Physico-Chemical and Melissopalynological Characterization of Czech Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physico-Chemical Analysis
2.1.1. Water Content
2.1.2. Electrical Conductivity
2.1.3. HMF Analysis
2.1.4. Diastase Activity
2.1.5. Free Acidity
2.1.6. Carbohydrate Content
2.1.7. Colour Measurement
2.2. Melissopalynological Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characterization of Czech Honey
3.2. Pollen Profile of Czech Honey
3.3. Comparison with European Honeys
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and Bioactive Properties of Six Honey Samples from Various Floral Origins from Tunisia. Arabian J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Shamala, T.R.; Shri Jyothi, Y.; Saibaba, P. Stimulatory Effect of Honey on Multiplication of Lactic Acid Bacteria under in Vitro and in Vivo Conditions. Lett. Appl. Microbiol. 2000, 30, 453–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naila, A.; Flint, S.H.; Sulaiman, A.Z.; Ajit, A.; Weeds, Z. Classical and Novel Approaches to the Analysis of Honey and Detection of Adulterants. Food Control 2018, 90, 152–165. [Google Scholar] [CrossRef]
- Vet, T.J.; Sci, A.; Dümen, E.; Akkaya, H.; Merve Öz, G.; Sezgin, F.H. Turkish Journal of Veterinary and Animal Sciences Microbiological and Parasitological Quality of Honey Produced in İstanbul. Turkish J. Veterinary Anim. Sci. 2013, 37, 602–607. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Dimou, M.; Liolios, V.; Kanelis, D.; Goras, G.; Thrasyvoulou, A. The Determination of the Botanical Origin in Honeys with Over-Represented Pollen: Combination of Melissopalynological, Sensory and Physicochemical Analysis. J. Sci. Food Agric. 2018, 98, 2705–2712. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piana, L.; Bogdanov, S.; Bentabol, A.; Gotsiou, P.; Kerkvliet, J.; Martin, P.; Morlot, M.; Ortiz Valbuena, A.; Ruoff, K.; et al. Botanical Species Giving Unifloral Honey in Europe. Apidologie 2004, 35, S82–S93. [Google Scholar] [CrossRef]
- Oddo, L.P.; Piazza, M.G.G.; Sabatini, A.G.G.; Accorti, M. Characterization of Unifloral Honeys. Apidologie 1995, 26, 453–465. [Google Scholar] [CrossRef] [Green Version]
- Persano Oddo, L.; Piro, R. Main European Unifloral Honeys: Descriptive Sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Atanassova, J.; Yurukova, L.; Lazarova, M. Pollen and Inorganic Characteristics of Bulgarian Unifloral Honeys. Czech J. Food Sci. 2012, 30, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Puusepp, L.; Koff, T. Pollen Analysis of Honey from the Baltic Region, Estonia. Grana 2014, 53, 54–61. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical composition of honey. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 43–82. ISBN 9783319596891. [Google Scholar]
- Stawiarz, E.; Wróblewska, A. Melissopalynological Analysis of Multifloral Honeys from the Sandomierska Upland Area of Poland. J. Apic. Sci. 2010, 54, 65–75. [Google Scholar]
- Feás, X.; Pires, J.; Iglesias, A.; Estevinho, M.L. Characterization of Artisanal Honey Produced on the Northwest of Portugal by Melissopalynological and Physico-Chemical Data. Food Chem. Toxicol. 2010, 48, 3462–3470. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and Classification of Greek Pine Honeys According to Their Geographical Origin Based on Volatiles, Physicochemical Parameters and Chemometrics. Food Chem. 2014. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Halatsi, E.Z.; Kontakos, S.; Kontominas, M.G. Characterization and Geographical Classification of Greek Fir Honeys Based on Physicochemical Parameters, Colour Attributes, and Volatile Compounds Using Chemometrics. IOSR J. Agric. Vet. Sci. 2017. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and Classification of Thymus Capitatus (L.) Honey According to Geographical Origin Based on Volatile Compounds, Physicochemical Parameters and Chemometrics. Food Res. Int. 2014, 55, 363–372. [Google Scholar] [CrossRef]
- Dobre, I.; Alexe, P.; Escuredo, O.; Seijo, C.M. Palynological Evaluation of Selected Honeys from Romania. Grana 2013, 52, 113–121. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Coll, F.V. Physico-Chemical Properties, Composition and Pollen Spectrum of French Lavender (Lavandula Stoechas L.) Honey Produced in Spain. Z. Lebensm. Unters. Forsch. 1993. [Google Scholar] [CrossRef]
- De La Fuente, E.; Ruiz-Matute, A.I.; Valencia-Barrera, R.M.; Sanz, J.; Martínez Castro, I. Carbohydrate Composition of Spanish Unifloral Honeys. Food Chem. 2011, 129, 1483–1489. [Google Scholar] [CrossRef] [Green Version]
- Downey, G.; Hussey, K.; Daniel Kelly, J.; Walshe, T.F.; Martin, P.G. Preliminary Contribution to the Characterisation of Artisanal Honey Produced on the Island of Ireland by Palynological and Physico-Chemical Data. Food Chem. 2005, 91, 347–354. [Google Scholar] [CrossRef]
- Čeksteryte, V.; Kurtinaitiene, B.; Balžekas, J. Pollen Diversity in Honey Collected from Lithuania’s Protected Landscape Areas. Proc. Est. Acad. Sci. 2013, 62, 277–282. [Google Scholar] [CrossRef]
- Beckh, G.; Camps, G. Neue Spezifikationen Für Trachthonige. Dtsch. Leb. 2009, 105, 105–110. [Google Scholar]
- Bogdanov, S. Harmonised Methods of the International Honey Commission; Swiss Bee Research Centre, FAM: Liebefeld, Switzerland, 2009. [Google Scholar]
- Phadebas Phadebas Instruction for Use. Available online: https://www.phadebas.com/wp-content/uploads/SPE9047-02-Bilaga-1.pdf (accessed on 14 April 2021).
