Application of Visible/Near Infrared Spectrometers to Quickly Detect the Nitrogen, Phosphorus, and Potassium Content of Chemical Fertilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
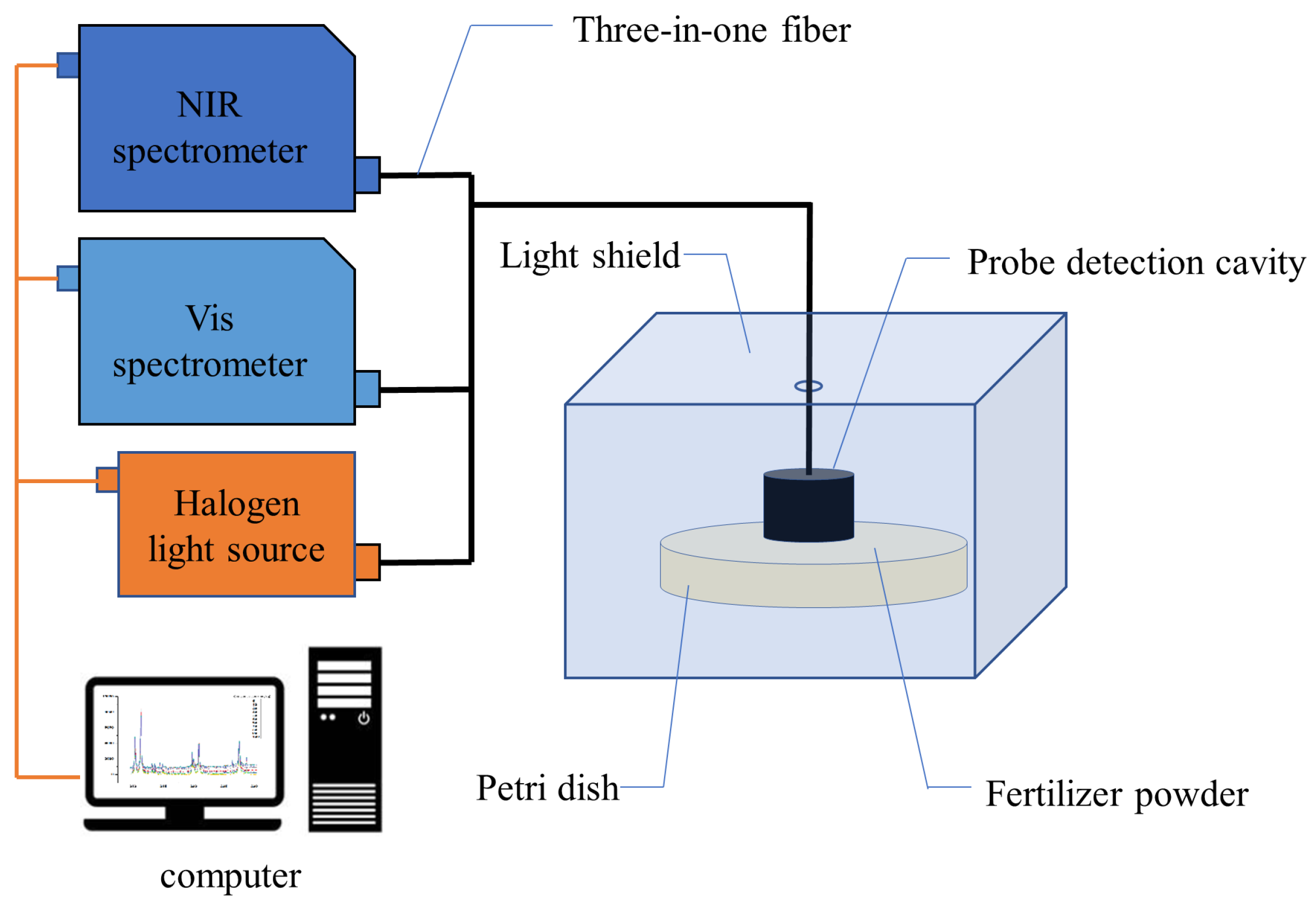
2.2. Vis/NIR Spectroscopy Instrumentation
2.3. Data Analysis
2.3.1. Data Preprocessing
2.3.2. Statistical Analysis
2.3.3. Performance Evaluation
3. Results
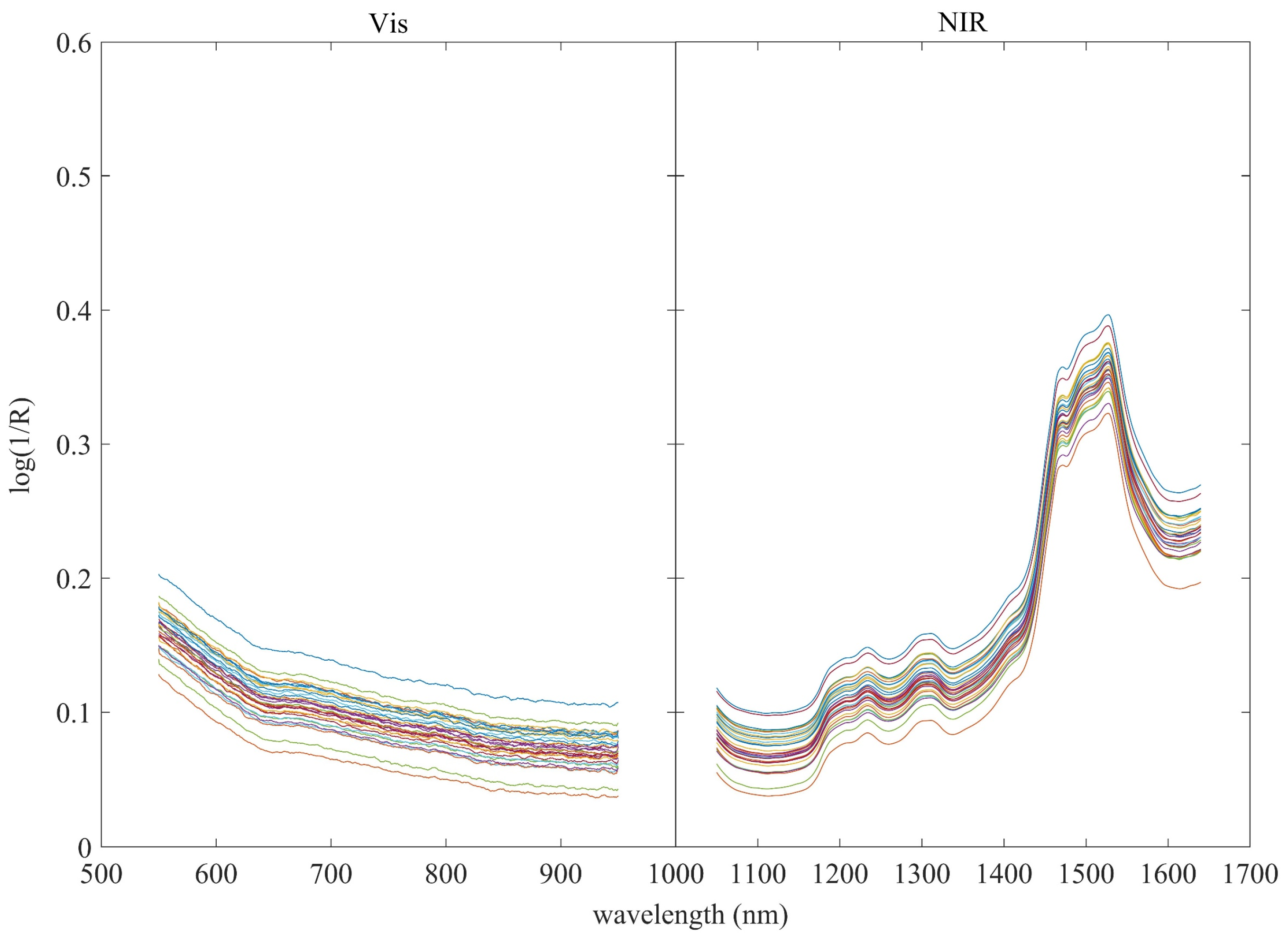
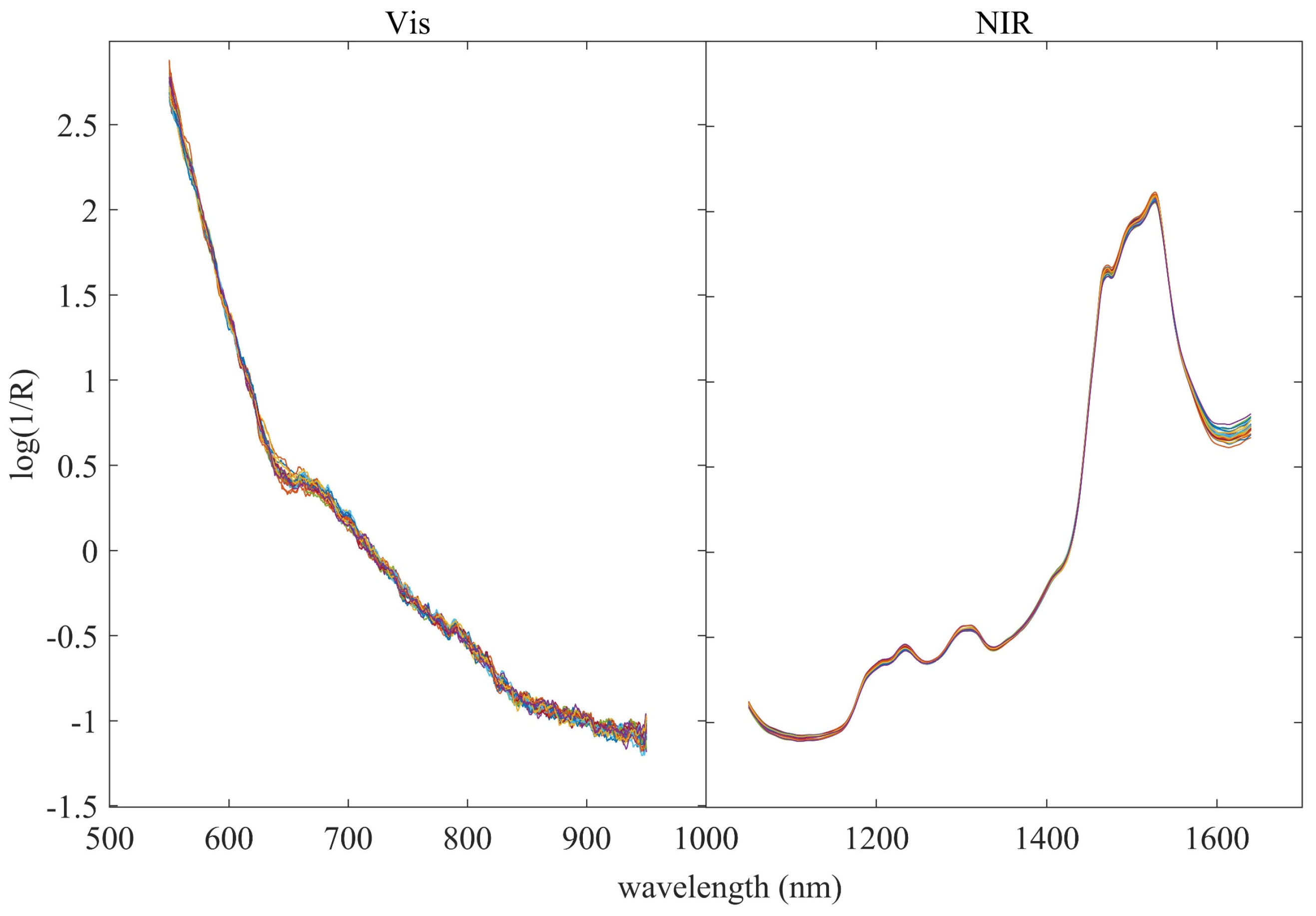
3.1. Spectral Analysis
3.2. Selection of Characteristic Wavelength
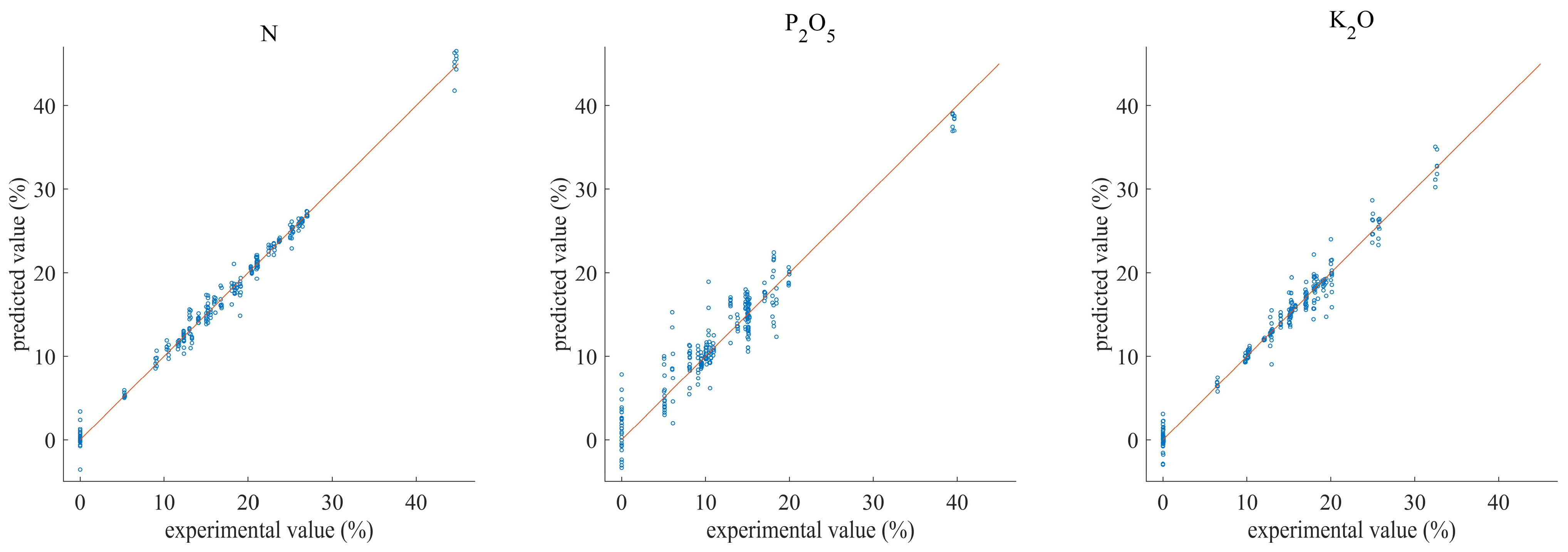
3.3. Multivariate Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–190. [Google Scholar]
- Prajapati, K.; Modi, H. The importance of potassium in plant growth—A review. Indian J. Plant Sci. 2012, 1, 177–186. [Google Scholar]
- Fan, B.; Chu, D.; Shang, Z. Improvement on determination of water-soluble potassium content in fertilizers by potassium tetraphenylborate gravimetric method and development of related international standard. Sci. Technol. Rev. 2014, 32, 32–38. [Google Scholar]
