Low-Intensity Whole-Body Vibration: A Useful Adjuvant in Managing Obesity? A Pilot Study
Abstract
:1. Introduction
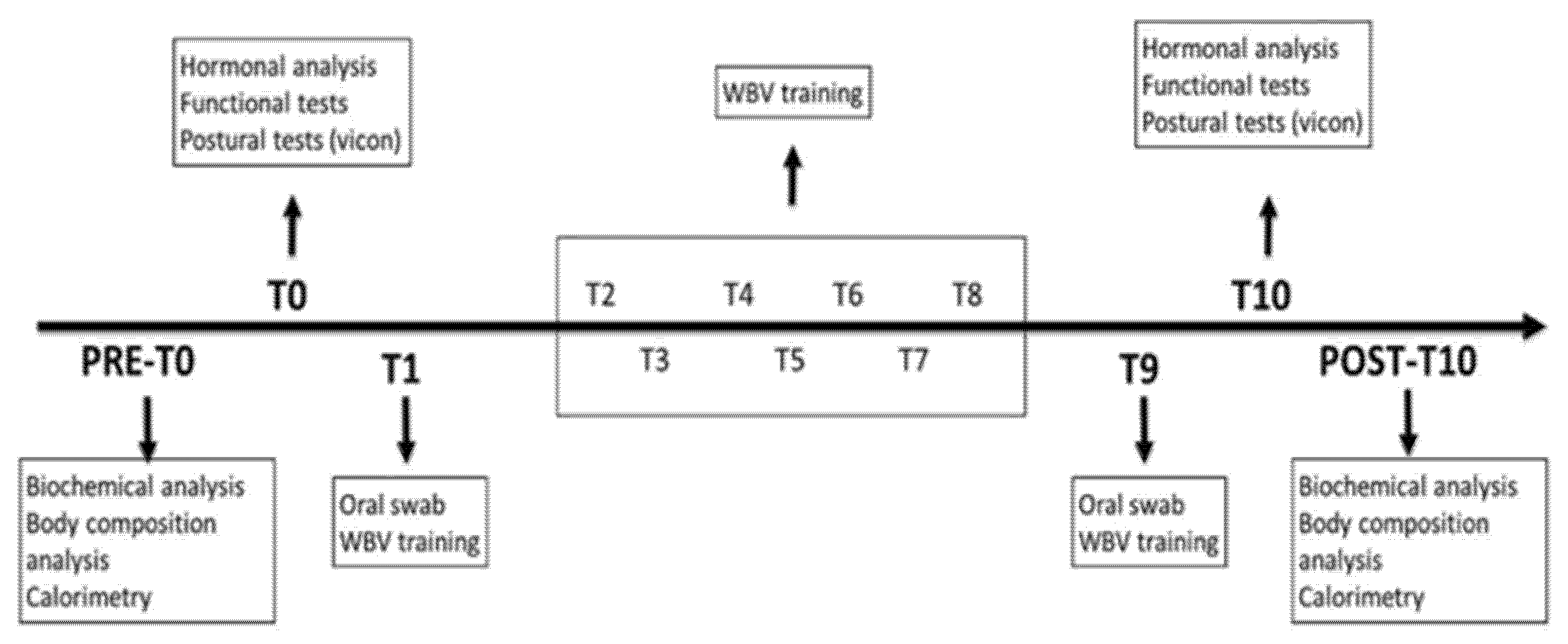
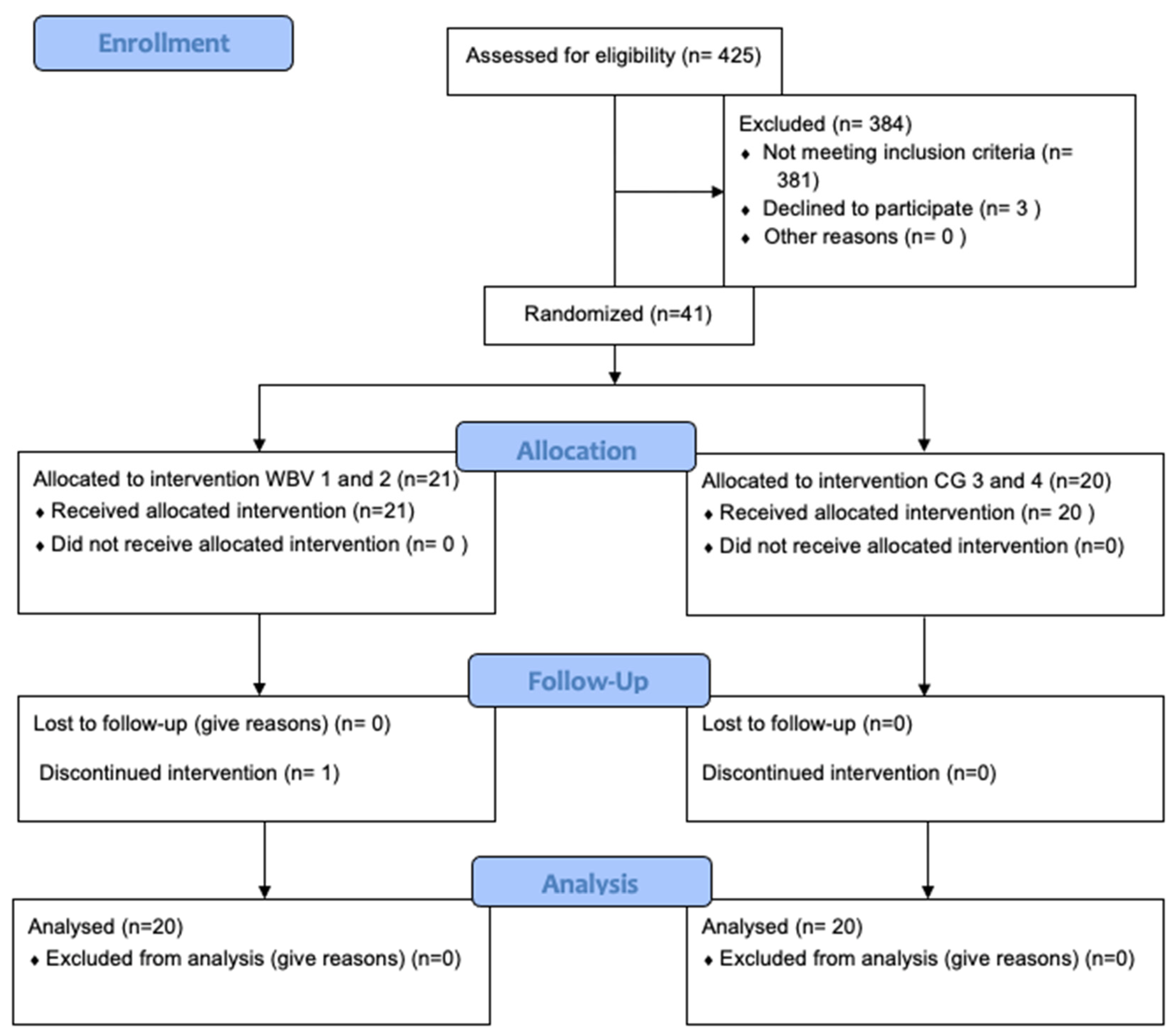
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric Measurement
2.3. Metabolic Measurement
2.4. Laboratory Analyses
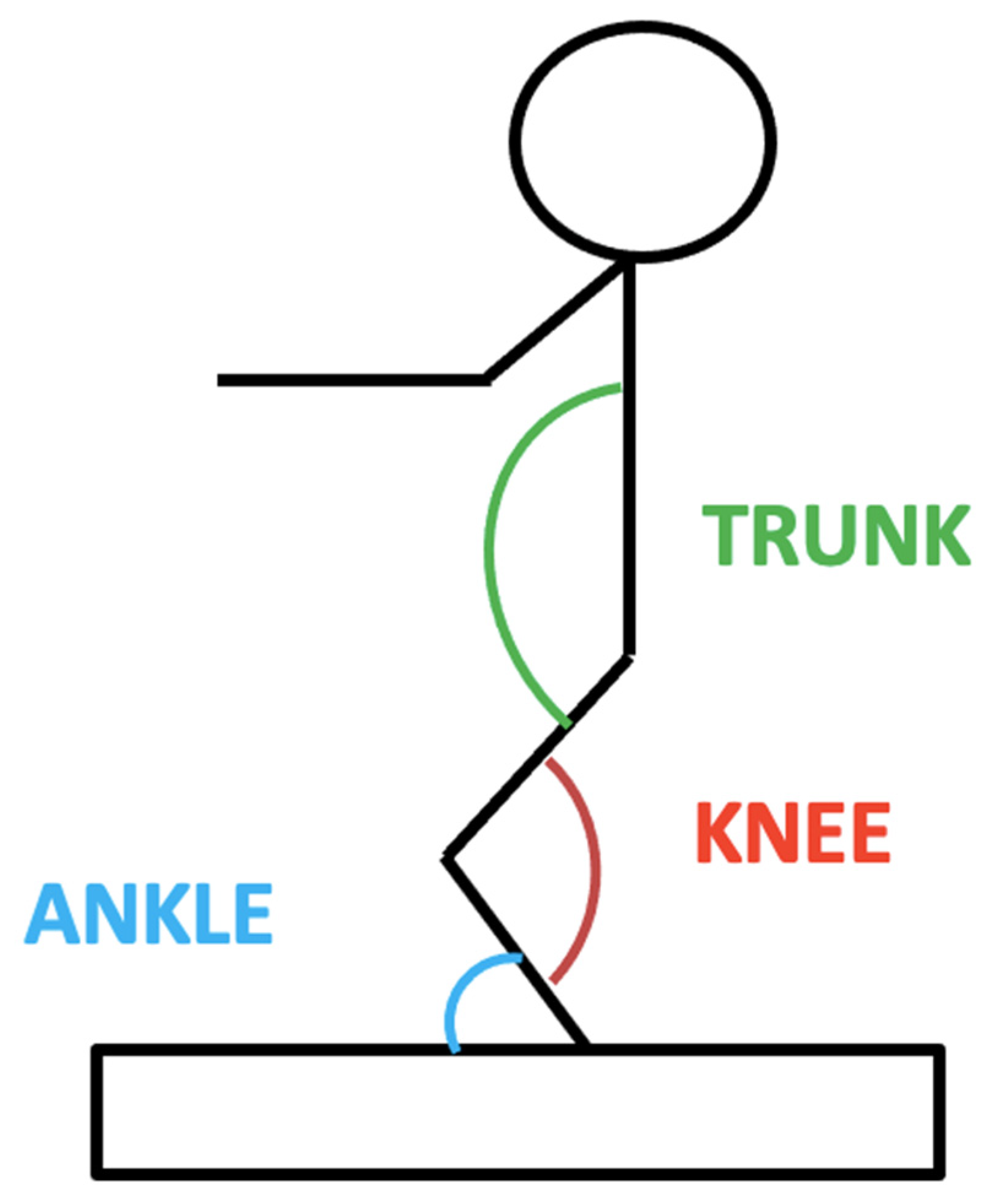
2.5. Muscle Strength and Function
2.6. Metabolic Analyses and Diet
2.7. Fitness Training
2.8. WBV
2.9. Statistical Analysis
3. Results
3.1. Metabolic Syndrome and Body Composition
3.2. Muscle Strength and Functional Components
3.3. Hormonal Components
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zdziarski, L.A.; Wasser, J.G.; Vincent, H.K. Chronic pain management in the obese patient: A focused review of key challenges and potential exercise solutions. J. Pain Res. 2015, 8, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.M.; Coutinho, S.R.; Silva, M.N.; Mata, J.; Vieira, P.N.; Minderico, C.S.; Melanson, K.J.; Baptista, F.; Sardinha, L.B.; Teixeira, P.J. The effect of physical activity on weight loss is mediated by eating self-regulation. Patient Educ. Couns. 2010, 79, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Ross, R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1999, 23, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Guérin, E.; Fortier, M.S. Situational motivation and perceived intensity: Their interaction in predicting changes in positive affect from physical activity. J. Obes. 2012, 2012, 269320. [Google Scholar] [CrossRef] [Green Version]
- Roelants, M.; Delecluse, C.; Verschueren, S.M. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J. Am. Geriatr. Soc. 2004, 52, 901–908. [Google Scholar] [CrossRef]
- Cardinale, M.; Bosco, C. The use of vibration as an exercise intervention. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef]
- Rittweger, J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef] [Green Version]
- Delecluse, C.; Roelants, M.; Verschueren, S. Strength increase after whole-body vibration compared with resistance training. Med. Sci. Sport Exerc. 2003, 1033–1041. [Google Scholar] [CrossRef]
