Multi-target Natural and Nature-Inspired Compounds against Neurodegeneration: A Focus on Dual Cholinesterase and Phosphodiesterase Inhibitors
Abstract
:1. Introduction
2. Multi-Target Natural and Nature-Inspired Compounds
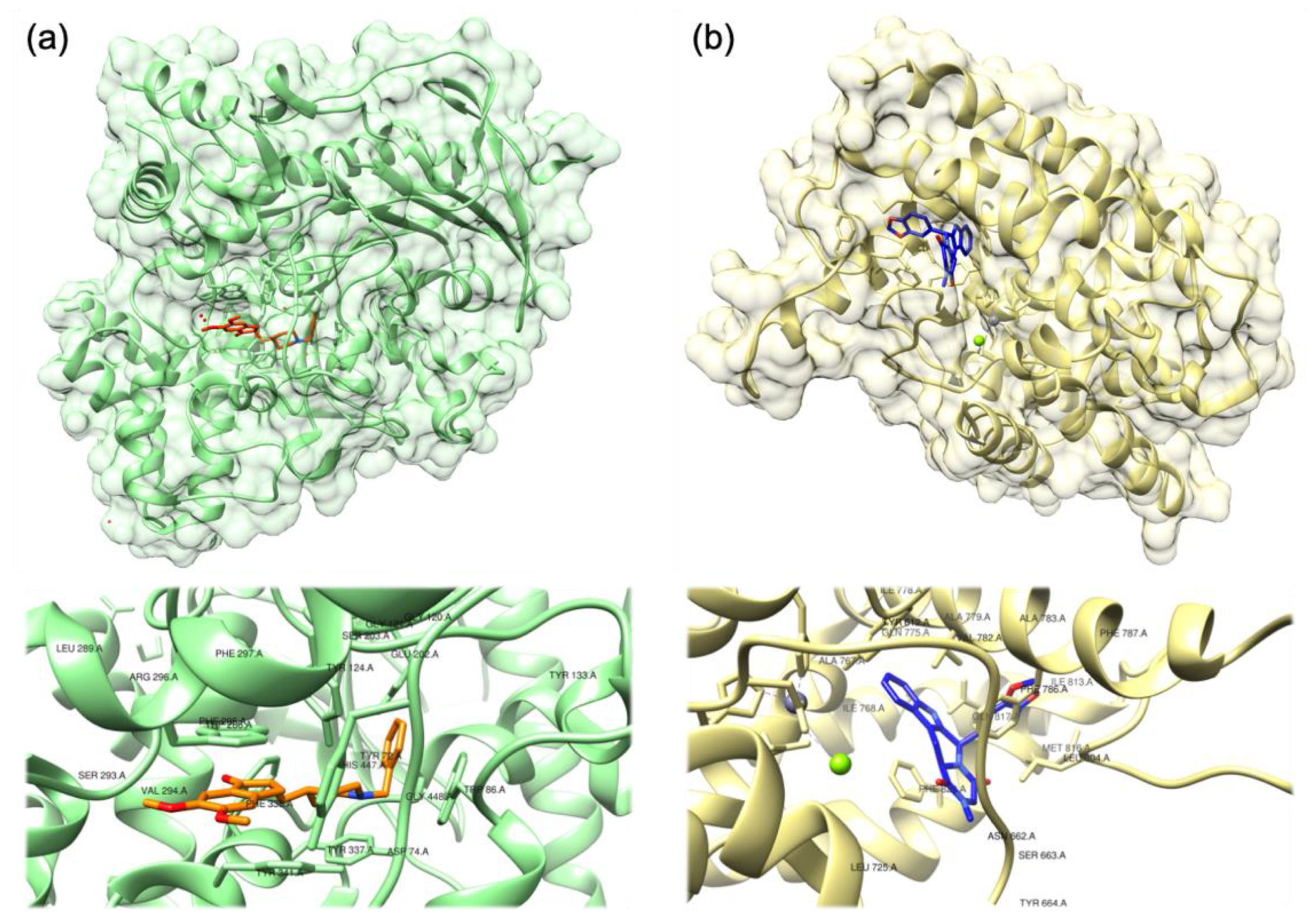
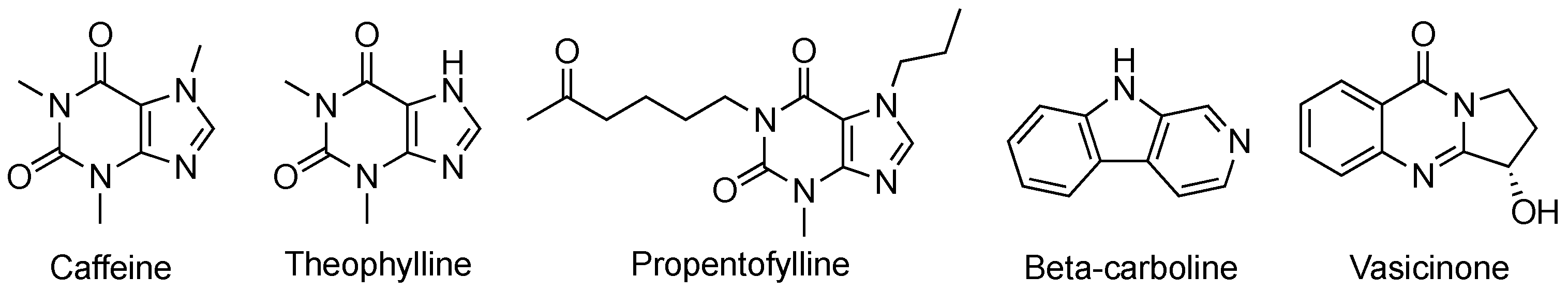
2.1. Xanthines and Other Alkaloids
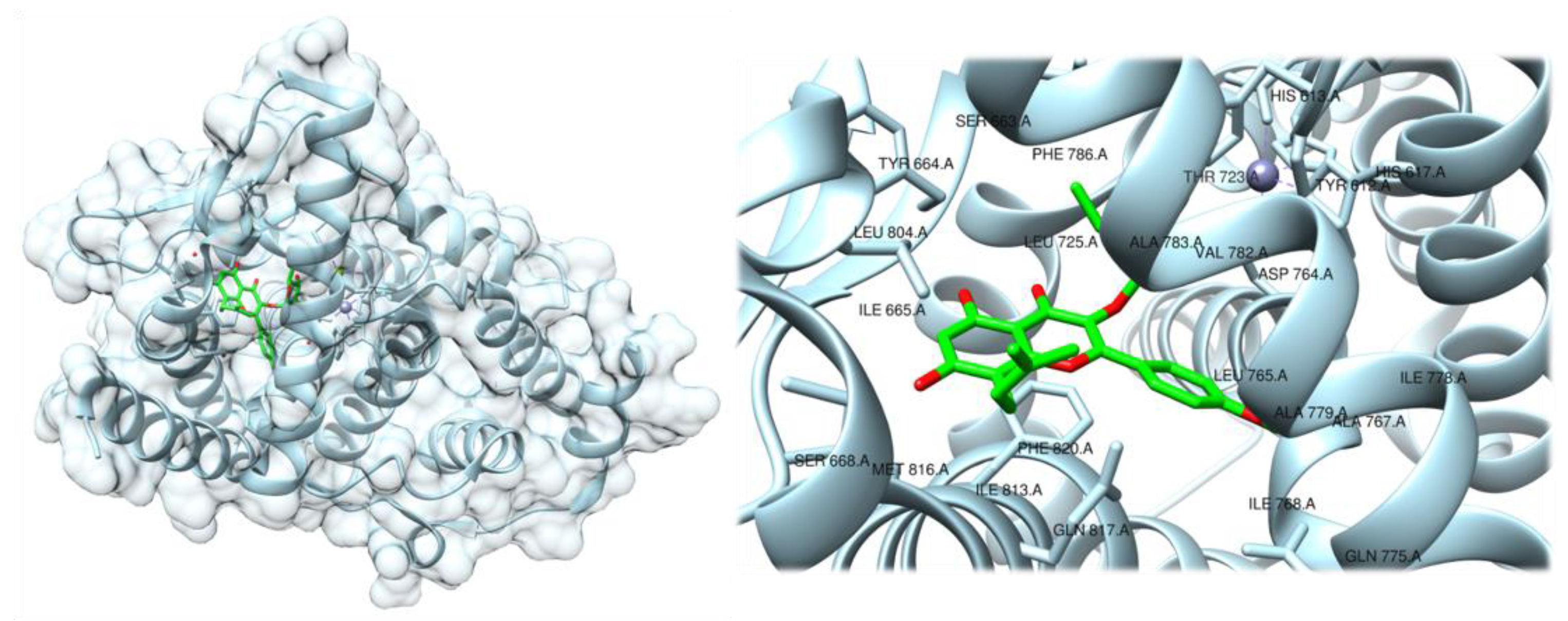
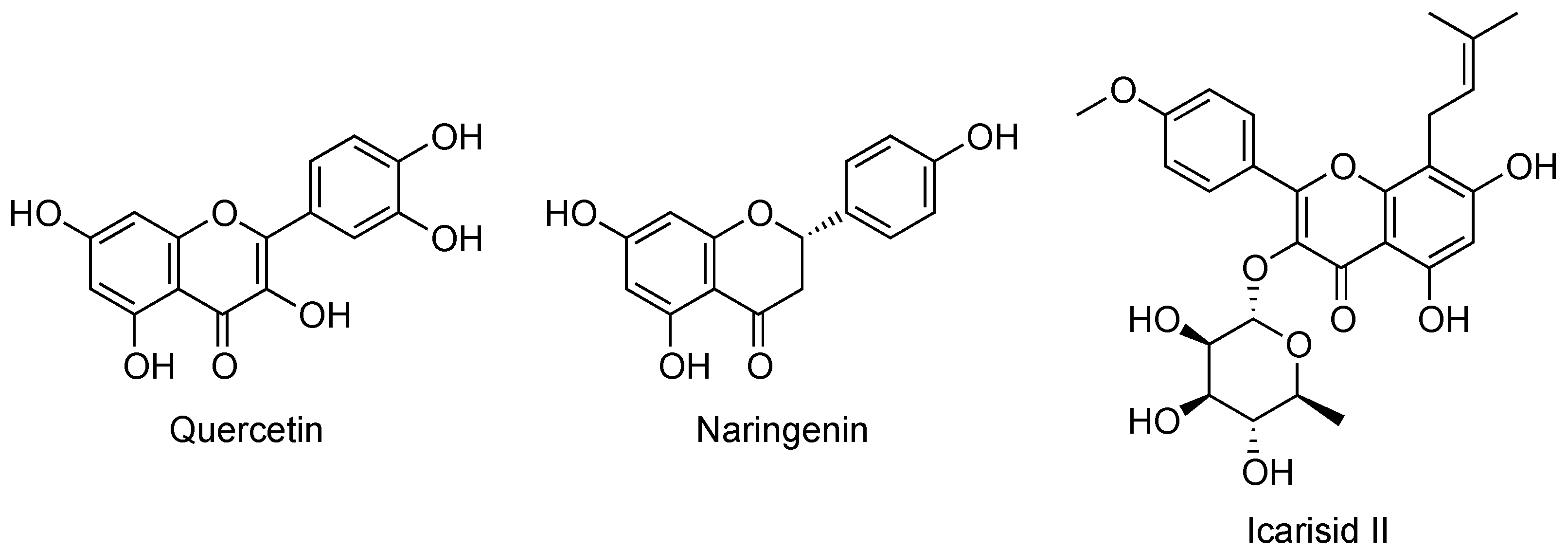
2.2. Flavonoids and Coumarins
2.3. Natural Extracts Containing Polyphenolic Acids
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castellani, R.J.; Rolston, R.K.; Smith, M.A. Alzheimer Disease. Disease 2010, 56, 484–546. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Chadha, N.; Silakari, O. Hybrids: A new paradigm to treat Alzheimer’s disease. Mol. Divers. 2015, 20, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Singh, N.; Silakari, O. Exploration of multi-target potential of chromen-4-one based compounds in Alzheimer’s disease: Design, synthesis and biological evaluations. Bioorganic Med. Chem. 2017, 25, 6273–6285. [Google Scholar] [CrossRef] [PubMed]
- Kaduszkiewicz, H.; Zimmermann, T.; Beck-Bornholdt, H.-P.; Bussche, H.V.D. Cholinesterase inhibitors for patients with Alzheimer’s disease: Systematic review of randomised clinical trials. BMJ 2005, 331, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuno, N. Donepezil in the treatment of patients with Alzheimer’s disease. Expert Rev. Neurother. 2009, 9, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.J. Phosphodiesterase inhibitors as potential cognition enhancing agents. Curr. Top. Med. Chem. 2010, 10, 222–230. [Google Scholar] [CrossRef]
- Kroker, K.S.; Rast, G.; Giovannini, R.; Marti, A.; Dorner-Ciossek, C.; Rosenbrock, H. Inhibition of acetylcholinesterase and phosphodiesterase-9A has differential effects on hippocampal early and late LTP. Neuropharmacology 2012, 62, 1964–1974. [Google Scholar] [CrossRef]
- Ribaudo, G.; Ongaro, A.; Zagotto, G.; Memo, M.; Gianoncelli, A. Therapeutic potential of phosphodiesterase inhibitors against neurodegeneration: The Perspective of the medicinal chemist. ACS Chem. Neurosci. 2020, 11, 1726–1739. [Google Scholar] [CrossRef]