- Xu, B. Rapid determination of phosphorus pentoxide in fertilizer by quinoline phosphomolybdate volumetric method. Phosphate Compd. Fertil. 2012, 27, 87. [Google Scholar]
- Sun, H.; Li, T.; Tao, L.; Gong, Y. Study on the determination of potassium ion concentration in compound fertilizer by temperature titration. Soil Fertil. Sci. China 2012, 4, 95–98. [Google Scholar]
- Lin, Z.; Wang, R.; Wang, Y.; Wang, L.; Lu, C.; Liu, Y.; Zhang, Z.; Zhu, L. Accurate and rapid detection of soil and fertilizer properties based on visible/near-infrared spectroscopy. Appl. Opt. 2018, 57, D69–D73. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Lu, C.; Wang, J.; Huang, W.; Jian, Q.; Wang, Y.; Lin, L.; Song, L. Quantitative analysis of total nitrogen content in monoammonium phosphate fertilizer using visible-near infrared spectroscopy and least squares support vector machine. J. Appl. Spectrosc. 2019, 86, 465–469. [Google Scholar] [CrossRef]
- Wang, C.; Huang, C.; Qian, J.; Xiao, J.; Li, H.; Wen, Y.; He, X.; Ran, W.; Shen, Q.; Yu, G. Rapid and accurate evaluation of the quality of commercial organic fertilizers using near infrared spectroscopy. PLoS ONE 2014, 9, e88279. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-L. Errors induced by spectral measurement positions and instrument noise in soil organic carbon prediction using vis-NIR on intact soil. Geoderma 2021, 382, 114731. [Google Scholar] [CrossRef]
- Bian, X.; Wang, K.; Tan, E.; Diwu, P.; Zhang, F.; Guo, Y. A selective ensemble preprocessing strategy for near-infrared spectral quantitative analysis of complex samples. Chemom. Intell. Lab. Syst. 2020, 197, 103916. [Google Scholar] [CrossRef]
- Alrezj, O.A.; Patchava, K.C.; Benaissa, M.; Alshebeili, S. Coupling scatter correction with bandpass filtering for preprocessing in the quantitative analysis of glucose from near infrared spectra. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 1800–1803. [Google Scholar]
