Relative Hand Grip and Back Muscle Strength, but Not Mean Muscle Strength, as Risk Factors for Incident Metabolic Syndrome and Its Metabolic Components: 16 Years of Follow-Up in a Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
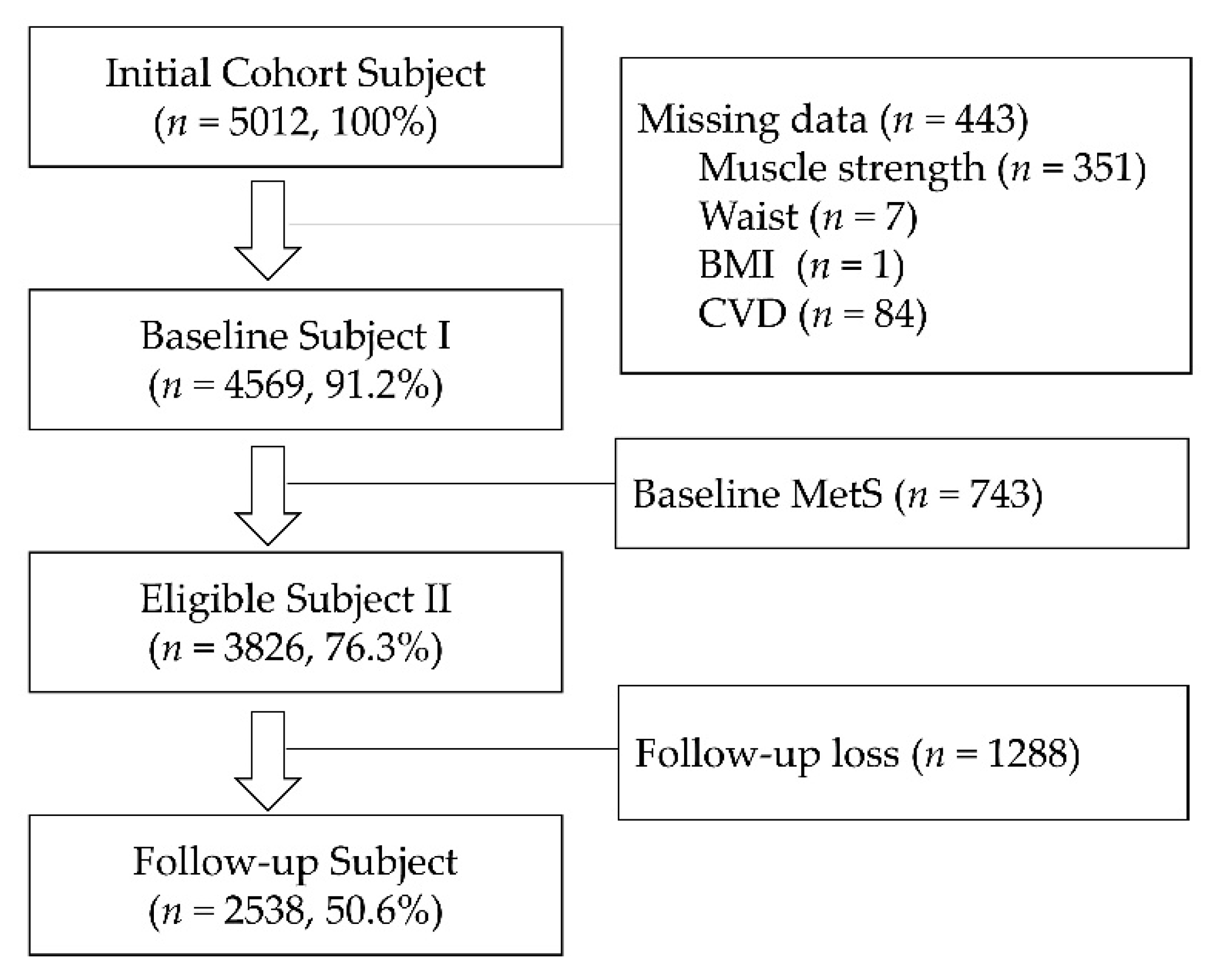
2.1. Study Participants and Population
2.2. Muscle Strength
2.3. Covariates
2.4. Definitions of Metabolic Syndrome
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Study Participants According to Gender
3.2. Characteristics of Study Participants in Normal and Incident Metabolic Syndrome According to Gender
3.3. Cox Proportional Hazard Ratios for Incident Metabolic Syndrome According to Inverse One Standard Deviation Increase in Mean Hand Grip Strength, Back Muscle Strength, and Relative Muscle Strength in Gender
3.4. Cox Proportional Hazard Ratios for Incident Metabolic Syndrome According to Quartile Groups of Mean Hand Grip Strength, Back Muscle Strength, and Relative Muscle Strength by Gender
3.5. Cox Proportional Hazard Ratios for Incident Metabolic Syndrome According to Delta Change of Mean Hand Grip Strength and Relative Hand Grip Strength in Gender
3.6. Cox Proportional Hazard Ratios for Incident Metabolic Syndrome Components According to Mean Hand Grip Strength, Back Muscle Strength, and Relative Muscle Strength in Gender
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lee, W.J.; Peng, L.N.; Chiou, S.T.; Chen, L.K. Relative Handgrip Strength Is a Simple Indicator of Cardiometabolic Risk among Middle-Aged and Older People: A Nationwide Population-Based Study in Taiwan. PLoS ONE 2016, 11, e0160876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Nilsson, P.M. The metabolic syndrome: A glance at its history. J. Hypertens. 2006, 24, 621–626. [Google Scholar] [CrossRef]
- Lim, S.; Shin, H.; Song, J.H.; Kwak, S.H.; Kang, S.M.; Won Yoon, J.; Choi, S.H.; Cho, S.I.; Park, K.S.; Lee, H.K.; et al. Increasing prevalence of metabolic syndrome in Korea: The Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care 2011, 34, 1323–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.R.; Jeon, Y.J.; Kim, M.C.; Jeong, T.; Koo, W.R. Reference values for hand grip strength in the South Korean population. PLoS ONE 2018, 13, e0195485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker for Older Adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbat-Artigas, S.; Plouffe, S.; Pion, C.H.; Aubertin-Leheudre, M. Toward a sex-specific relationship between muscle strength and appendicular lean body mass index? J. Cachexia Sarcopenia Muscle 2013, 4, 137–144. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Isiozor, N.M.; Khan, H.; Laukkanen, J.A. Handgrip strength-A risk indicator for type 2 diabetes: Systematic review and meta-analysis of observational cohort studies. Diabetes Metab. Res. Rev. 2021, 37, e3365. [Google Scholar] [CrossRef]