- Mao, F.; Wang, H.; Ni, W.; Zheng, X.; Wang, M.; Bao, K.; Ling, D.; Li, X.; Xu, Y.; Zhang, H.; et al. Design, synthesis, and biological evaluation of orally available first-generation dual-target selective inhibitors of acetylcholinesterase (AChE) and phosphodiesterase 5 (PDE5) for the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 328–345. [Google Scholar] [CrossRef]
- Pan, T.; Xie, S.; Zhou, Y.; Hu, J.; Luo, H.; Li, X.; Huang, L. Dual functional cholinesterase and PDE4D inhibitors for the treatment of Alzheimer’s disease: Design, synthesis and evaluation of tacrine-pyrazolo [3,4-b]pyridine hybrids. Bioorganic Med. Chem. Lett. 2019, 29, 2150–2152. [Google Scholar] [CrossRef]
- Ribaudo, G.; Memo, M.; Gianoncelli, A. A Perspective on natural and nature-inspired small molecules targeting phosphodiesterase 9 (PDE9): Chances and challenges against neurodegeneration. Pharmaceuticals 2021, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Lakics, V.; Karran, E.H.; Boess, F.G. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 2010, 59, 367–374. [Google Scholar] [CrossRef]
- Ji, H.-F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef]
- Gallant, M.; Aspiotis, R.; Day, S.; Dias, R.; Dube, D.; Dubé, L.; Friesen, R.W.; Girard, M.; Guay, D.; Hamel, P.; et al. Discovery of MK-0952, a selective PDE4 inhibitor for the treatment of long-term memory loss and mild cognitive impairment. Bioorganic Med. Chem. Lett. 2010, 20, 6387–6393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-Y.; Zhu, Y.; Jiang, Y.-R.; Zhao, X.-J.; Guo, D. Design, synthesis and biological evaluation of dual acetylcholinesterase and phosphodiesterase 5A inhibitors in treatment for Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2017, 27, 4180–4184. [Google Scholar] [CrossRef] [PubMed]
- Hutson, P.; Finger, E.; Magliaro, B.; Smith, S.; Converso, A.; Sanderson, P.; Mullins, D.; Hyde, L.; Eschle, B.; Turnbull, Z.; et al. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-pyran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology 2011, 61, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Klett, J.; Pilarzyk, K.; Lee, D.I.; Kass, D.; Menniti, F.S.; Kelly, M.P. Identification of new PDE9A isoforms and how their expression and subcellular compartmentalization in the brain change across the life span. Neurobiol. Aging 2018, 65, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Van der Staay, F.J.; Rutten, K.; Bärfacker, L.; DeVry, J.; Erb, C.; Heckroth, H.; Karthaus, D.; Tersteegen, A.; van Kampen, M.; Blokland, A.; et al. The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents. Neuropharmacology 2008, 55, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Schwam, E.M.; Nicholas, T.; Chew, R.; Billing, C.B.; Davidson, W.; Ambrose, D.; Altstiel, L.D. A multicenter, double-blind, placebo-controlled trial of the PDE9A inhibitor, PF-04447943, in Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 413–421. [Google Scholar] [CrossRef]
- Moschetti, V.; Boland, K.; Feifel, U.; Hoch, A.; Zimdahl-Gelling, H.; Sand, M. First-in-human study assessing safety, tolerability and pharmacokinetics of BI 409306, a selective phosphodiesterase 9A inhibitor, in healthy males. Br. J. Clin. Pharmacol. 2016, 82, 1315–1324. [Google Scholar] [CrossRef]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. The medicinal chemistry of natural and semisynthetic compounds against Parkinson’s and Huntington’s diseases. ACS Chem. Neurosci. 2017, 8, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Zagotto, G.; Ribaudo, G. An overview of new possible treatments of Alzheimer’s disease, based on natural products and semi-synthetic compounds. Curr. Med. Chem. 2017, 24, 3749–3773. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Diniz, B.S.; Pinto, J.A.; Gonzaga, M.L.C.; Guimarães, F.M.; Gattaz, W.F.; Forlenza, O.V. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.; Zagotto, G.; Memo, M.; Gianoncelli, A.; Ribaudo, G. Natural phosphodiesterase 5 (PDE5) inhibitors: A computational approach. Nat. Prod. Res. 2019, 35, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. The perspective of caffeine and caffeine derived compounds in therapy. Bratisl. Lek. List. 2015, 116, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Szadujkis-Szadurska, K.; Grzesk, G.; Szadujkis-Szadurski, L.; Gajdus, M.; Matusiak, G. Role of acetylcholine and calcium ions in three vascular contraction models: Angiotensin II, phenylephrine and caffeine. Exp. Ther. Med. 2012, 4, 329–333. [Google Scholar] [CrossRef]