- Ghazi, M.; Rippetoe, J.; Chandrashekhar, R.; Wang, H. Focal vibration therapy: Vibration parameters of effective wearable devices. Appl. Sci. 2021, 11, 2969. [Google Scholar] [CrossRef]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox control of skeletal muscle regeneration. Antioxidants Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef]
- Beck, B.R.; Norling, T.L. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am. J. Phys. Med. Rehabil. 2010, 89, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- Wuestefeld, A.; Fuermaier, A.B.M.; Bernardo-Filho, M.; da Cunha de Sá-Caputo, D.; Rittweger, J.; Schoenau, E.; Stark, C.; Marin, P.J.; Seixas, A.; Judex, S.; et al. Towards reporting guidelines of research using whole-body vibration as training or treatment regimen in human subjects-A Delphi consensus study. PLoS ONE 2020, 15, e0235905. [Google Scholar] [CrossRef]
- Rauch, F.; Sievanen, H.; Boonen, S.; Cardinale, M.; Degens, H.; Felsenberg, D.; Roth, J.; Schoenau, E.; Verschueren, S.; Rittweger, J. Reporting whole-body vibration intervention studies: Recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J. Musculoskelet. Neuronal Interact. 2010, 10, 193–198. [Google Scholar]
- Cardinale, M.; Pope, M.H. The effects of whole body vibration on humans: Dangerous or advantageous? Acta Physiol. Hung. 2003, 90, 195–206. [Google Scholar] [CrossRef]
- Garcia-Mendez, Y.; Pearlman, J.L.; Boninger, M.L.; Cooper, R.A. Health risks of vibration exposure to wheelchair users in the community. J. Spinal Cord Med. 2013, 36, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Muir, J.; Kiel, D.P.; Rubin, C.T. Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. J. Sci. Med. Sport 2013, 16, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Stoyneva, Z.; Lyapina, M.; Tzvetkov, D.; Vodenicharov, E. Current pathophysiological views on vibration-induced Raynaud’s phenomenon. Cardiovasc. Res. 2002, 57, 615–624. [Google Scholar] [CrossRef]
