Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies
Abstract
1. Introduction
2. Classic Extraction
3. Enzymatic Extraction
4. Pressurized Systems
5. Wave-Energy-Based Cell Disruption
6. Electroextraction
7. Supercritical Fluid Extraction
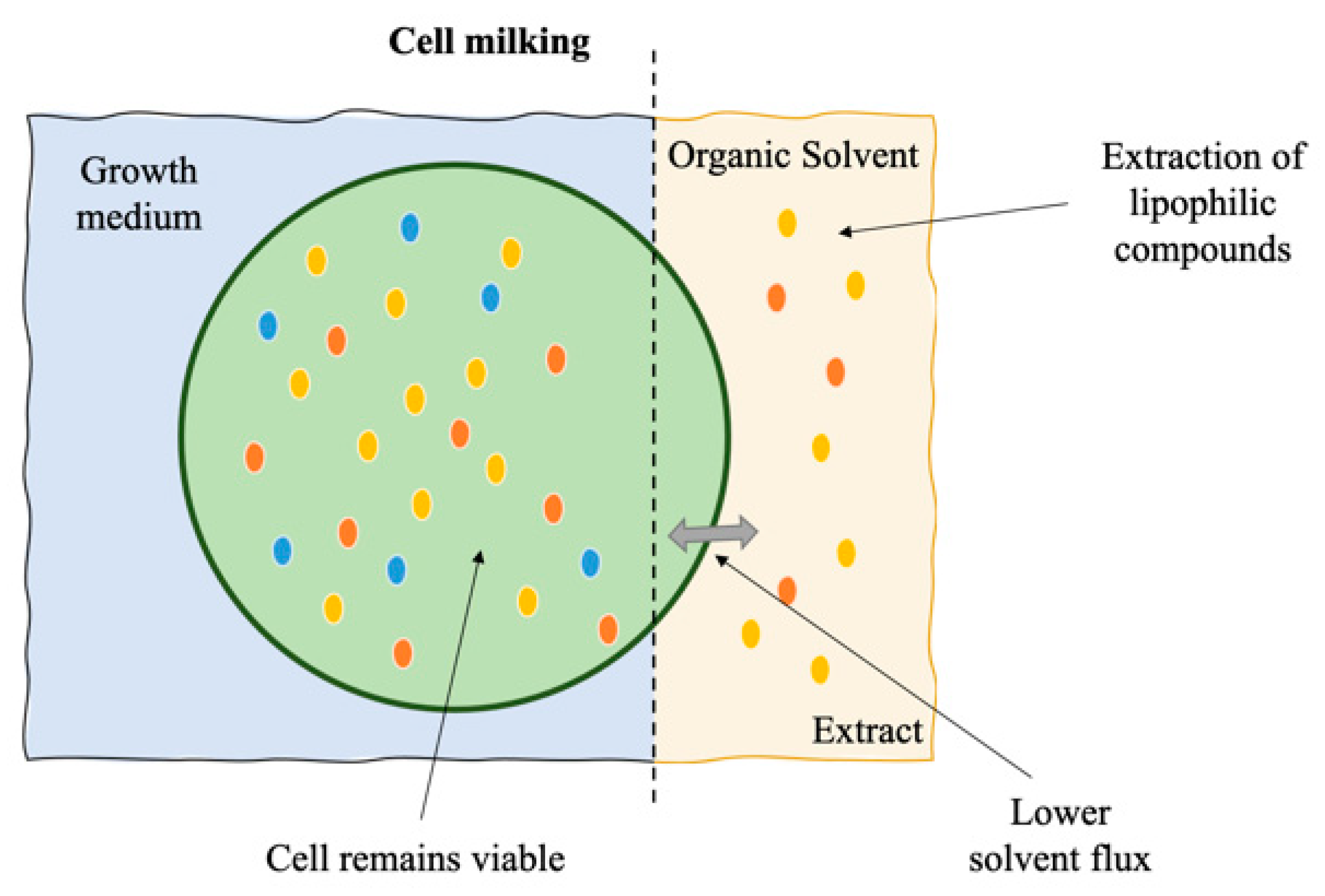
8. Cell Milking
9. Novel Extraction Methodologies
10. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; 736p. [Google Scholar] [CrossRef]
- Masojdek, J.; Koblek, M.; Torzillo, G. Photosynthesis in Microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2007; pp. 21–36. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 465–492. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Singh, D.; Saxena, R.; Rani, R.; Gupta, R.P.; Puri, S.K.; Mathur, A.S. High-value coproducts from algae—An innovational way to deal with advance algal industry. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 343–363. [Google Scholar] [CrossRef]
- Tafreshi, A.H.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol. 2009, 107, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.A.; Bahri, P.A.; Moheimani, N.R. Repetitive non-destructive milking of hydrocarbons from Botryococcus braunii. Renew. Sustain. Energy Rev. 2017, 79, 1229–1240. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Saul Garcia-Perez, J.; Chandra, R.; Castillo-Zacarias, C.; Iqbal, H.M.N.; Parra-Saldívar, R. Methods for Extraction of Valuable Products from Microalgae Biomass BT—Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 245–263. [Google Scholar] [CrossRef]
- Grima, E.M.; Fernández, F.G.A.; Medina, A.R. Downstream Processing of Cell Mass and Products. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 266–267. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Kwon, O.N.; Chung, D.; Pan, C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Vijayan, D.; Praveenkumar, R.; Han, J.-I.; Lee, K.; Park, J.-Y.; Chang, W.-S.; Lee, J.-S.; Oh, Y.-K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Zou, T.B.; Jia, Q.; Li, H.W.; Wang, C.X.; Wu, H.F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.D.; Sim, S.J.; Chang, D.K.; Sang, J.S. Selective extraction of free Astaxanthin from Haematococcus culture using a tandem organic solvent system. Biotechnol. Prog. 2007, 23, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Machado, D.; Morales-Oyervides, L.; Contreras-Esquivel, J.C.; Aguilar, C.; Méndez-Zavala, A.; Raso, J.; Montañez, J. Effect of ohmic heating processing conditions on color stability of fungal pigments. Food Sci. Technol. Int. 2017, 23, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Richmond, A.E. Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 1979, 120, 155–159. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Aarthy, A.; Kumari, S.; Turkar, P.; Subramanian, S. An insight on algal cell disruption for biodiesel production. Asian J. Pharm. Clin. Res. 2018, 11, 21–26. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Sierra, L.S.; Dixon, C.K.; Wilken, L.R. Enzymatic cell disruption of the microalgae Chlamydomonas reinhardtii for lipid and protein extraction. Algal Res. 2017, 25, 149–159. [Google Scholar] [CrossRef]
- Vernès, L.; Li, Y.; Chemat, F.; Abert-Vian, M. Biorefinery Concept as a Key for Sustainable Future to Green Chemistry—The Case of Microalgae BT—Plant Based “Green Chemistry 2.0”: Moving from Evolutionary to Revolutionary. In Plant Based “Green Chemistry 2.0.; Li, Y., Chemat, F., Eds.; Springer: Singapore, 2019; pp. 15–50. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Optimization of enzyme-assisted lipid extraction from Nannochloropsis microalgae. J. Taiwan Inst. Chem. Eng. 2016, 67, 106–114. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Vanjari, P.; Raghavarao, K.S.M.S. Synergistic method for extraction of high purity Allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Spiden, E.M.; Yap, B.H.J.; Hill, D.R.A.; Kentish, S.E.; Scales, P.J.; Martin, G.J.O. Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour. Technol. 2013, 140, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, K.; Kar, J.R.; Singhal, R.S. Extraction of lipids from Chlorella saccharophila using high-pressure homogenization followed by three phase partitioning. Appl. Biochem. Biotechnol. 2015, 176, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, T.M.M.; Verstreken, H.; Dejonghe, C.; Gheysen, L.; Foubert, I.; Grauwet, T.; Van Loey, A.M. Cell disruption of Nannochloropsis sp. improves in vitro bioaccessibility of carotenoids and ω3-LC-PUFA. J. Funct. Foods 2020, 103770. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Das, A.K. Classification of Extraction Methods. In Essentials of Botanical Extraction; Mandal, S.C., Mandal, V., Das, A.K., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 83–136. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Jaime, L.; Martín-Álvarez, P.J.; Cifuentes, A.; Ibáñez, E. Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J. Agric. Food Chem. 2006, 54, 5597–5603. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Pressurized fluid extraction of bioactive compounds from Phormidium species. J. Agric. Food Chem. 2008, 56, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Amaro, H.M.; Catarina Guedes, A.; Preto, M.A.C.; Sousa-Pinto, I.; Xavier Malcata, F. Gloeothece sp. as a nutraceutical source-an improved method of extraction of carotenoids and fatty acids. Mar. Drugs 2018, 16, 327. [Google Scholar] [CrossRef] [PubMed]
- Juin, C.; Chérouvrier, J.-R.; Thiéry, V.; Gagez, A.-L.; Bérard, J.-B.; Joguet, N.; Kaas, R.; Cadoret, J.-P.; Picot, L. Microwave-assisted extraction of phycobiliproteins from Porphyridium purpureum. Appl. Biochem. Biotechnol. 2015, 175, 1–15. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Nogara, G.P.; Menezes, C.R.; Cichoski, A.J.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Identification of chlorophyll molecules with peroxyl radical scavenger capacity in microalgae Phormidium autumnale using ultrasound-assisted extraction. Food Res. Int. 2017, 99, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Ang, W.M.R.; Chen, X.; Voigtmann, M.; Lau, R. Lipid releasing characteristics of microalgae species through continuous ultrasonication. Bioresour. Technol. 2014, 158, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Moraes, L.; Zaparoli, M.; Morais, M.G. Progress in the physicochemical treatment of microalgae biomass for value-added product recovery. Bioresour. Technol. 2020, 122727. [Google Scholar] [CrossRef]
- Sastry, S.K.; Heskitt, B.F.; Sarang, S.S.; Somavat, R.; Ayotte, K. Why ohmic heating? Advantages, applications, technology, and limitations. In Ohmic Heating in Food Processing; Ramaswamy, H.S., Marcotte, M., Sastry, S., Abdelrahim, K., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 7–14. [Google Scholar] [CrossRef]
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.A.; Vasconcelos, V.; Vicente, A.A. Electrotechnologies applied to microalgal biotechnology—Applications, techniques and future trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of pulsed electric field treatments on permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef]
- Luengo, E.; Martínez, J.M.; Bordetas, A.; Álvarez, I.; Raso, J. Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Luengo, E.; Martínez, J.M.; Coustets, M.; Álvarez, I.; Teissié, J.; Rols, M.P.; Raso, J. A comparative study on the effects of millisecond- and microsecond-pulsed electric field treatments on the permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 2015, 248, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Martínez, J.M.; Gojkovic, Z.; Ferro, L.; Maza, M.; Álvarez, I.; Raso, J.; Funk, C. Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121694. [Google Scholar] [CrossRef]