- Huang, J.; Shi, T.; Gong, B.; Li, X.; Liao, G.; Tang, Z. Fitting an optical fiber background with a weighted Savitzky–Golay smoothing filter for Raman spectroscopy. Appl. Spectrosc. 2018, 72, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, Q.; Tang, G.; Feng, Q.; Lin, L. The combined optimization of Savitzky-Golay smoothing and multiplicative scatter correction for FT-NIR PLS models. Int. Sch. Res. Not. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Roy, I.G. On computing first and second order derivative spectra. J. Comput. Phys. 2015, 295, 307–321. [Google Scholar] [CrossRef]
- Lin, Z. Research on the Rapid Detection Models of Fertilizer and Soil Parameters Based on the Visible/Near Infrared Spectral; University of Science and Technology of China: Hefei, China, 2016. [Google Scholar]
- Huang, G.; Huang, G.-B.; Song, S.; You, K. Trends in extreme learning machines: A review. Neural Netw. 2015, 61, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-B.; Zhu, Q.-Y.; Siew, C.-K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
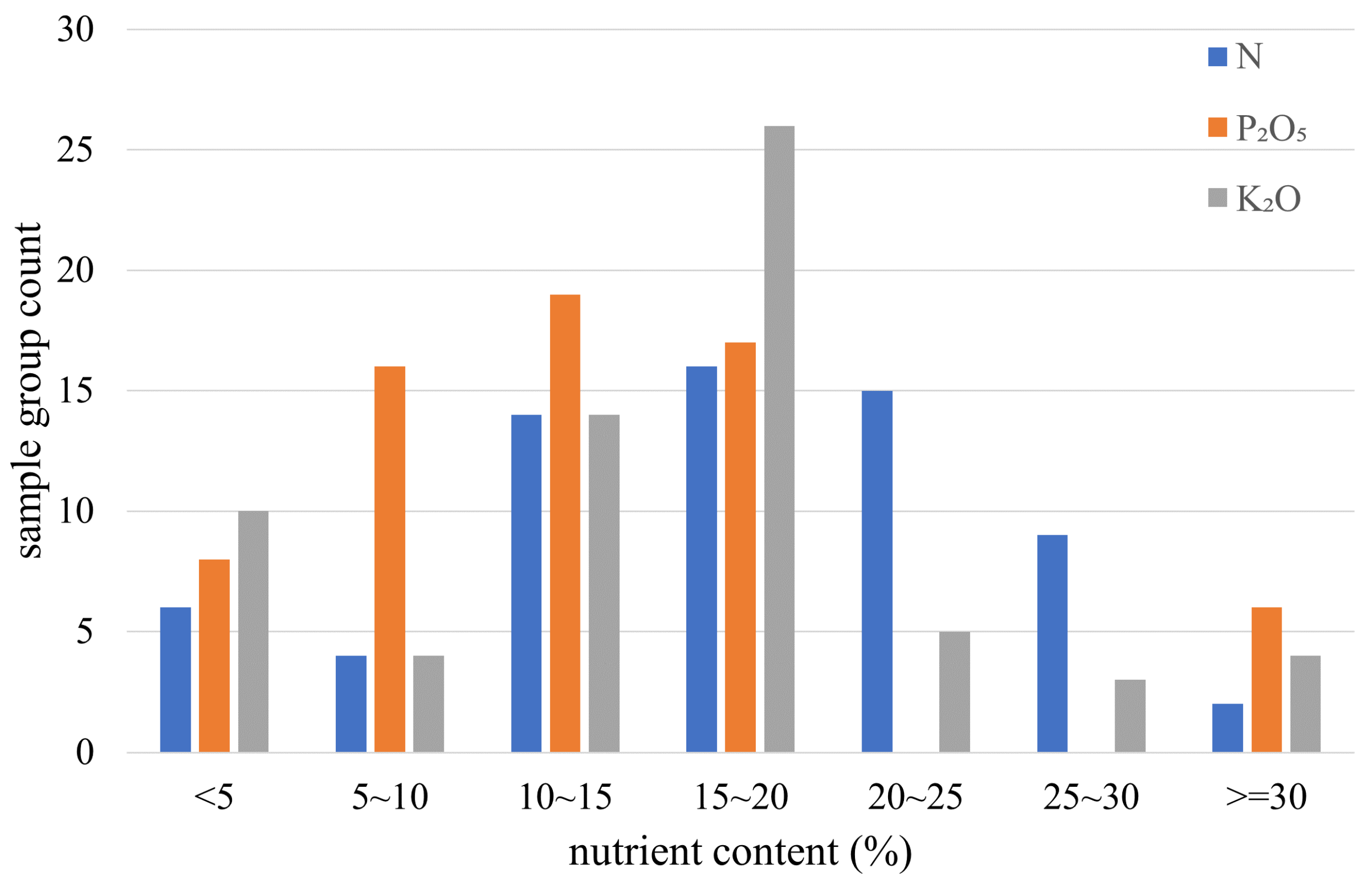