- Zago, M.; Capodaglio, P.; Ferrario, C.; Tarabini, M.; Galli, M. Whole-body vibration training in obese subjects: A systematic review. PLoS ONE 2018, 13, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Prisby, R.D.; Lafage-Proust, M.-H.; Malaval, L.; Belli, A.; Vico, L. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: What we know and what we need to know. Ageing Res. Rev. 2008, 7, 319–329. [Google Scholar] [CrossRef]
- Sañudo, B.; Alfonso-Rosa, R.; Del Pozo-Cruz, B.; Del Pozo-Cruz, J.; Galiano, D.; Figueroa, A. Whole body vibration training improves leg blood flow and adiposity in patients with type 2 diabetes mellitus. Eur. J. Appl. Physiol. 2013, 113, 2245–2252. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, A.; Kalfon, R.; Wong, A. Whole-body vibration training decreases ankle systolic blood pressure and leg arterial stiffness in obese postmenopausal women with high blood pressure. Menopause 2015, 22, 423–427. [Google Scholar] [CrossRef]
- Maddalozzo, G.F.; Iwaniec, U.T.; Turner, R.T.; Rosen, C.J.; Widrick, J.J. Whole-body vibration slows the acquisition of fat in mature female rats. Int. J. Obes. 2008, 32, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, C.; Iacovelli, M.; Tsarpela, O.; Cardinale, M.; Bonifazi, M.; Tihanyi, J.; Viru, M.; De Lorenzo, A.; Viru, A. Hormonal responses to whole-body vibration in men. Eur. J. Appl. Physiol. 2000, 81, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Skraparlis, A.; Kabasakalis, A.; Mantzoros, C.S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism 2014, 63, 918–921. [Google Scholar] [CrossRef]
- Vissers, D.; Verrijken, A.; Mertens, I.; Van Gils, C.; Van De Sompel, A.; Truijen, S.; Van Gaal, L. Effect of long-term whole body vibration training on visceral adipose tissue: A preliminary report. Obes. Facts 2010, 3, 93–100. [Google Scholar] [CrossRef]
- Milanese, C.; Piscitelli, F.; Zenti, M.G.; Moghetti, P.; Sandri, M.; Zancanaro, C. Ten-week whole-body vibration training improves body composition and muscle strength in obese women. Int. J. Med. Sci. 2013, 10, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Kokkoris, P.; Pi-Sunyer, F.X. Obesity and endocrine disease. Endocrinol. Metab. Clin. N. Am. 2003, 32, 895–914. [Google Scholar] [CrossRef]
