; Ripamonti, A.; Roveri, N.; Foresti, E. Magnesium and strontium interaction with carbonate-containing hydroxyapatite in aqueous medium. J. Inorg. Biochem. 1981, 15, 317–327. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S.; Sandri, M.; Logroscino, G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007, 3, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Ciocca, L.; Donati, D.; Fantini, M.; Landi, E.; Piattelli, A.; Iezzi, G.; Tampieri, A.; Spadari, A.; Romagnoli, N.; Scotti, R. CAD–CAM-generated hydroxyapatite scaffold to replace the mandibular condyle in sheep: Preliminary results. J. Biomater. Appl. 2013, 28, 207–218. [Google Scholar] [CrossRef]
- Zhu, Y.; Goh, C.; Shrestha, A. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci. 2021, 21, 2000365. [Google Scholar] [CrossRef]
- Le Geroz, R. Calcium Phosphates in Oral Biology and Medicine. In Monographs in Oral Science; Myers, H., Ed.; Karger Publishers: Basel, Switzerland, 1991; pp. 82–107. [Google Scholar]
- Cai, Y.; Zhang, S.; Ong, S.; Zeng, X.; Wang, W. Simultaneous Incorporation of Magnesium and Fluorine Ions in Hydroxyapatite Coatings on Metallic Implant for Osseointegration and Stability. In Hydroxyapatite Coatings for Biomedical Applications; Zhang, S., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 55–144. [Google Scholar]
- Driessens, F.; Verbeek, R. Effects of chloride on the calcium rich calcium phosphates of the system Ca(OH)2-H3PO4 –H2O. In Biominerals; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Cho, J.S.; Yoo, D.S.; Chung, Y.-C.; Rhee, S.-H. Enhanced bioactivity and osteoconductivity of hydroxyapatite through chloride substitution. J. Biomed. Mater. Res. Part A 2014, 102, 455–469. [Google Scholar] [CrossRef]