- Ji, C.; Zheng, L.; Zhang, R.; Wu, Q.; Zhao, Y. Handgrip strength is positively related to blood pressure and hypertension risk: Results from the National Health and nutrition examination survey. Lipids Health Dis. 2018, 17, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef]
- Vancampfort, D.; Stubbs, B.; Firth, J.; Smith, L.; Swinnen, N.; Koyanagi, A. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low- and middle-income countries. Int. J. Geriatr. Psychiatry 2019, 34, 609–616. [Google Scholar] [CrossRef]
- Crewther, B.T.; Gill, N.; Weatherby, R.P.; Lowe, T. A comparison of ratio and allometric scaling methods for normalizing power and strength in elite rugby union players. J. Sports Sci. 2009, 27, 1575–1580. [Google Scholar] [CrossRef]
- Maranhao Neto, G.A.; Oliveira, A.J.; Pedreiro, R.C.; Pereira-Junior, P.P.; Machado, S.; Marques Neto, S.; Farinatti, P.T. Normalizing handgrip strength in older adults: An allometric approach. Arch. Gerontol. Geriatr. 2017, 70, 230–234. [Google Scholar] [CrossRef]
- Choquette, S.; Bouchard, D.R.; Doyon, C.Y.; Senechal, M.; Brochu, M.; Dionne, I.J. Relative strength as a determinant of mobility in elders 67-84 years of age. a nuage study: Nutrition as a determinant of successful aging. J. Nutr. Health Aging 2010, 14, 190–195. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Lee, S.K.; Shin, C. Normalized Hand Grip and Back Muscle Strength as Risk Factors for Incident Type 2 Diabetes Mellitus: 16 Years of Follow-Up in a Population-Based Cohort Study. Diabetes Metab. Syndr. Obes. 2021, 14, 741–750. [Google Scholar] [CrossRef]
- Kawamoto, R.; Ninomiya, D.; Kasai, Y.; Kusunoki, T.; Ohtsuka, N.; Kumagi, T.; Abe, M. Handgrip strength is associated with metabolic syndrome among middle-aged and elderly community-dwelling persons. Clin. Exp. Hypertens 2016, 38, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.Y.; Lee, M.K.; Yu, M.S.; Kang, M.J.; Lee, D.H.; Kim, K.C.; Im, J.A.; Kim, S.H.; Jeon, J.Y. Lower Relative Handgrip Strength is Significantly Associated with a Higher Prevalence of the Metabolic Syndrome in Adults. Metab. Syndr. Relat. Disord. 2019, 17, 280–288. [Google Scholar] [CrossRef]
- Chun, S.W.; Kim, W.; Choi, K.H. Comparison between grip strength and grip strength divided by body weight in their relationship with metabolic syndrome and quality of life in the elderly. PLoS ONE 2019, 14, e0222040. [Google Scholar] [CrossRef] [PubMed]
- Hong, S. Association of Relative Handgrip Strength and Metabolic Syndrome in Korean Older Adults: Korea National Health and Nutrition Examination Survey VII-1. J. Obes. Metab. Syndr. 2019, 28, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, M.; Chi, V.T.Q.; Wang, J.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Bao, X.; Gu, Y.; et al. Handgrip strength is inversely associated with metabolic syndrome and its separate components in middle aged and older adults: A large-scale population-based study. Metabolism 2019, 93, 61–67. [Google Scholar] [CrossRef]