| Detection Indicator | N (%) | P2O5 (%) | K2O (%) |
|---|---|---|---|
| Max value | 44.77 | 60.22 | 52.12 |
| Min value | 0 | 0 | 0 |
| Average value | 17.06 | 14.24 | 15.09 |
| Standard deviation | 79.38 | 170.75 | 102.09 |
| Nutrient | 550~950 nm | 1050~1640 nm | Full Spectrum |
|---|---|---|---|
| N | 44 | 117 | 161 |
| P2O5 | 32 | 197 | 229 |
| K2O | 59 | 102 | 161 |
| Nutrient | Spectral Band | Algorithm | Calibration Set | Prediction Set | ||||
|---|---|---|---|---|---|---|---|---|
| RMSECV % | RMSEP % | RPD | ||||||
| N | 550~950 nm | PLS | 0.653 | 5.211 | 0.596 | 5.620 | 1.29 | |
| ELM | 0.854 | 3.382 | 0.784 | 4.111 | 2.02 | |||
| 1050~1640 nm | PLS | 0.891 | 2.920 | 0.881 | 3.056 | 2.74 | ||
| ELM | 0.991 | 0.823 | 0.984 | 1.116 | 7.91 | |||
| full spectrum | PLS | 0.933 | 2.284 | 0.908 | 2.676 | 3.159 | ||
| ELM | 0.996 | 0.537 | 0.989 | 0.910 | 9.71 | |||
| characteristic wavelength | PLS | 0.931 | 2.317 | 0.906 | 2.709 | 3.10 | ||
| ELM | 0.995 | 0.659 | 0.986 | 1.033 | 8.59 | |||
| P2O5 | 550~950 nm | PLS | 0.333 | 10.587 | 0.330 | 10.618 | 0.69 | |