- Castro, R.M.; Escamilla, M.J.; Reig, F.B. Evaluation of the Color of Some Spanish Unifloral Honey Types as a Characterization Parameter. J. Aoac Int. 1992, 75, 537–542. [Google Scholar] [CrossRef]
- European Union. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, 10, 47–52. [Google Scholar]
- Pharmacopoea Bohemica MMXVII Český Lékopis; Grada Publishing: Holešovice, Czech Republic, 2017.
- Pharmacopoeia, T.B. Honey—British Pharmacopoeia. Med. Healthcare Prod. Regul. Agency 2021, 2, 4. [Google Scholar]
- Louppis, A.P.; Karabagias, I.K.; Kontakos, S.; Kontominas, M.G.; Papastephanou, C.; Konstantinos Karabagias, I.; Kontakos, S.; Kontominas, M.G.; Papastephanou, C. Botanical Discrimination of Greek Unifloral Honeys Based on Mineral Content in Combination with Physicochemical Parameter Analysis, Using a Validated Chemometric Approach. Microchem. J. 2017, 135, 180–189. [Google Scholar] [CrossRef]
- Shaaban, B.; Seeburger, V.; Schroeder, A.; Lohaus, G. Sugar, Amino Acid and Inorganic Ion Profiling of the Honeydew from Different Hemipteran Species Feeding on Abies Alba and Picea Abies. PLoS ONE 2020, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, S.; Ruoff, K.; Persano Oddo, L. Physico-Chemical Methods for the Characterisation of Unifloral Honeys: A Review. Apidologie 2004. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, R.; Hebbar, H.U.; Rastogi, N.K. Processing of Honey: A Review. Int. J. Food Prop. 2007, 10, 127–143. [Google Scholar] [CrossRef]
- Abu-Tarboush, H.M.; Al-Kahtani, H.A.; El-Sarrage, M.S. Floral-Type Identification and Quality Evaluation of Some Honey Types. Food Chem. 1993, 46, 13–17. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Weston, R.J.; Brocklebank, L.K. The Oligosaccharide Composition of Some New Zealand Honeys. Food Chem. 1999, 64, 33–37. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-Chemical and Bioactive Properties of Different Floral Origin Honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Canini, A.; Pichichero, E.; Alesian, D.; Canuti, L.; Leonardi, D. Nutritional and Botanical Interest of Honey Collected from Protected Natural Areas. Plant Biosyst. 2009, 143, 62–70. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1970, 51, 125–138. [Google Scholar] [CrossRef]
- Demianowicz, Z. Charakteristik der einartenhonige. Ann. de l’Abeille 1964, 7, 273–288. [Google Scholar] [CrossRef]
- El-Labban, M. Beekeepers’ Guide for Pollen Identification of Honey; Mohammad El-Labban: Lebanon, Switzerland, 2020; ISBN 978-9953-0-5184-0. [Google Scholar]
- Danner, N.; Molitor, A.M.; Schiele, S.; Härtel, S.; Steffan-Dewenter, I. Season and Landscape Composition Affect Pollen Foraging Distances and Habitat Use of Honey Bees. Ecol. Appl. 2016, 26, 1920–1929. [Google Scholar] [CrossRef]
- Sant’Ana, L.D.O.; Sousa, J.P.L.M.; Salgueiro, F.B.; Lorenzon, M.C.A.; Castro, R.N. Characterization of Monofloral Honeys with Multivariate Analysis of Their Chemical Profile and Antioxidant Activity. J. Food Sci. 2012, 77, C135–C140. [Google Scholar] [CrossRef]
- Olga, E.; María, F.-G.G.; María Carmen, S.; Carmen, S.M. Differentiation of Blossom Honey and Honeydew Honey from Northwest Spain. Agriculture 2012, 2, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Physicochemical Properties and Pollen Profile of Oak Honeydew and Evergreen Oak Honeydew Honeys from Spain: A Comparative Study. Foods 2019, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Karabournioti, S.; Karabagias, V.K.; Badeka, A.V. Palynological, Physico-Chemical and Bioactivity Parameters Determination, of a Less Common Greek Honeydew Honey: “Dryomelo. ” Food Control 2020, 109. [Google Scholar] [CrossRef]
- van der Ham, R.W.J.M.; Kaas, J.P.; Kerkvliet, J.D. Pollenanalyse, Stuifmeelonderzoek van Honing Voor Imkers, Scholen En Laboratoria, 1st ed.; Proefbedrijf voor Insektenbestuiving en Bijenhouderij Ambrosiushoeve; Stichting Landelijk: Hilvarenbeek, The Netherlands, 1999; ISBN 9789080543812. [Google Scholar]