- Cummings, K.J.; Commons, K.G.; Trachtenberg, F.L.; Li, A.; Kinney, H.C.; Nattie, E.E. Caffeine improves the ability of serotonin-deficient (Pet-1−/−) mice to survive episodic asphyxia. Pediatr. Res. 2013, 73, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Gołembiowska, K.; Dziubina, A. The effect of adenosine A2A receptor antagonists on hydroxyl radical, dopamine, and glutamate in the striatum of rats with altered function of VMAT. Neurotox. Res. 2012, 22, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.P. Caffeine’s mechanisms of action and its cosmetic use. Ski. Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimer’s Dis. 2010, 20, S85–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohanka, M. Alzheimer’s disease and oxidative stress: A review. Curr. Med. Chem. 2013, 21, 356–364. [Google Scholar] [CrossRef]
- Rivera-Oliver, M.; Díaz-Ríos, M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: A review. Life Sci. 2014, 101, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Li, S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson’s disease. Geriatr. Gerontol. Int. 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Kobal, J.; Melik, Z.; Cankar, K.; Strucl, M. Cognitive and autonomic dysfunction in presymptomatic and early Huntington’s disease. J. Neurol. 2014, 261, 1119–1125. [Google Scholar] [CrossRef]
- Sanders, O.; Rajagopal, L. Phosphodiesterase inhibitors for Alzheimer’s Disease: A systematic review of clinical trials and epidemiology with a mechanistic rationale. J. Alzheimer’s Dis. Rep. 2020, 4, 185–215. [Google Scholar] [CrossRef]
- Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Seyfried, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Unique role of caffeine compared to other methylxanthines (theobromine, theophylline, pentoxifylline, propentofylline) in regulation of AD relevant genes in neuroblastoma SH-SY5Y wild type cells. Int. J. Mol. Sci. 2020, 21, 9015. [Google Scholar] [CrossRef]
- Mohamed, T.; Osman, W.; Tin, G.; Rao, P.P. Selective inhibition of human acetylcholinesterase by xanthine derivatives: In vitro inhibition and molecular modeling investigations. Bioorganic Med. Chem. Lett. 2013, 23, 4336–4341. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Saravanan, P.; Thakur, M.; Patra, S.; Parameswaran, S. Development of xanthine based inhibitors targeting phosphodiesterase 9A. Lett. Drug Des. Discov. 2017, 14, 1122–1137. [Google Scholar] [CrossRef]