- Abrahamsson, V.; Cunico, L.P.; Andersson, N.; Nilsson, B.; Turner, C. Multicomponent inverse modeling of supercritical fluid extraction of carotenoids, chlorophyll a, ergosterol and lipids from microalgae. J. Supercrit. Fluids 2018, 139, 53–61. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Serrano, C.M.; Rodríguez, M.R.; de la Ossa, E.M.; Lubián, L.M.; Montero, O. Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J. Sep. Sci. 2008, 31, 1352–1362. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC—Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Machmudah, S.; Diono, W.; Kanda, H.; Goto, M. Supercritical fluids extraction of valuable compounds from algae: Future perspectives and challenges. Eng. J. 2018, 22, 13–30. [Google Scholar] [CrossRef]
- Jaime, L.; Mendiola, J.A.; Ibáñez, E.; Martin-Álvarez, P.J.; Cifuentes, A.; Reglero, G.; Señoráns, F.J. β-Carotene isomer composition of sub- and supercritical carbon dioxide extracts. Antioxidant activity measurement. J. Agric. Food Chem. 2007, 55, 10585–10590. [Google Scholar] [CrossRef]
- Hosseini, S.R.P.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Liau, B.C.; Shen, C.T.; Liang, F.P.; Hong, S.E.; Hsu, S.L.; Jong, T.T.; Chang, C.M.J. Supercritical fluids extraction and anti-solvent purification of carotenoids from microalgae and associated bioactivity. J. Supercrit. Fluids 2010, 55, 169–175. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E.; Lubián, L.M.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. J. Food Eng. 2005, 66, 245–251. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Fernandez-Sevilla, J.M.; Fernández, F.G.A.; García, M.C.C.; Grima, E.M. Supercritical fluid extraction of carotenoids from Scenedesmus almeriensis. Food Chem. 2010, 123, 928–935. [Google Scholar] [CrossRef]
- Guedes, A.C.; Gião, M.S.; Matias, A.A.; Nunes, A.V.M.; Pintado, M.E.; Duarte, C.M.M.; Malcata, F.X. Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. J. Food Eng. 2013, 116, 478–482. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef]