- Guyot, C.; Bouseta, A.; Scheirman, V.; Collin, S. Floral Origin Markers of Chestnut and Lime Tree Honeys. J. Agric. Food Chem. 1998, 46, 625–633. [Google Scholar] [CrossRef]
- Gašić, U.; Šikoparija, B.; Tosti, T.; Trifković, J.; Milojković-Opsenica, Maja Natić, D.; Tešić, Ž.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. Phytochemical Fingerprints of Lime Honey Collected in Serbia. J. AOAC Int. 2014, 97, 1259–1267. [Google Scholar] [CrossRef]
- Uršulin-Trstenjak, N.; Hrga, I.; Stjepanović, B.; Dragojlović, D.; Levanic, D. DETERMINATION OF BOTANIC ORIGIN OF THE CROATIAN BLACK LOCUST HONEY (Istria Region) USING MELISSOPALYNOLOGICAL ANALYSIS. J. Hyg. Eng. Design 2013, 4, 122–126. [Google Scholar]
- Dobre, I.; Georgescu, L.A.; Alexe, P.; Escuredo, O.; Seijo, M.C. Rheological Behavior of Different Honey Types from Romania. Food Res. Int. 2012, 49, 126–132. [Google Scholar] [CrossRef]
- KOÇYİĞİT, M.; Keskin, T. DAŞTAN Pollen Morphology of Some Trifolium Species Which Are Favorite Plants of Honey Bees in Istanbul. J. Fac. Pharm. Istanbul 2013, 43, 85–94. [Google Scholar]
- Silici, S.; Gökceoglu, M. Pollen Analysis of Honeys from Mediterranean Region of Anatolia. Grana 2007, 46, 57–64. [Google Scholar] [CrossRef]
- Stacherzak, A.; Hájek, L.; Hełdak, M. Changes in the Use of Agricultural Land in Poland and Czech Republic. J. Ecol. Eng. 2019, 20, 211–221. [Google Scholar] [CrossRef]
- Knollová, I.; Chytrý, M. Oak-Hornbeam Forests of the Czech Republic: Geographical and Ecological Approaches to Vegetation Classification. Preslia 2004, 76, 291–311. [Google Scholar]
- Salonen, A.; Ollikka, T.; Grönlund, E.; Ruottinen, L.; Julkunen-Tiitto, R. Pollen Analyses of Honey from Finland. Grana 2009, 48, 281–289. [Google Scholar] [CrossRef]