- Capodaglio, P.; De Souza, S.A.; Parisio, C.; Precilios, H.; Vismara, L.; Cimolin, V.; Brunani, A. Reference values for the 6-Min Walking Test in obese subjects. Disabil. Rehabil. 2013, 35, 1199–1203. [Google Scholar] [CrossRef]
- Kawanabe, K.; Kawashima, A.; Sashimoto, I.; Takeda, T.; Sato, Y.; Iwamoto, J. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J. Med. 2007, 56, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, D.E.; Lindstrom, J.; Lakka, T.A.; Eriksson, J.G.; Niskanen, L.; Wikstrom, K.; Aunola, S.; Keina¨nen-Kiukaanniemi, S.; Laakso, M.; Valle, T.T.; et al. Physical activity in the prevention of type 2 diabetes the Finnish diabetes prevention study David. Br. J. Nutr. 2000, 83. [Google Scholar] [CrossRef] [Green Version]
- Laaksonen, D.E.; Lakka, H.M.; Salonen, J.T.; Niskanen, L.K.; Rauramaa, R.; Lakka, T.A. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002, 25, 1612–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1a dependent myokine that derives browning of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Mai, S.; Grugni, G.; Mele, C.; Vietti, R.; Vigna, L.; Sartorio, A.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Irisin levels in genetic and essential obesity: Clues for a potential dual role. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Aydin, S.; Aydin, S.; Kuloglu, T.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Cicek, D. Alterations of irisin concentrations in saliva and serum of obese and normal-weight subjects, before and after 45 min of a Turkish bath or running. Peptides 2013, 50, 13–18. [Google Scholar] [CrossRef]
- Gröschl, M.; Topf, H.G.; Rauh, M.; Kurzai, M.; Rascher, W.; Köhler, H. Postprandial response of salivary ghrelin and leptin to carbohydrate uptake. Gut 2006, 55, 433–434. [Google Scholar] [CrossRef] [Green Version]
| Group | N (M/F) | Age [years] | Height [cm] | Body Mass [kg] | BMI [kg/m2] |
|---|---|---|---|---|---|
| 1 | 11 (6/5) | 42.7 (13.0) | 168.5 (10.5) | 124.5 (19.3) | 44.2 (7.1) |
| 2 | 10 (5/5) | 38.5 (12.3) | 165.7 (9.1) | 118.3 (16.1) | 43.2 (6.4) |
| 3 | 10 (5/5) | 42.7 (9.6) | 168.2 (7.7) | 132.5 (18.3) | 46.8 (5.2) |