- Montero, O.; Macìas-Sánchez, M.D.; Lama, C.M.; Lubián, L.M.; Mantell, C.; Rodríguez, M.; De La Ossa, E.M. Supercritical CO2 extraction of β-carotene from a marine strain of the cyanobacterium Synechococcus species. J. Agric. Food Chem. 2005, 53, 9701–9707. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E.; Lubián, L.M.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Synechococcus sp. J. Supercrit. Fluids 2007, 39, 323–329. [Google Scholar] [CrossRef]
- Gillet, J.N. Ultrafast molecular dynamics of biofuel extraction for microalgae and bacteria milking: Blocking membrane folding pathways to damaged lipid-bilayer conformations with nanomicelles. J. Biomol. Struct. Dyn. 2015, 33, 690–705. [Google Scholar] [CrossRef]
- Hejazi, M.A.; De Lamarliere, C.; Rocha, J.M.S.; Vermuë, M.; Tramper, J.; Wijffels, R.H. Selective extraction of carotenoids from the microalga Dunaliella salina with retention of viability. Biotechnol. Bioeng. 2002, 79, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kleinegris, D.M.M.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. The selectivity of milking of Dunaliella salina. Mar. Biotechnol. 2010, 12, 14–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, C.D.; Sim, S.J. Direct extraction of astaxanthin from Haematococcus culture using vegetable oils. Biotechnol. Lett. 2008, 20, 441–444. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Lee, I.; Han, J.-I. Simultaneous treatment (cell disruption and lipid extraction) of wet microalgae using hydrodynamic cavitation for enhancing the lipid yield. Bioresour. Technol. 2015, 186, 246–251. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Ooi, C.W.; Ong, H.C.; Ling, T.C.; Show, P.L. Extraction of natural astaxanthin from Haematococcus pluvialis using liquid biphasic flotation system. Bioresour. Technol. 2019, 290, 121794. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng. Rev. 2014, 7, 45–62. [Google Scholar] [CrossRef]
- Zhang, R.; Marchal, L.; Lebovka, N.; Vorobiev, E.; Grimi, N. Two-step procedure for selective recovery of bio-molecules from microalga Nannochloropsis oculata assisted by high voltage electrical discharges. Bioresour. Technol. 2020, 302, 122893. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Mannozzi, C.; Fauster, T.; Haas, K.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Jaeger, H. Role of thermal and electric field effects during the pre-treatment of fruit and vegetable mash by pulsed electric fields (PEF) and ohmic heating (OH). Innov. Food Sci. Emerg. Technol. 2018, 48, 131–137. [Google Scholar] [CrossRef]
- Yildiz, H.; Icier, F.; Baysal, T. Changes in β-carotene, chlorophyll and color of spinach puree during ohmic heating. J. Food Process Eng. 2010, 33, 763–779. [Google Scholar] [CrossRef]