- Varis, A.L. Influence of Changes in Crop Cultivation Areas on Pollen Contents of Honey. Agric. Food Sci. Finl. 2000, 9, 253–256. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Melissopalynology, Antioxidant Activity and Multielement Analysis of Two Types of Early Spring Honeys from Hungary. Food Biosci. 2020, 35, 100587. [Google Scholar] [CrossRef]
- Rašić, S.; Štefanić, E.; Antunović, S.; Jović, J.; Kristek, S. Pollen Analysis of Honey from North-Eastern Croatia. Poljoprivreda 2018, 24, 43–49. [Google Scholar] [CrossRef]
- Truzzi, C.; Illuminati, S.; Annibaldi, A.; Finalea, C.; Rossetti, M.; Scarponi, G. Physicochemical Properties of Honey from Marche, Central Italy: Classification of Unifloral and Multifloral Honeys by Multivariate Analysis. Nat. Prod. Commun. 2014, 9, 1595–1602. [Google Scholar] [CrossRef] [Green Version]
- Šarić, G.; Matković, D.; Hruškar, M.; Vahčić, N. Characterisation and Classification of Croatian Honey by Physicochemical Parameters. Food Technol. Biotechnol. 2008, 46, 355–367. [Google Scholar]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Azeredo, L.D.C.; Azeredo, M.A.A.; De Souza, S.R.; Dutra, V.M.L. Protein Contents and Physicochemical Properties in Honey Samples of Apis Mellifera of Different Floral Origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
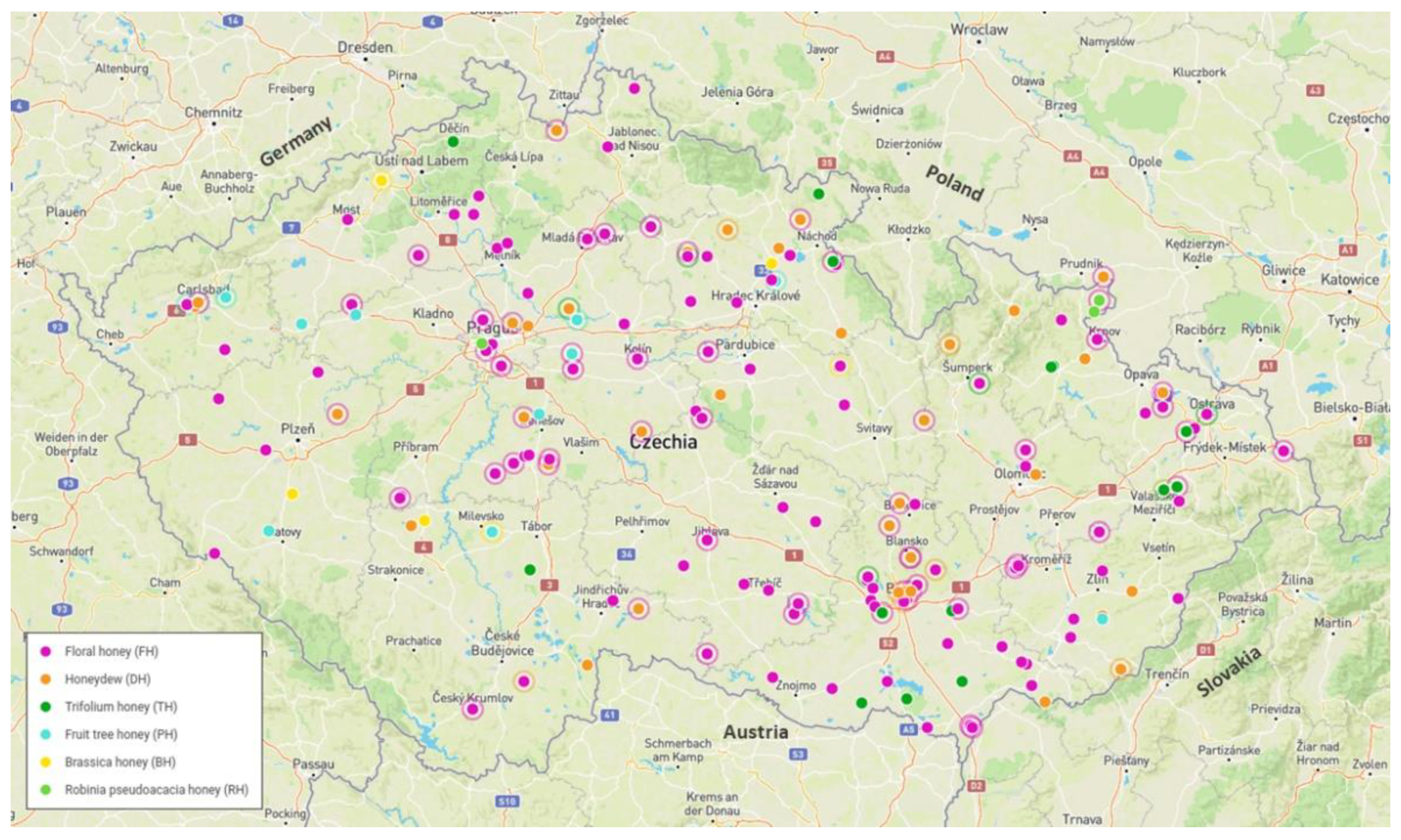
| Sample | Frequency | Threshold Criteria |
|---|---|---|
| Brassica honey (BH) | 10 | <0.8 mS/cm E. Cond. >70% specific pollen |
| Floral honey (FH) * | 207 | <0.8 mS/cm E. Cond. |
| Fruit tree honey (PH) * | 15 | <0.8 mS/cm E. Cond. >20% specific pollen or >15% when is in majority |
| Honeydew (HD) * | 57 | >0.8 mS/cm E. Cond. without specific pollen |
| Lime tree honey (LH) | 5 | >10% specific pollen |
| Robinia pseudoacacia honey (RH) ** | 4 | <0.8 mS/cm E. Cond. >10% specific pollen |
| Trifolium honey (TH) ** | 18 | >20% specific pollen |
| BH | FH | PH | HD | LH | RH | TH | |
|---|---|---|---|---|---|---|---|
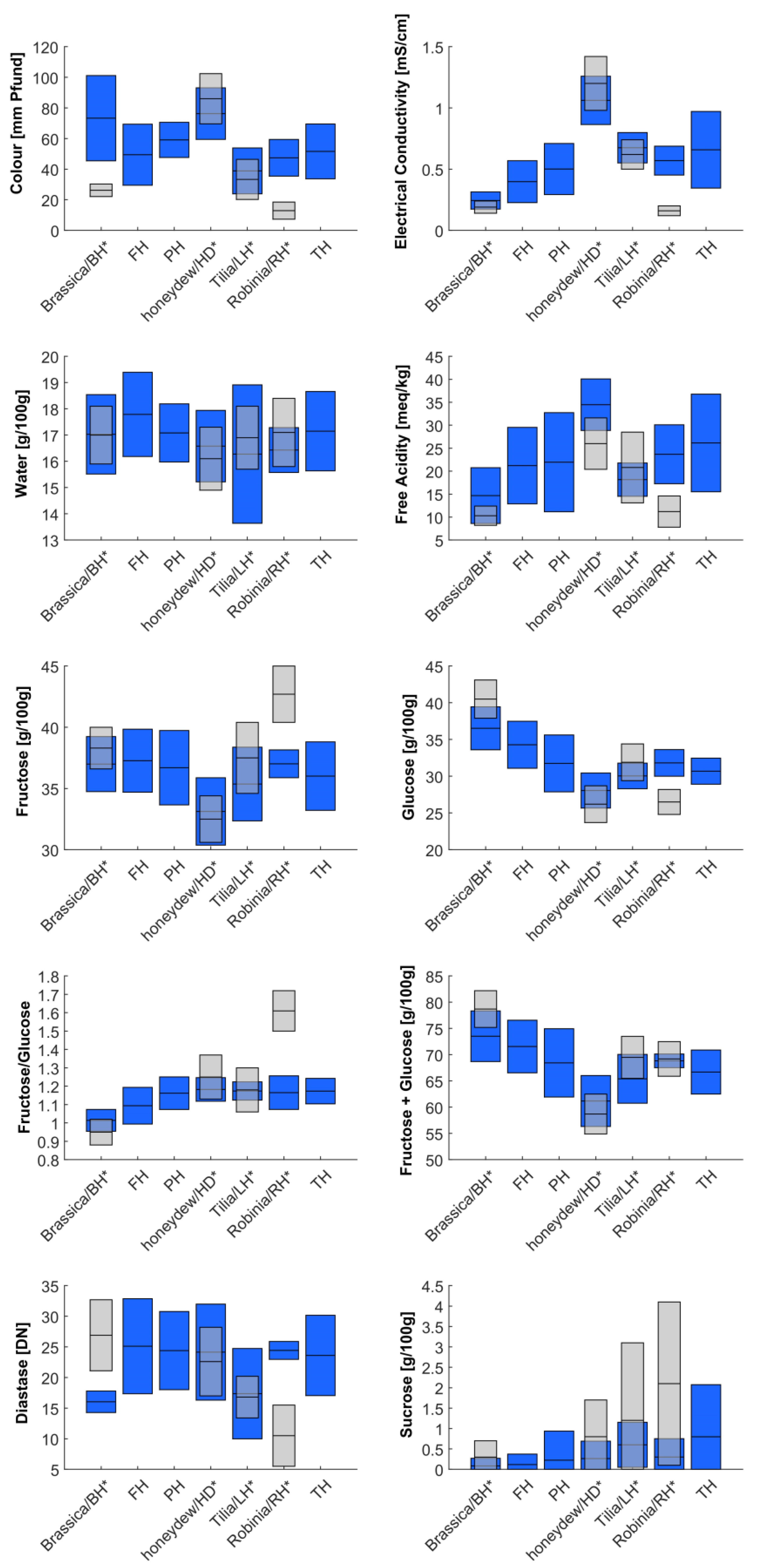
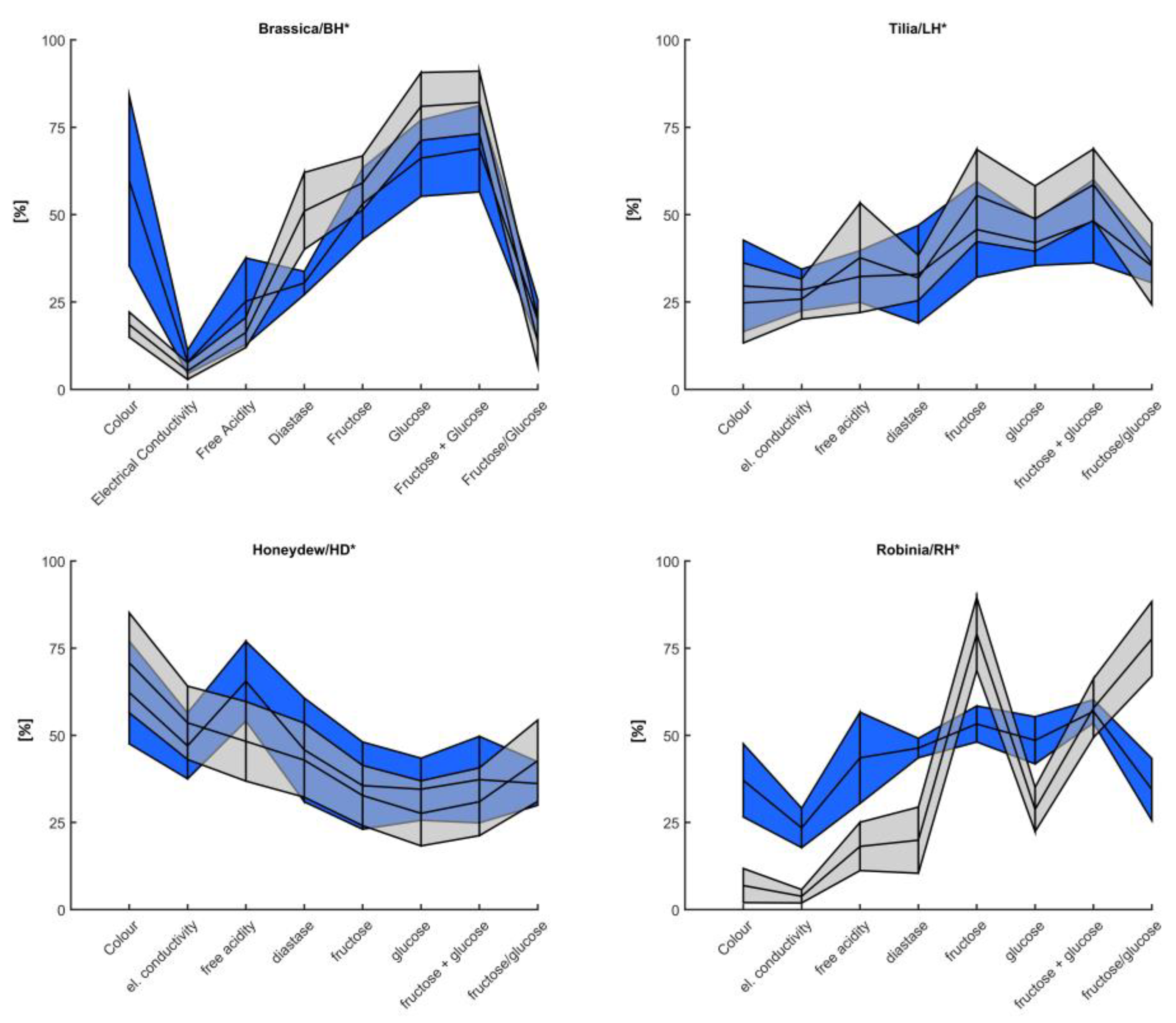
| E. Cond. (mS/cm) | 0.24 ± 7 a | 0.4 ± 17.1 a | 0.5 ± 20.9 ab | 1.1 ± 19.7 c | 0.7 ± 13.5 bc | 0.6 ± 11.8 ab | 0.6 ± 31.8 b |