| 4 | 10 (5/5) | 48.0 (9.4) | 167.2 (10.6) | 123.6 (27.0) | 43.9 (7.2) |
| Variable | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Mass [kg] | Post | 118.45 (18.29) | 111.28 (14.26) | 125.22 (16.29) | 117.92 (23.89) |
| Pre | 124.47 (19.28) | 118.27 (16.05) | 132.5 (18.32) | 123.61 (26.96) | |
| BMI [kg/m2] | Post | 41.82 (5.28) | 53.90 (42.10) | 44.28 (4.96) | 41.95 (6.48) |
| Pre | 44.23 (7.05) | 43.21 (6.40) | 46.80 (5.21) | 43.89 (7.18) | |
| Abdomen circumference [cm] | Post | 115.30 (11.37) | 124.50 (8.82) | 132.40 (10.18) | 130.8 (17.92) |
| Pre | 120.60 (11.63) | 126.70 (9.51) | 135.20 (10.40) | 134.05 (17.89) | |
| PAS [mm/hg] | Post | 123.64 (10.98) | 120.50 (6.85) | 130.00 (12.25) | 119.44 (12.86) |
| Pre | 137.73 (19.15) | 137.50 (19.61) | 144.00 (16.47) | 142.00 (15.49) | |
| PAD [mm/hg] | Post | 78.64 (6.74) | 76.00 (5.16) | 85.00 (5.00) | 75.56 (8.82) |
| Pre | 80.00 (8.94) | 84.50 (10.66) | 92.50 (17.20) | 87.00 (8.23) | |
| FC [bpm] | Post | 72.55 (8.38) | 76.70 (9.37) | 76.11 (7.94) | 77.78 (9.01) |
| Pre | 85.82 (14.07) | 83.20 (9.91) | 88.60 (15.99) | 82.90 (6.54) | |
| FFM [kg] | Post | 66.33 (16.05) | 62.11 (13.01) | 68.42 (14.37) | 66.24 (14.29) |
| Pre | 67.98 (17.73) | 64.70 (12.65) | 70.96 (16.46) | 66.48 (16.75) | |
| FFM [%] | Post | 55.45 (7.08) | 55.55 (8.80) | 54.06 (7.88) | 56.70 (5.45) |
| Pre | 54.34 (7.9) | 54.91 (7.82) | 53.50 (7.57) | 54.03 (6.95) | |
| CHO [mg/dL] | Post | 147.00 (47.9) | 153.30 (28.11) | 171.10 (26.61) | 146.40 (40.20) |
| Pre | 200.60 (42.6) | 185.50 (25.89) | 180.90 (34.20) | 201.30 (27.29) | |
| HDL [mg/dL] | Post | 39.09 (10.25) | 34.60 (8.26) | 37.90 (8.76) | 38.60 (5.46) |
| Pre | 42.36 (9.98) | 37.50 (7.35) | 40.60 (9.06) | 44.80 (7.57) | |
| LDL [mg/dL] | Post | 115.2 (54.1) | 100.70 (22.02) | 120.60 (23.57) | 91.40 (38.4) |
| Pre | 143.2 (41.4) | 131.60 (20.93) | 126.90 (33.70) | 140.30 (20.72) | |
| HbA1c [%] | Post | 5.34 (0.34) | 5.41 (0.38) | 5.43 (0.28) | 5.62 (0.46) |
| Pre | 5.51 (0.29) | 5.52 (0.34) | 5.57 (0.44) | 5.78 (0.36) | |
| Insulin [mU/L] | Post | 14.32 (6.47) | 19.03 (14.82) | 21.11 (12.52) | 15.46 (6.29) |