- Huang, M.; Shao, Y.; Hou, J.; Cui, W.; Liang, B.; Huang, Y.; Li, Z.; Wu, Y.; Zhu, X.; Liu, P.; et al. Structural asymmetry of phosphodiesterase-9A and a unique pocket for selective binding of a potent enantiomeric inhibitor. Mol. Pharmacol. 2015, 88, 836–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huai, Q.; Wang, H.; Zhang, W.; Colman, R.W.; Robinson, H.; Ke, H. Crystal structure of phosphodiesterase 9 shows orientation variation of inhibitor 3-isobutyl-1-methylxanthine binding. Proc. Natl. Acad. Sci. USA 2004, 101, 9624–9629. [Google Scholar] [CrossRef] [Green Version]
- Ribaudo, G.; Coghi, P.; Zanforlin, E.; Law, B.Y.K.; Wu, Y.Y.J.; Han, Y.; Qiu, A.C.; Qu, Y.Q.; Zagotto, G.; Wong, V.K.W.; et al. Semi-synthetic isoflavones as BACE-1 inhibitors against Alzheimer’s disease. Bioorganic Chem. 2019, 87, 474–483. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Ribaudo, G.; Pagano, M.A.; Pavan, V.; Redaelli, M.; Zorzan, M.; Pezzani, R.; Mucignat-Caretta, C.; Vendrame, T.; Bova, S.; Zagotto, G. Semi-synthetic derivatives of natural isoflavones from Maclura pomifera as a novel class of PDE-5A inhibitors. Fitoterapia 2015, 105, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, G.; Vendrame, T.; Bova, S. Isoflavones from Maclura pomifera: Structural elucidation and in silico evaluation of their interaction with PDE. Nat. Prod. Res. 2017, 31, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, G.; Ongaro, A.; Zagotto, G. 5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one. Molbank 2018, 2018, M1004. [Google Scholar] [CrossRef] [Green Version]
- Gianoncelli, A.; Ongaro, A.; Zagotto, G.; Memo, M.; Ribaudo, G. 2-(3,4-Dihydroxyphenyl)-4-(2-(4-nitrophenyl)hydrazono)-4H-chromene-3,5,7-triol. Molbank 2020, 2020, M1144. [Google Scholar] [CrossRef]
- Oselladore, E.; Ongaro, A.; Zagotto, G.; Memo, M.; Ribaudo, G.; Gianoncelli, A. Combinatorial library generation, molecular docking and molecular dynamics simulations for enhancing the isoflavone scaffold in phosphodiesterase inhibition. N. J. Chem. 2020, 44, 19472–19488. [Google Scholar] [CrossRef]