| ELM | 0.921 | 3.649 | 0.841 | 5.179 | 2.38 | |||
| 1050~1640 nm | PLS | 0.779 | 6.100 | 0.745 | 6.553 | 1.76 | ||
| ELM | 0.980 | 1.850 | 0.897 | 4.170 | 3.18 | |||
| full spectrum | PLS | 0.899 | 4.123 | 0.853 | 4.636 | 2.55 | ||
| ELM | 0.968 | 2.324 | 0.945 | 2.933 | 4.45 | |||
| characteristic wavelength | PLS | 0.905 | 3.987 | 0.844 | 5.124 | 2.46 | ||
| ELM | 0.974 | 1.439 | 0.963 | 1.724 | 5.09 | |||
| K2O | 550~950 nm | PLS | 0.595 | 6.383 | 0.522 | 6.932 | 1.16 | |
| ELM | 0.880 | 3.468 | 0.730 | 5.214 | 2.00 | |||
| 1050~1640 nm | PLS | 0.825 | 4.189 | 0.804 | 4.434 | 2.01 | ||
| ELM | 0.996 | 0.667 | 0.975 | 1.565 | 6.70 | |||
| full spectrum | PLS | 0.955 | 2.117 | 0.906 | 3.070 | 3.23 | ||
| ELM | 0.990 | 0.999 | 0.981 | 1.393 | 7.29 | |||
| characteristic wavelength | PLS | 0.913 | 2.959 | 0.901 | 3.153 | 3.05 | ||