- Yodsuwan, N.; Kamonpatana, P.; Chisti, Y.; Sirisansaneeyakul, S. Ohmic heating pretreatment of algal slurry for production of biodiesel. J. Biotechnol. 2018, 267, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M.; Barbosa, M.; Gouveia, L.; Sepúlveda, C.; Bazaes, J.; Arbib, Z. Economics of microalgae production. In Microalgae-Based Biofuels and Bioproducts; Woodhead Publishing Series in Energy; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 485–503. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- De Morais, M.G.; da Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from microalgae: Properties, extraction and purification, with some recent applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Bastiaens, L.; Van Roy, S.; Thomassen, G.; Elst, K. Biorefinery of algae: Technical and economic considerations. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 327–345. [Google Scholar] [CrossRef]
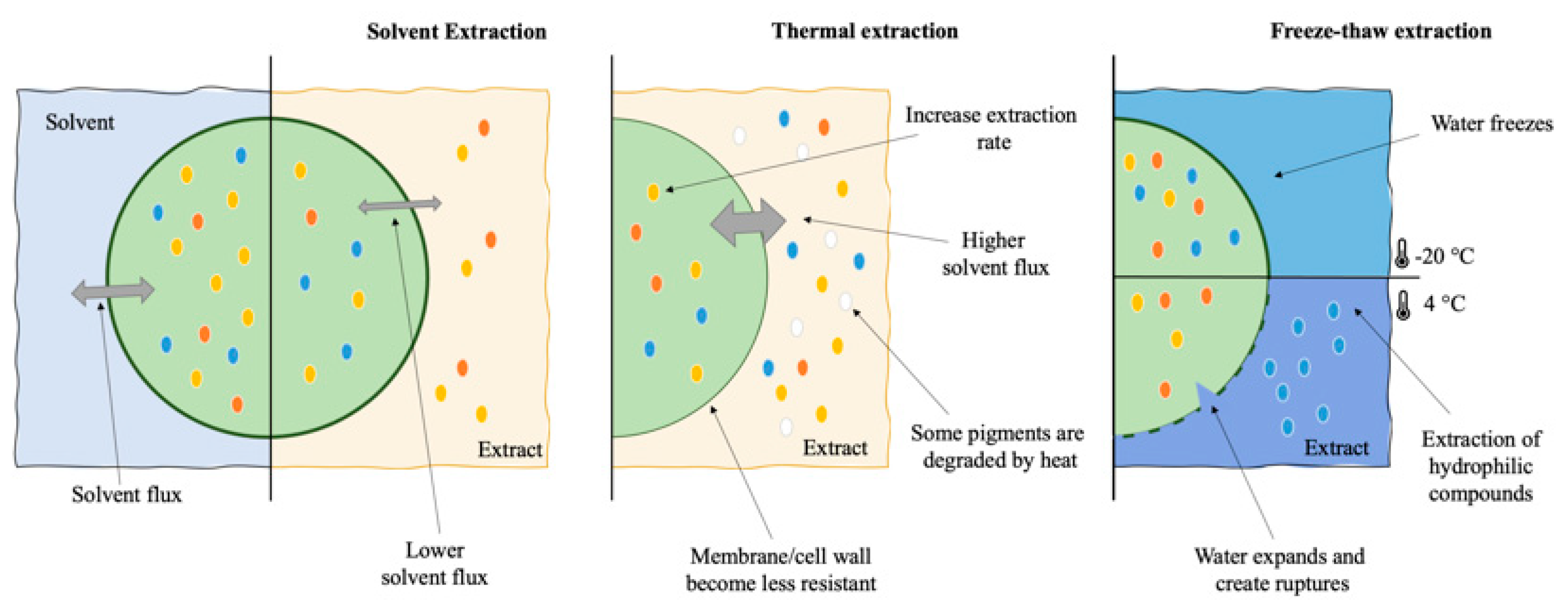
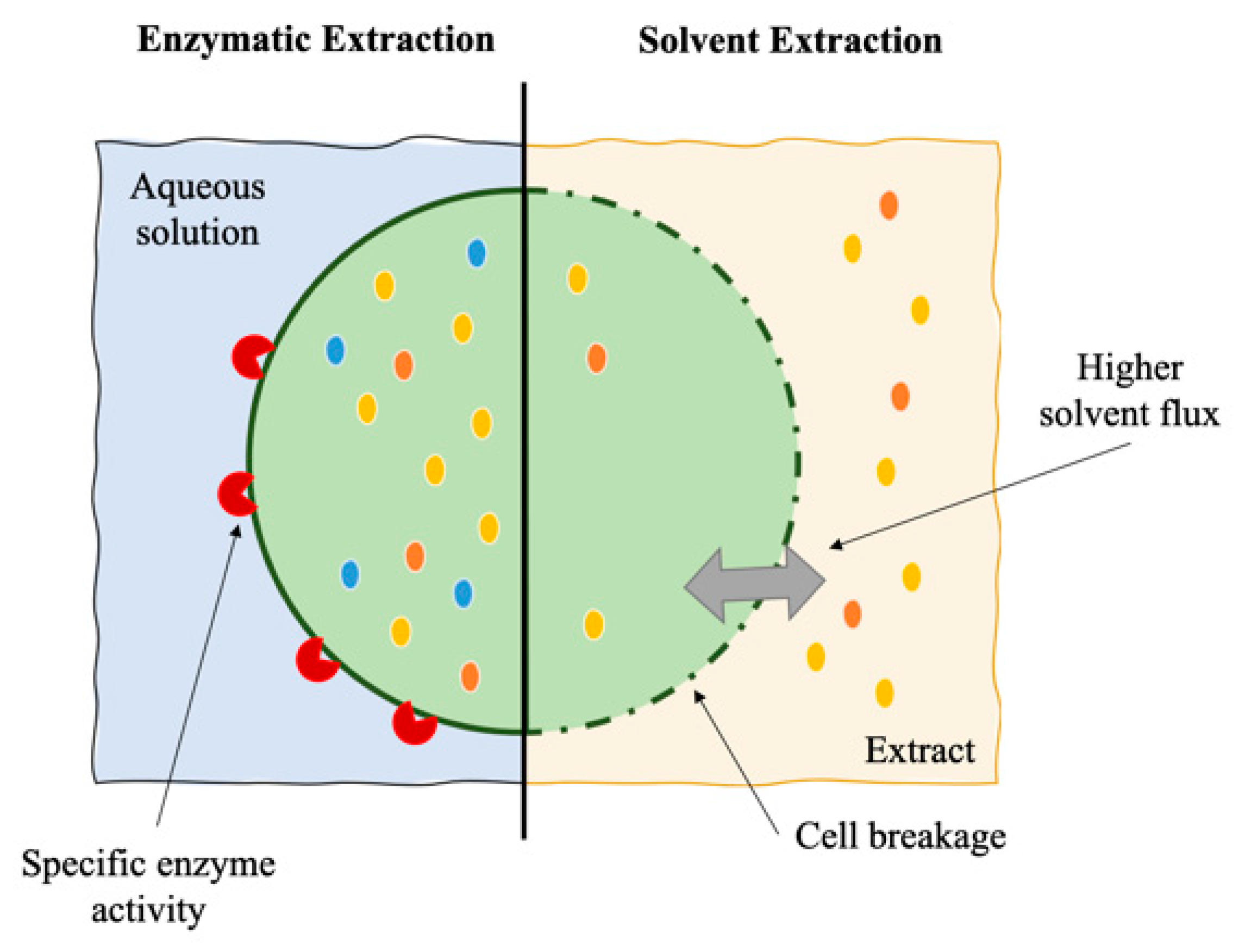
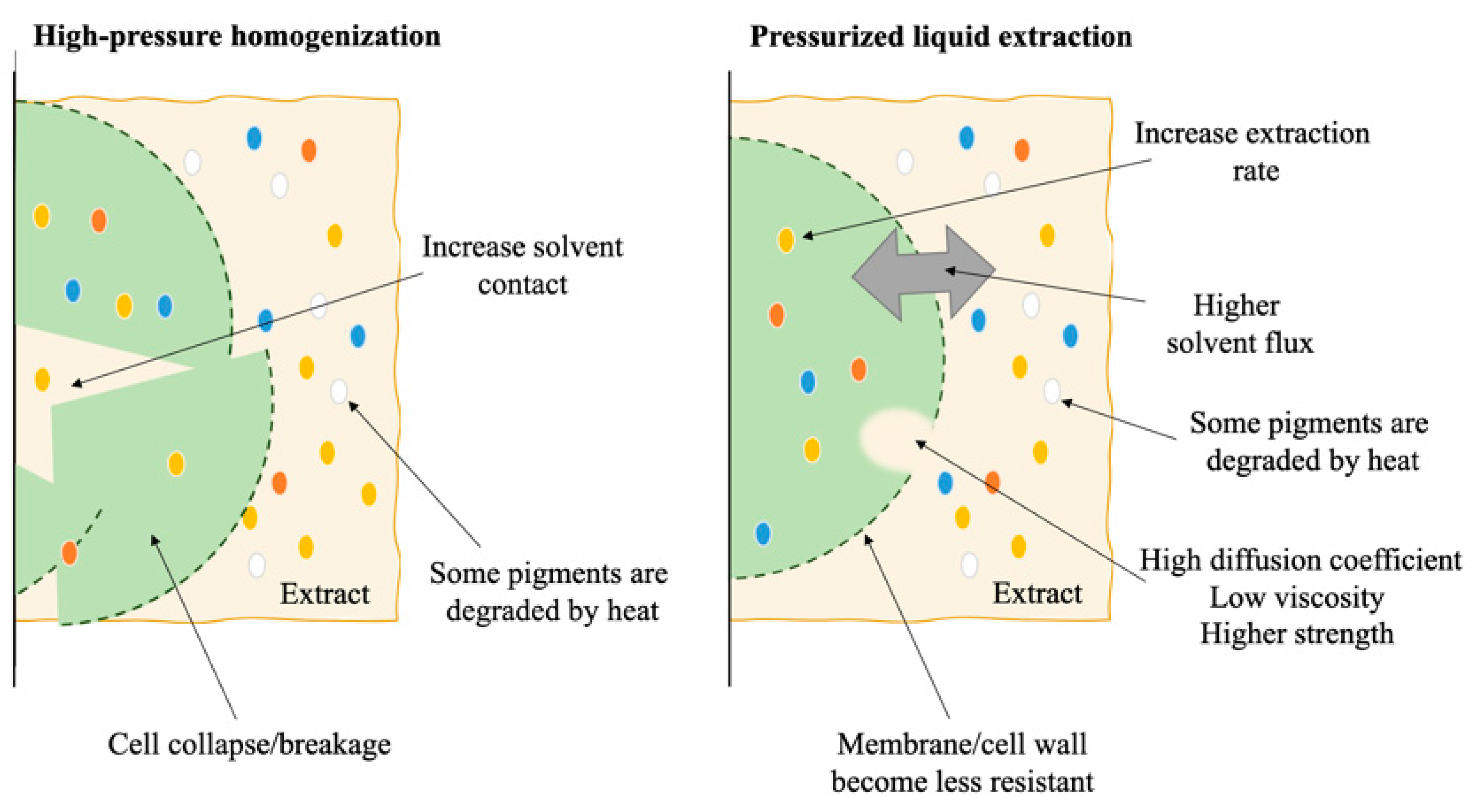


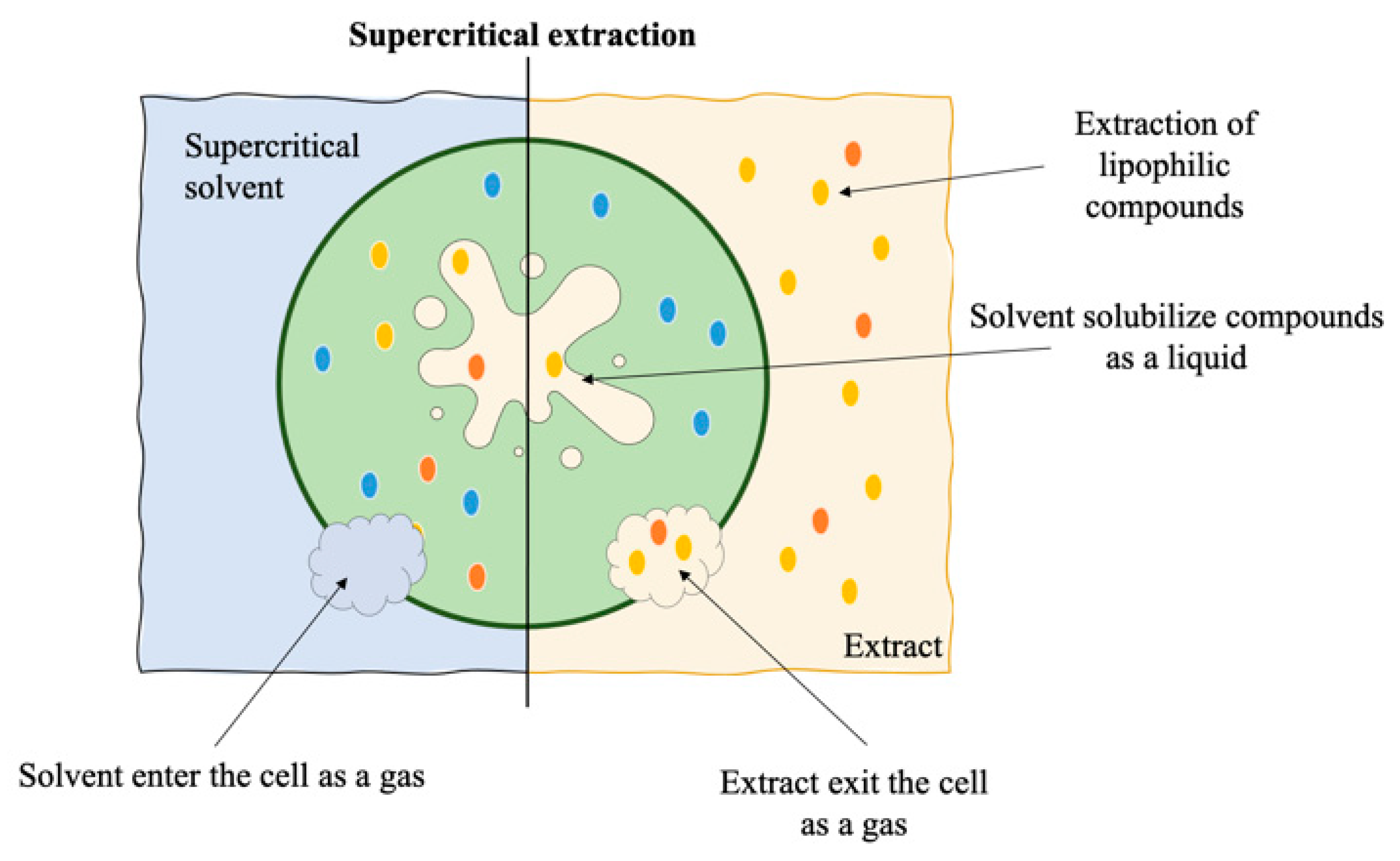
| Extraction Method a | Product | Species | Processing Parameters b | Extraction Yield (mg.gDW) | Reference |
|---|---|---|---|---|---|
| Classic solvent extraction | Fucoxanthin | Isochrysis galbana | S: ethanol; T: RT; t: 60 min | 18.23 ± 0.54 | [14] |
| Classic solvent extraction | Astaxanthin | Haematococcus pluvialis | S: acetone; T: RT; t: 16 h | ca. 5.00 | [16] |
| Classic solvent extraction | Astaxanthin | H. pluvialis | S: ethanol:ethyl acetate (1:1, v:v); T: RT; t: 90 min | 16.48 ± 0.67 | [17] |
| Classic solvent extraction | Phycobiliproteins | Arthrospira platensis | S: water or sodium phosphate buffer; T: 25 °C; t: 4 h | 92.00 | [22] |
| Enzymatic | Allophycocyanin | A. platensis | S: lysozyme + surfactants; T: 37 °C; t: 20 h | 32.27 | [29] |
| HPH | Carotenoids | Nannochloropsis sp. | S: water/recovered with hexane:isopropanol (3:2, v:v) p: 1000 bar; T: n.s.; c: 4 cycles | Violoxanthin—2.50 ± 0.24; Antheraxanthin—1.74 ± 0.34; Zeaxanthin—1.93 ± 0.24; β-carotene—10.07 ± 1.70 c | [34] |
| PLE | Carotenoids and chlorophyll | Chlorella vulgaris | S: ethanol:water (9:1, v:v); p: 100 bar; Lutein—T: 148 °C; t: 35 min; β-carotene– T: 117 °C; t: 25 min; Chlorophyll a—T: 173 °C; t: 15 min; Chlorophyll b—T: 170 °C; t: 3 min | Lutein—3.70 β-carotene—0.67 Chlorophyll a—10.83 Chlorophyll b—6.81 | [36] |