| Pre | 18.69 (7.76) | 26.82 (17.44) | 17.58 (7.68) | 21.94 (6.86) | |
| Glucose [mg/dL] | Post | 89.27 (8.46) | 86.00 (6.55) | 90.10 (4.58) | 90.40 (8.22) |
| pre | 92.45 (5.56) | 102.40 (25.05) | 90.20 (5.12) | 97.40 (8.46) |
| Variable | Factor | F | P | Partial η2 |
|---|---|---|---|---|
| Mass [kg] | Time | 2.26 | 0.14 | 0.03 |
| Group | 1.74 | 0.17 | 0.07 | |
| Group × Time | 0.01 | 0.99 | 0.0003 | |
| BMI [kg/m2] | Time | 0.08 | 0.78 | 0.001 |
| Group | 0.58 | 0.63 | 0.02 | |
| Group × Time | 0.86 | 0.47 | 0.034 | |
| Abdomen circumference [cm] | Time | 1.43 | 0.24 | 0.02 |
| Group | 6.59 | 0.001 | 0.22 | |
| Group × Time | 0.06 | 0.98 | 0.002 | |
| PAS [mm/hg] | Time | 25.8 | 0.29 × 10−5 | 0.26 |
| Group | 1.09 | 0.36 | 0.04 | |
| Group × Time | 0.35 | 0.79 | 0.01 | |
| PAD [mm/hg] | Time | 11.24 | 0.001 | 0.14 |
| Group | 3.95 | 0.01 | 0.04 | |
| Group × Time | 1.02 | 0.39 | 0.04 | |
| FC [bpm] | Time | 15.3 | 0.21 × 10−3 | 0.17 |
| Group | 0.32 | 0.81 | 0.01 | |
| Group × Time | 0.75 | 0.53 | 0.03 | |
| FFM [kg] | Time | 0.27 | 0.61 | 0.004 |
| Group | 0.57 | 0.64 | 0.02 | |
| Group × Time | 0.03 | 0.99 | 0.001 | |
| FFM [%] | Time | 0.57 | 0.45 | 0.008 |
| Group | 0.18 | 0.91 | 0.007 | |
| Group × Time | 0.09 | 0.97 | 0.003 | |
| CHO [mg/dL] | Time | 21.6 | 0.17 × 10−4 | 0.24 |
| Group | 0.15 | 0.93 | 0.007 | |
| Group × Time | 1.89 | 0.14 | 0.08 | |
| HDL [mg/dL] | Time | 4.01 | 0.05 | 0.05 |
| Group | 1.7 | 0.18 | 0.06 | |
| Group × Time | 0.19 | 0.91 | 0.007 | |
| LDL [mg/dL] | Time | 14.1 | 0.34 × 10−3 | 0.16 |
| Group | 0.74 | 0.53 | 0.03 | |
| Group × Time | 1.29 | 0.28 | 0.05 | |
| HB1Ac [%] | Time | 3.22 | 0.08 | 0.04 |
| Group | 2.29 | 0.09 | 0.09 | |
| Group × Time | 0.03 | 0.99 | 0.001 | |
| Insulin [mU/L] | Time | 2.56 | 0.11 | 0.03 |
| Group | 1.29 | 0.28 | 0.05 | |
| Group × Time | 1.13 | 0.34 | 0.04 | |
| Glucose [mg/dL] | Time | 7.71 | 0.007 | 0.09 |
| Group | 0.74 | 0.53 | 0.03 | |
| Group × Time | 2.14 | 0.1 | 0.08 |
| Variable | Time | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| GH [μg/L] | Post | 0.29 (0.28) | 0.36 (0.32) | 0.40 (0.32) | 0.58 (0.69) |
| Pre | 0.55 (0.95) | 1.02 (2.16) | 0.57 (0.74) | 0.33 (0.46) | |