| ELM | 0.994 | 0.709 | 0.980 | 1.237 | 7.17 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Qiao, W.; Chen, H.; Zhou, J.; Liu, F. Application of Visible/Near Infrared Spectrometers to Quickly Detect the Nitrogen, Phosphorus, and Potassium Content of Chemical Fertilizers. Appl. Sci. 2021, 11, 5103. https://doi.org/10.3390/app11115103
Shen J, Qiao W, Chen H, Zhou J, Liu F. Application of Visible/Near Infrared Spectrometers to Quickly Detect the Nitrogen, Phosphorus, and Potassium Content of Chemical Fertilizers. Applied Sciences. 2021; 11(11):5103. https://doi.org/10.3390/app11115103
Chicago/Turabian StyleShen, Jiangang, Weiming Qiao, Huizhe Chen, Jun Zhou, and Fei Liu. 2021. "Application of Visible/Near Infrared Spectrometers to Quickly Detect the Nitrogen, Phosphorus, and Potassium Content of Chemical Fertilizers" Applied Sciences 11, no. 11: 5103. https://doi.org/10.3390/app11115103
APA StyleShen, J., Qiao, W., Chen, H., Zhou, J., & Liu, F. (2021). Application of Visible/Near Infrared Spectrometers to Quickly Detect the Nitrogen, Phosphorus, and Potassium Content of Chemical Fertilizers. Applied Sciences, 11(11), 5103. https://doi.org/10.3390/app11115103