| PLE | β-carotene | Dunaliella salina | S: ethanol; p: 100 bar; T: 160 °C; t: 30 min | 34.6 | [37] |
| PLE | Carotenoids | Phormidium spp. | S: ethanol; p: 100 bar; T: 150 °C; t: 20 min | n.s. | [38] |
| CPSE | Carotenoids | Gloeothece sp. | S: ethanol; p: 180 bar; T: 60 °C; c: 3 cycles | Lutein—2.9 ± 0.1; β-carotene—1.5 ± 0.1 c | [39] |
| MAE | Phycobiliproteins | Porphyridium purpureum | S: water; P: n.s.; t: 10 s; Phycoerythrin—T: 40 °C; Phycocyanin/Allophycocyanin—T: 100 °C | Phycoerythrin—73.7 ± 2.3; Phycocyanin—34.8 ± 6.4; Allophycocyanin—32.3 ± 1.2 | [40] |
| MAE | Fucoxanthin | Cylindrotheca closterium | S: acetone; P: 50 W; T: 56 °C t: 5 min | 4.24 ± 0.09 | [41] |
| UAE | β-carotene | A. platensis | S: heptane; Pd: 167 W.cm−2; T: 30 °C t: 8 min | ca. 1.00 | [42] |
| UAE | Carotenoids | H. pluvialis | S: ethanol:ethyl acetate (1:1, v:v); P: 200 W; F: 40 kHz; T: 41.1 °C t: 16 min | 27.58 ± 0.40 | [17] |
| PEF | Carotenoids | C. vulgaris | S: citrate- phosphate McIlvaine buffer/recovered with ethanol; E: 20 kV.cm−1; T: n.s.; t: 25 pulses of 3 μs | ca. 1.00 | [49] |
| PEF | Phycocyanin | A. platensis | S: water E: 15 kV.cm−1; T: 40 °C; t: 50 pulses of 3 μs | 100.00 | [52] |
| PEF | Phycocyanin | Nostoc commune | S: water E: 5 kV.cm−1; F: 2 Hz; T: 40 °C; t: 1500 pulses of 1 μs | 29.66 ± 0.52 | [53] |
| PEF | Phycoerythrin | Porphyridium cruentum | S: citrate- phosphate McIlvaine buffer; T: 20 to 30 °C; E: 10 kV.cm−1; F: 0.5 Hz; t: 50 pulses of 3 μs | 32.00 | [54] |
| PEF pre-treatment | Carotenoids | Nannochloropsis spp. | 1st stage—S: water; T: 20 °C; E: 20 kV.cm−1, t: 400 pulses of 10 μs) (2nd stage) S: recovered in DMSO or ethanol + water (1:1, v:v); T: 20 °C | n.s. | [55] |
| PEF pre-treatment | Astaxanthin | H. pluvialis | S: culture medium/recovered with ethanol; T: 20 °C; E: 1 kV·cm−1; t: 10 pulses of 5 ms | 18.3 | [56] |
| SC-CO2 | β-carotene | D. salina | S: SC-CO2; p: 443 bar; T: 27.5 °C; t: 100 min | n.s. | [61] |
| SC-CO2 | Carotenoids | D. salina | S: SC-CO2; p: 400 bar; T: 30 °C; t: 90 min | 115.43 | [62] |
| SC-CO2 | Carotenoids | Nannochloropsis oculata | S: SC-CO2 + Ethanol; p: 350 bar; T: 50 °C; t: 30 min | 7.61 | [63] |
| SC-CO2 | Carotenoids and chlorophyll | Nannochloropsis gaditana | S: SC-CO2; p: 400 bar; T: 60 °C; t: 3 h | Carotenoids—0.34; Chlorophylls—2.23 | [64] |
| SC-CO2 | Lutein and β-carotene | Scenedesmus almeriensis | S: SC-CO2; p: 400 bar; T: 60 °C; t: 5 h | Lutein—0.04; β-carotene—1.50 | [65] |