- Guicciardi, S.; Galassi, C.; Landi, E.; Tampieri, A.; Satou, K.; Pezzotti, G. Rheological characteristics of slurry controlling the microstructure and the compressive strength behavior of biomimetic hydroxyapatite. J. Mater. Res. 2001, 16, 163–170. [Google Scholar] [CrossRef]
- Liebschner, M.A.K. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar] [CrossRef]
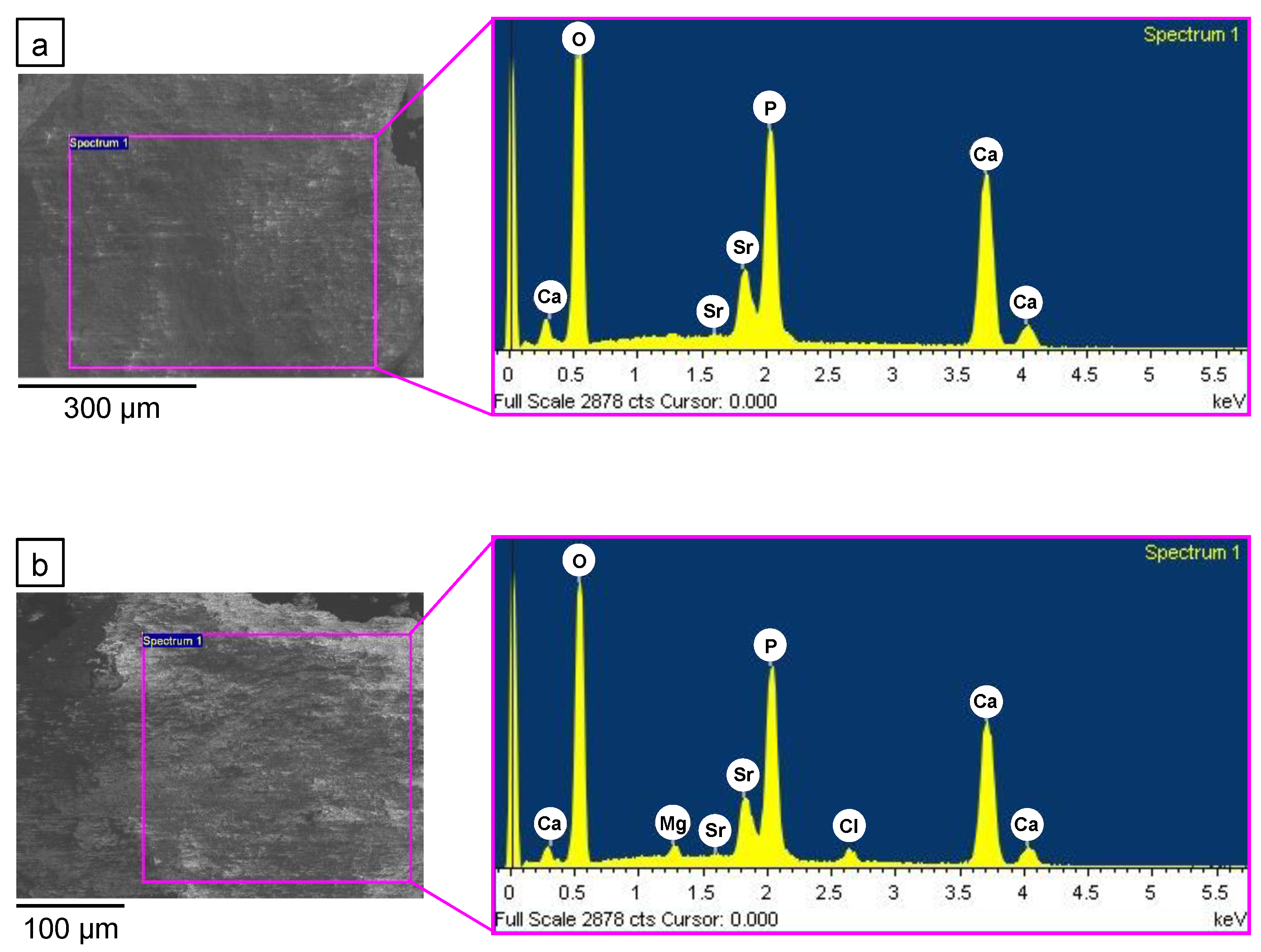

| a (Å) | c (Å) | V (Å3) | |
|---|---|---|---|
| SrHA | 9.462 | 6.913 | 536.0 |
| SM1d1 | 9.418 | 6.880 | 528.4 |
| SM1d5 | 9.342 | 6.827 | 516.0 |
| SM2d1 | 9.407 | 6.879 | 527.2 |
| SM2d5 | 9.352 | 6.819 | 516.5 |
| Ca (mol%) | P (mol%) | Mg (mol%) | Sr (mol%) | Ca/P | (cat)/P | Mg/Ca | Sr/Ca | Mg/Sr | |
|---|---|---|---|---|---|---|---|---|---|
| SrHA | 21.87 | 14.45 | - | 2.84 | 1.51 | 1.71 | - | 0.130 | - |
| SM1d1 | 21.83 | 13.80 | 0.74 | 2.64 | 1.58 | 1.83 | 0.034 | 0.121 | 0.280 |
| SM1d5 | 21.03 | 13.90 | 1.00 | 2.69 | 1.51 | 1.78 | 0.048 | 0.128 | 0.372 |
| SM2d1 | 22.00 | 13.52 | 0.89 | 2.59 | 1.63 | 1.88 | 0.040 | 0.118 | 0.344 |
| SM2d5 | 20.46 | 13.53 | 1.79 | 2.57 | 1.51 | 1.83 | 0.087 | 0.126 | 0.696 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, E.; Guizzardi, S.; Papa, E.; Galli, C. Mg,Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties. Appl. Sci. 2021, 11, 4930. https://doi.org/10.3390/app11114930
Landi E, Guizzardi S, Papa E, Galli C. Mg,Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties. Applied Sciences. 2021; 11(11):4930. https://doi.org/10.3390/app11114930
Chicago/Turabian StyleLandi, Elena, Stefano Guizzardi, Elettra Papa, and Carlo Galli. 2021. "Mg,Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties" Applied Sciences 11, no. 11: 4930. https://doi.org/10.3390/app11114930