- Orallo, F.; Camiña, M.; Álvarez, E.; Basaran, H.; Lugnier, C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (±)-naringenin. Planta Med. 2005, 71, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.L.; Lugnier, C.; Keravis, T.; Lopes, M.J.; Fantini, F.A.; Schmitt, M.; Cortes, S.F.; Lemos, V.S. The flavonoid dioclein is a selective inhibitor of cyclic nucleotide phosphodiesterase type 1 (PDE1) and a cGMP-dependent protein kinase (PKG) vasorelaxant in human vascular tissue. Eur. J. Pharmacol. 2009, 620, 78–83. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oyeleye, S.I.; Dada, F.A.; Olasehinde, T.A.; Oboh, G. Modulatory effect of quercetin and its glycosylated form on key enzymes and antioxidant status in rats penile tissue of paroxetine-induced erectile dysfunction. Biomed. Pharmacother. 2018, 107, 1473–1479. [Google Scholar] [CrossRef]
- Bortoli, M.; Tiezza, M.D.; Muraro, C.; Pavan, C.; Ribaudo, G.; Rodighiero, A.; Tubaro, C.; Zagotto, G.; Orian, L. Psychiatric disorders and oxidative injury: Antioxidant effects of zolpidem therapy disclosed in silico. Comput. Struct. Biotechnol. J. 2019, 17, 311–318. [Google Scholar] [CrossRef]
- Muraro, C.; Tiezza, M.D.; Pavan, C.; Ribaudo, G.; Zagotto, G.; Orian, L. Major depressive disorder and oxidative stress: In Silico investigation of fluoxetine activity against ROS. Appl. Sci. 2019, 9, 3631. [Google Scholar] [CrossRef] [Green Version]
- Ribaudo, G.; Bortoli, M.; Pavan, C.; Zagotto, G.; Orian, L. Antioxidant potential of psychotropic drugs: From clinical evidence to in vitro and in vivo assessment and toward a new challenge for in silico molecular design. Antioxidants 2020, 9, 714. [Google Scholar] [CrossRef]
- Xie, S.-S.; Wang, X.; Jiang, N.; Yu, W.; Wang, K.D.; Lan, J.-S.; Li, Z.-R.; Kong, L.-Y. Multi-target tacrine-coumarin hybrids: Cholinesterase and monoamine oxidase B inhibition properties against Alzheimer’s disease. Eur. J. Med. Chem. 2015, 95, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumbar, M.N.; Kamble, R.R.; Kamble, A.A.; Salian, S.R.; Kumari, S.; Nair, R.; Kalthur, G.; Adiga, S.K.; Prasad, D.J. Design and microwave assisted synthesis of coumarin derivatives as PDE inhibitors. Int. J. Med. Chem. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Huang, Q.; Liu, J.; Liang, N.; Li, Q.; Li, Q.; Xie, S.