| SC-CO2 | Carotenoids | Scenedesmus obliquus | S: SC-CO2; p: 250 bar; T: 60 °C; t: 4 h | 0.18 | [66] |
| SC-CO2 | Astaxanthin | H. pluvialis | S: SC-CO2; p: 550 bar; T: 50 °C; t: 100 min | 19.72 | [68] |
| SC-CO2 | Astaxanthin | H. pluvialis | S: SC-CO2 with addition of Ethanol; p: 300 bar; T: 60 °C; t: n.s. | 1.8 | [67] |
| SC-CO2 | β-carotene | Synechococcus sp. | S: SC-CO2; p: 358 bar; T: 50 °C; t: 2 h | 0.49 ± 0.10 | [69] |
| SC-CO2 | Carotenoids | Synechococcus sp. | S: SC-CO2; p: 300 bar; T: 50 °C t: 3 h | 1.51 | [70] |
| Cell Milking | β-carotene | D. salina | S: dodecane | 0.25 d | [72] |
| Cell Milking | β-carotene | D. salina | S: dodecane | 5.30 d | [73] |
| Cell Milking | Astaxanthin | H. pluvialis | S: dodecane | 85.00 e | [18] |
| Cell Milking | Astaxanthin | H. pluvialis | S: vegetable oils | 76.00 e | [74] |
| Laser | n.s. | N. oculata | S: water; P: 10 W; F: 20 kHz; t: 1 min | n.s. | [75] |
| Hydrodynamic cavitation | Lipidic extract | N. salina | S: culture medium/recovered with hexane | n.s. | [76] |
| Liquid biphasic flotation | Astaxanthin | H. pluvialis | S: 2-propanol as organic phase and (NH4)2SO4 as salt for the aqueous phase | 95.11 d | [77] |
| HVED | Chlorophyll, carotenoids | N. oculata | S: water; E: 40 kV.cm−1; T: 20 to 30 °C; t: 400 pulses of 4ms | n.s. | [79] |
| OH pre-treatment | Lipidic extract | Chlorella sp. | S: water (wet biomass); E: 0.015 kV.cm−1; F: 0.005 kHz; T: 70 °C; t: 2 min | n.s. | [83] |
| Methodology | Advantages | Limitations |
|---|---|---|
| Classic solvent extraction |
|
|
| Enzymatic |
|
|
| Pressure |
|
|
| Wave energy-based |
|
|
| Electric fields |
|
|
| Supercritical extraction |
|
|
| Cell milking |
|
|
| Laser |
|
|
| Hydrodynamic cavitation |
|
|
| Flotation |
|
|
| High voltage electrical discharge |
|
|
| Ohmic heating |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Appl. Sci. 2021, 11, 5187. https://doi.org/10.3390/app11115187
Pagels F, Pereira RN, Vicente AA, Guedes AC. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Applied Sciences. 2021; 11(11):5187. https://doi.org/10.3390/app11115187
Chicago/Turabian StylePagels, Fernando, Ricardo N. Pereira, António A. Vicente, and A. Catarina Guedes. 2021. "Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies" Applied Sciences 11, no. 11: 5187. https://doi.org/10.3390/app11115187
APA StylePagels, F., Pereira, R. N., Vicente, A. A., & Guedes, A. C. (2021). Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Applied Sciences, 11(11), 5187. https://doi.org/10.3390/app11115187