| Colour (Pfund) | 73.3 ± 27.8 ab | 49.4 ± 20 a | 59.1 ± 11.5 ab | 76.3 ± 16.8 b | 45.6 ± 21.2 a | 47.4 ± 12 a | 50 ± 17.1 a |
| HMF (mg/kg) | 2.5 ± 1.9 | 3.1 ± 3.9 | 4 ± 3.4 | 2.5 ± 3.5 | 2.5 ± 2.8 | 1.7 ± 1.3 | 3.5 ± 6.2 |
| Diastase (DN) | 16 ± 1.8 a | 25.1 ± 7.8 b | 24.4 ± 6.4 b | 24.1 ± 7.8 b | 18.6 ± 7.2 ab | 24.4 ± 1.5 b | 23.5 ± 6.8 ab |
| Water (g/100 g) | 17 ± 1.5 | 17.8 ± 1.6 | 17.1 ± 1.1 | 16.6 ± 1.4 | 16.2 ± 2.4 | 16.4 ± 0.9 | 17.2 ± 1.5 |
| F. Ac. (meq/kg) | 14.7 ± 6.1 a | 21.2 ± 8.3 ab | 22 ± 10.8 ab | 34.5 ± 5.6 c | 20.5 ± 6.6 ab | 23.7 ± 6.4 ab | 25.8 ± 10.8 b |
| Fructose (g/100 g) | 37 ± 2.2 b | 37.3 ± 2.6 b | 36.7 ± 3 b | 33.1 ± 2.8 a | 34.5 ± 3.4 ab | 37 ± 1.1 b | 36.3 ± 2.5 b |
| Glucose (g/100 g) | 36.5 ± 2.9 c | 34.3 ± 3.2 bc | 31.7 ± 3.9 b | 28.1 ± 2.4 a | 30 ± 1.6 ab | 31.8 ± 1.8 bc | 30.7 ± 1.8 ab |
| F + G (g/100 g) | 73.5 ± 4.8 b | 71.6 ± 5 b | 68.4 ± 6.5 b | 61.2 ± 4.9 a | 64.6 ± 4.6 ab | 68.8 ± 1.3 ab | 67.1 ± 4 ab |
| F/G (g/100 g) | 1 ± 0.1 a | 1.1 ± 0.1 ab | 1.2 ± 0.1 bc | 1.2 ± 0.1 c | 1.1 ± 0.1 abc | 1.2 ± 0.1 bc | 1.2 ± 0.1 c |
| Sucrose (g/100 g) | 0.1 ± 0.2 a | 0.1 ± 0.3 a | 0.2 ± 0.7 a | 0.3 ± 0.4 a | 0.5 ± 0.6 ab | 0.3 ± 0.4 ab | 0.8 ± 1.3 b |
| Maltose (g/100 g) | 2.9 ± 1 | 3.2 ± 1 | 4 ± 1.3 | 3.5 ± 0.7 | 4.6 ± 1.9 | 4.5 ± 1.1 | 3.7 ± 1 |
| Melibiose (g/100 g) | <0.2 | <0.2 | <0.2 | 0 ± 0.1 b | <0.2 | <0.2 | 0 ± 0.1 b |
| Turanose (g/100 g) | 1.3 ± 0.4 a | 1.7 ± 0.5 ab | 1.9 ± 0.5 bc | 2 ± 0.4 c | 2.4 ± 1 c | 2.2 ± 0.4 c | 2.1 ± 0.6 c |
| Trehalose (g/100 g) | 0.4 ± 0.4 a | 0.6 ± 0.4 a | 0.4 ± 0.4 a | 1.3 ± 0.6 b | 0.5 ± 0.4 a | 0.6 ± 0.4 ab | 0.8 ± 0.3 ab |
| Maltotriose (g/100 g) | 0 ± 0 | 0 ± 0.1 | 0.1 ± 0.2 | 0.3 ± 0.4 | 0.1 ± 0.1 | <0.1 | 0.1 ± 0.3 |
| Melezitose (g/100 g) | 0.6 ± 0.8 a | 0.6 ± 1 a | 0.7 ± 0.6 a | 4.9 ± 3.3 b | 0.8 ± 0.8 ab | 0.8 ± 0.8 a | 1.3 ± 2.2 a |
| BH | FH | PH | HD | LH | RH | TH | |
|---|---|---|---|---|---|---|---|