-S. Design, synthesis and biological evaluation of new coumarin-dithiocarbamate hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 146, 287–298. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Oyeleye, S.I.; Olasehinde, T.A.; Boligon, A.A. Modulation of some markers of erectile dysfunction and malonaldehyde levels in isolated rat penile tissue with unripe and ripe plantain peels: Identification of the constituents of the plants using HPLC. Pharm. Biol. 2017, 55, 1920–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajiboye, B.O.; Ojo, O.A.; Fatoba, B.; Afolabi, O.B.; Olayide, I.; Okesola, M.A.; Oyinloye, B.E. In vitro antioxidant and enzyme inhibitory properties of the n-butanol fraction of Senna podocarpa (Guill. and Perr.) leaf. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef]
- Dada, F.A.; Oyeleye, S.I.; Adefegha, S.A.; Oboh, G. Extracts from almond (Terminalia catappa) leaf and stem bark mitigate the activities of crucial enzymes and oxidative stress associated with hypertension in cyclosporine A-stressed rats. J. Food Biochem. 2021, 45. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Okeke, B.M.; Oyeleye, S.I. Comparative Effects of alkaloid extracts from Aframomum melegueta (Alligator Pepper) and Aframomum danielli (Bastered Melegueta) on enzymes relevant to erectile dysfunction. J. Diet. Suppl. 2017, 14, 542–552. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oyeleye, S.I.; Ogunsuyi, O.B.; Oboh, G. Phenolic analysis and erectogenic function of African Walnut (Tetracarpidium conophorum) seeds: The impact of the seed shell on biological activity. J. Food Biochem. 2019, 43, e12815. [Google Scholar] [CrossRef] [PubMed]
- Baratelli, T.D.G.; Gomes, A.C.C.; Wessjohann, L.A.; Kuster, R.M.; Simas, N.K. Phytochemical and allelopathic studies of Terminalia catappa L. (Combretaceae). Biochem. Syst. Ecol. 2012, 41, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.A.; Ojo, A.B.; Oyinloye, B.E.; Ajiboye, B.O.; Anifowose, O.O.; Akawa, A.; Olaiya, O.E.; Olasehinde, O.R.; Kappo, A.P. Ocimum gratissimum Linn. Leaves reduce the key enzymes activities relevant to erectile dysfunction in isolated penile and testicular tissues of rats. BMC Complement. Altern. Med. 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Salawu, S.O.; Bester, M.; Duodu, K.G. Phenolic composition and bioactive properties of cell wall preparations and whole grains of selected cereals and legumes. J. Food Biochem. 2014, 38, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar] [PubMed]