| Testosterone [nmol/L] | Post | 7.14 (6.91) | 10.49 (12.04) | 7.06 (7.11) | 6.67 (6.83) |
| Pre | 6.6 (7.28) | 8.39 (10.18) | 8.24 (8.40) | 5.81 (5.75) |
| Variable | Factor | F | P | Partial η2 |
|---|---|---|---|---|
| GH [μg/L] | Time | 0.23 | 0.63 | 0.003 |
| Group | 0.39 | 0.76 | 0.02 | |
| Group × Time | 0.87 | 0.46 | 0.46 | |
| Testosterone [nmol/L] | Time | 0.1 | 0.75 | 0.001 |
| Group | 0.57 | 0.64 | 0.02 | |
| Group × Time | 0.13 | 0.94 | 0.005 |
| T1 | T9 | |||||
|---|---|---|---|---|---|---|
| Salivary Irisin (ng/mL) | Salivary Irisin (ng/mL) | |||||
| PRE (SD) | POST (SD) | ∆% PRE (SD) | PRE (SD) | POST (SD) | ∆% POST (SD) | |
| Group 2 | 4.26 (3.31) | 10.80 (5.70) | 839.14 (1984.41) | 2.99 (2.10) | 8.79 (3.80) | 478.01 (823.44) |
| Group 4 | 25.15 (0.68) | 43.09 (40.67) | 578.72 (2630.94) | 22.06 (29.46) | 71.48 (246.77) | 509.58 (351.21) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbi, M.; Ferrario, C.; Tarabini, M.; Annino, G.; Cau, N.; Zago, M.; Marzullo, P.; Mai, S.; Galli, M.; Capodaglio, P. Low-Intensity Whole-Body Vibration: A Useful Adjuvant in Managing Obesity? A Pilot Study. Appl. Sci. 2021, 11, 5101. https://doi.org/10.3390/app11115101
Gobbi M, Ferrario C, Tarabini M, Annino G, Cau N, Zago M, Marzullo P, Mai S, Galli M, Capodaglio P. Low-Intensity Whole-Body Vibration: A Useful Adjuvant in Managing Obesity? A Pilot Study. Applied Sciences. 2021; 11(11):5101. https://doi.org/10.3390/app11115101
Chicago/Turabian StyleGobbi, Michele, Cristina Ferrario, Marco Tarabini, Giuseppe Annino, Nicola Cau, Matteo Zago, Paolo Marzullo, Stefania Mai, Manuela Galli, and Paolo Capodaglio. 2021. "Low-Intensity Whole-Body Vibration: A Useful Adjuvant in Managing Obesity? A Pilot Study" Applied Sciences 11, no. 11: 5101. https://doi.org/10.3390/app11115101
APA StyleGobbi, M., Ferrario, C., Tarabini, M., Annino, G., Cau, N., Zago, M., Marzullo, P., Mai, S., Galli, M., & Capodaglio, P. (2021). Low-Intensity Whole-Body Vibration: A Useful Adjuvant in Managing Obesity? A Pilot Study. Applied Sciences, 11(11), 5101. https://doi.org/10.3390/app11115101