| Aceraceae | |||||||
| Acer sp. (n = 106) | 0.3 ± 0.4 | 1.2 ± 2.8 | 2.9 ± 8.7 | 0.3 ± 0.8 | <0.1 | <0.1 | 1 ± 1.8 |
| Asteraceae | |||||||
| Achillea sp. (n = 20) | <0.1 | 0.7 ± 4.6 | 0.1 ± 0.3 | 0.1 ± 0.2 | <0.1 | <0.1 | 0.1 ± 0.5 |
| Artemisia sp. * (n = 163) | 0.8 ± 1.3 | 1.8 ± 3.1 | 4.6 ± 7.7 | 2.8 ± 4 | 1.2 ± 1.9 | <0.1 | 0.4 ± 1 |
| Bellis sp. * (n = 47) | 0 ± 0 | 0.1 ± 0.5 | 0.3 ± 0.6 | 0.4 ± 1.4 | 0.6 ± 0.9 | 0.3 ± 0.5 | 0.0 ± 0.1 |
| Helianthum sp. (n = 36) | 0.1 ± 0.2 | 0.2 ± 1 | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.1 ± 0.2 | 0.5 ± 1 | 0.3 ± 1 |
| Taraxacum sp. (n = 89) | 0.1 ± 0.2 | 0.4 ± 1.6 | 0.2 ± 0.5 | 0.5 ± 1.4 | <0.1 | 0.1 ± 0.2 | 0.6 ± 0.6 |
| Betulaceae | |||||||
| Alnus sp. * (n = 23) | <0.1 | 0.1 ± 0.3 | 0.0 ± 0.1 | 0.1 ± 0.3 | <0.1 | <0.1 | 0.1 ± 0.2 |
| Corylus sp. * (n = 28) | 0.2 ± 0.8 | 0.2 ± 0.8 | 0.8 ± 2.1 | 0.8 ± 5.2 | <0.1 | <0.1 | <0.1 |
| Boraginaceae | |||||||
| Myosotis sp. (n = 274) | 1.9 ± 1.9 a | 6.9 ± 10.4 a | 1.9 ± 1.5 a | 16.1 ± 17.9 b | 9.4 ± 5.3 b | 14.7 ± 15.6 b | 10.7 ± 12.2 ab |
| Brassicaceae | |||||||
| Brassica sp. (n = 291) | 79.5 ± 7.1 c | 41.6 ± 22.5 b | 24.1 ± 23.6 b | 21.7 ± 23.2 a | 6.8 ± 7.3 a | 15 ± 14.4 a | 10.84 ± 9.5 a |
| Hydrophyllaceae | |||||||
| Phacelia sp. (n = 184) | 1.1 ± 1.5 | 4.3 ± 8.1 | 7.8 ± 10.7 | 5.6 ± 7.8 | 12.3 ± 19.2 | 0.3 ± 0.6 | 2.7 ± 3.4 |
| Fagaceae | |||||||
| Quercus sp. * (n = 59) | 0.9 ± 2.8 | 0.4 ± 1.8 | 1 ± 1.9 | 0.3 ± 0.7 | <0.1 | <0.1 | 0.3 ± 0.9 |
| Rhamnaceae | |||||||
| Rhamnus sp. (n = 35) | <0.1 | 0.19 ± 1.4 a | 0.48 ± 0.88 b | 0.22 ± 0.86 ab | <0.1 | <0.1 | 0.12 ± 0.49 a |
| Rosaceae | |||||||
| Pyrus/Prunus sp. (n = 253) | 1 ± 1 a | 3.9 ± 4.4 a | 31.7 ± 13.9 b | 6.4 ± 8.2 a | 1.6 ± 2.5 a | 1.5 ± 3 a | 3.7 ± 4.9 a |
| Rubus sp. (n = 81) | <0.1 | 0.5 ± 1.9 | 0.8 ± 1.8 | 0.4 ± 1.2 | 1.8 ± 3.8 | 0.5 ± 1 | 1 ± 2.9 |
| Salicaceae | |||||||
| Salix sp. (n = 164) | 3.2 ± 5.1 | 4.7 ± 7.3 | 0.5 ± 1.3 | 0.7 ± 1.6 | 0.7 ± 1.1 | 2.9 ± 1.9 | 1.4 ± 2.3 |