- García-Barroso, C.; Ricobaraza, A.; Pascual-Lucas, M.; Unceta, N.; Rico, A.J.; Goicolea, M.A.; Sallés, J.; Lanciego, J.L.; Oyarzabal, J.; Franco, R.; et al. Tadalafil crosses the blood–brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology 2013, 64, 114–123. [Google Scholar] [CrossRef]
- Ribaudo, G.; Pagano, M.A.; Bova, S.; Zagotto, G. New therapeutic applications of phosphodiesterase 5 inhibitors (PDE5-Is). Curr. Med. Chem. 2016, 23, 1239–1249. [Google Scholar] [CrossRef]
- Prickaerts, J.; Şık, A.; Van Der Staay, F.J.; De Vente, J.; Blokland, A. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: Acquisition versus consolidation. Psychopharmacology 2005, 177, 381–390. [Google Scholar] [CrossRef]
- Beato, A.; Gori, A.; Boucherle, B.; Peuchmaur, M.; Haudecoeur, R. β-Carboline as a privileged scaffold for multitarget strategies in Alzheimer’s disease therapy. J. Med. Chem. 2021, 64, 1392–1422. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribaudo, G.; Memo, M.; Gianoncelli, A. Multi-target Natural and Nature-Inspired Compounds against Neurodegeneration: A Focus on Dual Cholinesterase and Phosphodiesterase Inhibitors. Appl. Sci. 2021, 11, 5044. https://doi.org/10.3390/app11115044
Ribaudo G, Memo M, Gianoncelli A. Multi-target Natural and Nature-Inspired Compounds against Neurodegeneration: A Focus on Dual Cholinesterase and Phosphodiesterase Inhibitors. Applied Sciences. 2021; 11(11):5044. https://doi.org/10.3390/app11115044
Chicago/Turabian StyleRibaudo, Giovanni, Maurizio Memo, and Alessandra Gianoncelli. 2021. "Multi-target Natural and Nature-Inspired Compounds against Neurodegeneration: A Focus on Dual Cholinesterase and Phosphodiesterase Inhibitors" Applied Sciences 11, no. 11: 5044. https://doi.org/10.3390/app11115044
APA StyleRibaudo, G., Memo, M., & Gianoncelli, A. (2021). Multi-target Natural and Nature-Inspired Compounds against Neurodegeneration: A Focus on Dual Cholinesterase and Phosphodiesterase Inhibitors. Applied Sciences, 11(11), 5044. https://doi.org/10.3390/app11115044