| Fabaceae | |||||||
| Robinia pseudoacacia (n = 117) | 0.0 ± 0.1 a | 0.8 ± 1.6 a | 1.2 ± 1.8 ab | 1.6 ± 3.3 a | 0.3 ± 0.7 a | 12.7 ± 0.9 b | 1.2 ± 2.6 a |
| Trifolium sp. (n = 238) | 0.6 ± 0.7 a | 3.7 ± 4.4 ab | 1.9 ± 2.8 ab | 3.6 ± 4.7 ab | 18.6 ± 21.1 bc | 8.6 ± 7 bc | 33 ± 12 c |
| Vicia sp. (n = 223) | 2.5 ± 4.4 | 2.3 ± 4.4 | 4.3 ± 4.7 | 3.3 ± 4.6 | 2 ± 3.8 | 9.7 ± 6.9 | 5.1 ± 7.1 |
| Tiliaceae | |||||||
| Tilia sp. (n = 183) | 0.1 ± 0.1 a | 1.1 ± 1.8 a | 2.4 ± 4.2 ab | 1.9 ± 3.2 ab | 22.8 ± 14.4 b | 2.3 ± 2.1 ab | 3.8 ± 4 b |
| Apiaceae (Umbelliferae) | |||||||
| Apiales *,# (n = 282) | 2.8 ± 2.9 | 6.4 ± 8.4 | 3.4 ± 4.8 | 6.9 ± 8 | 4.2 ± 3.8 | 8.5 ± 5.5 | 5.8 ± 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pospiech, M.; Javůrková, Z.; Hrabec, P.; Čížková, H.; Titěra, D.; Štarha, P.; Ljasovská, S.; Kružík, V.; Podskalská, T.; Bednář, J.; et al. Physico-Chemical and Melissopalynological Characterization of Czech Honey. Appl. Sci. 2021, 11, 4989. https://doi.org/10.3390/app11114989
Pospiech M, Javůrková Z, Hrabec P, Čížková H, Titěra D, Štarha P, Ljasovská S, Kružík V, Podskalská T, Bednář J, et al. Physico-Chemical and Melissopalynological Characterization of Czech Honey. Applied Sciences. 2021; 11(11):4989. https://doi.org/10.3390/app11114989
Chicago/Turabian StylePospiech, Matej, Zdeňka Javůrková, Pavel Hrabec, Helena Čížková, Dalibor Titěra, Pavel Štarha, Simona Ljasovská, Vojtěch Kružík, Tereza Podskalská, Josef Bednář, and et al. 2021. "Physico-Chemical and Melissopalynological Characterization of Czech Honey" Applied Sciences 11, no. 11: 4989. https://doi.org/10.3390/app11114989
APA StylePospiech, M., Javůrková, Z., Hrabec, P., Čížková, H., Titěra, D., Štarha, P., Ljasovská, S., Kružík, V., Podskalská, T., Bednář, J., Burešová, P. K., & Tremlová, B. (2021). Physico-Chemical and Melissopalynological Characterization of Czech Honey. Applied Sciences, 11(11), 4989. https://doi.org/10.3390/app11114989