- Ji, C.; Xia, Y.; Tong, S.; Wu, Q.; Zhao, Y. Association of handgrip strength with the prevalence of metabolic syndrome in US adults: The national health and nutrition examination survey. Aging 2020, 12, 7818–7829. [Google Scholar] [CrossRef]
- Kang, Y.; Park, S.; Kim, S.; Koh, H. Handgrip Strength among Korean Adolescents with Metabolic Syndrome in 2014–2015. J. Clin. Densitom. 2020, 23, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y. Relationship of Handgrip Strength to Metabolic Syndrome among Korean Adolescents 10–18 Years of Age: Results from the Korean National Health and Nutrition Examination Survey 2014–18. Metab. Syndr. Relat. Disord. 2021, 19, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, J.P.; Cohen, D.D.; Ney-Salazar, D.; Martinez, D.; Otero, J.; Gomez-Arbelaez, D.; Camacho, P.A.; Sanchez-Vallejo, G.; Arcos, E.; Narvaez, C.; et al. The prediction of Metabolic Syndrome alterations is improved by combining waist circumference and handgrip strength measurements compared to either alone. Cardiovasc. Diabetol. 2021, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Han, P.; Zhao, Y.; Zhang, Y.; Wang, L.; Tao, Z.; Jiang, Z.; Shen, S.; Wu, Y.; Wu, J.; et al. Muscle mass rather than muscle strength or physical performance is associated with metabolic syndrome in community-dwelling older Chinese adults. BMC Geriatr. 2021, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Lu, J.; Xu, Z.; Xu, Y.; Yang, Y. Association between handgrip strength and the risk of new-onset metabolic syndrome: A population-based cohort study. BMJ Open 2020, 10, e041384. [Google Scholar] [CrossRef] [PubMed]
- McCowan, T.C.; Ferris, E.J.; Baker, M.L.; Robbins, K.V.; Reifsteck, J.E.; Fleisher, H.L.; Barnes, R.W. Human percutaneous laser angioplasty. J. Ark Med. Soc. 1986, 82, 594–596. [Google Scholar]
- Jurca, R.; Lamonte, M.J.; Barlow, C.E.; Kampert, J.B.; Church, T.S.; Blair, S.N. Association of muscular strength with incidence of metabolic syndrome in men. Med. Sci. Sports Exerc. 2005, 37, 1849–1855. [Google Scholar] [CrossRef] [Green Version]
- LaMonte, M.J.; Barlow, C.E.; Jurca, R.; Kampert, J.B.; Church, T.S.; Blair, S.N. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: A prospective study of men and women. Circulation 2005, 112, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Kasukawa, Y.; Miyakoshi, N.; Hongo, M.; Ishikawa, Y.; Kudo, D.; Suzuki, M.; Mizutani, T.; Kimura, R.; Ono, Y.; Shimada, Y. Age-related changes in muscle strength and spinal kyphosis angles in an elderly Japanese population. Clin. Interv. Aging 2017, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, H.; Hoshino, M.; Ohyama, S.; Terai, H.; Suzuki, A.; Yamada, K.; Takahashi, S.; Hayashi, K.; Tamai, K.; Hori, Y.; et al. The association of back muscle strength and sarcopenia-related parameters in the patients with spinal disorders. Eur. Spine J. 2019, 28, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Lakka, H.M.; Salonen, J.T.; Niskanen, L.K.; Rauramaa, R.; Lakka, T.A. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002, 25, 1612–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, N.; Wada, J.; Saito, T.; Nishikawa, H.; Matsumoto, S.; Miyachi, M.; Makino, H.; Numata, T. Comparison of muscle strength between Japanese men with and without metabolic syndrome. Acta Med. Okayama 2007, 61, 99–102. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ahn, S.; Kim, Y.J.; Ji, M.J.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lim, S. Comparison between Dual-Energy X-ray Absorptiometry and Bioelectrical Impedance Analyses for Accuracy in Measuring Whole Body Muscle Mass and Appendicular Skeletal Muscle Mass. Nutrients 2018, 10, 738. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cuthbertson, D.J.; Bell, J.A.; Ng, S.Y.; Kemp, G.J.; Kivimaki, M.; Hamer, M. Dynapenic obesity and the risk of incident Type 2 diabetes: The English Longitudinal Study of Ageing. Diabet. Med. 2016, 33, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Larsen, B.A.; Wassel, C.L.; Kritchevsky, S.B.; Strotmeyer, E.S.; Criqui, M.H.; Kanaya, A.M.; Fried, L.F.; Schwartz, A.V.; Harris, T.B.; Ix, J.H.; et al. Association of Muscle Mass, Area, and Strength with Incident Diabetes in Older Adults: The Health ABC Study. J. Clin. Endocrinol. Metab. 2016, 101, 1847–1855. [Google Scholar] [CrossRef]
- Bai, T.; Fang, F.; Li, F.; Ren, Y.; Hu, J.; Cao, J. Sarcopenia is associated with hypertension in older adults: A systematic review and meta-analysis. BMC Geriatr. 2020, 20, 279. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, S.; Gao, T.; Zhong, F.; Cai, J.; Sun, Y.; Ma, A. Association between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artero, E.G.; Lee, D.C.; Lavie, C.J.; Espana-Romero, V.; Sui, X.; Church, T.S.; Blair, S.N. Effects of muscular strength on cardiovascular risk factors and prognosis. J. Cardiopulm Rehabil. Prev. 2012, 32, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieland, M.; Verdijk, L.B.; de Groot, L.C.; van Loon, L.J. Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.R.; Shin, A.; Kim, J.; Jee, S.H.; Sung, J. Leisure-time physical activity is associated with a reduced risk for metabolic syndrome. Ann. Epidemiol. 2009, 19, 784–792. [Google Scholar] [CrossRef]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Santos, A.R.; Amaral, T.F. Differences in handgrip strength protocols to identify sarcopenia and frailty—A systematic review. BMC Geriatr. 2017, 17, 238. [Google Scholar] [CrossRef] [Green Version]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Lind, L. Genome-Wide Association Study of the Metabolic Syndrome in UK Biobank. Metab. Syndr. Relat. Disord. 2019, 17, 505–511. [Google Scholar] [CrossRef]
- Misselbeck, K.; Parolo, S.; Lorenzini, F.; Savoca, V.; Leonardelli, L.; Bora, P.; Morine, M.J.; Mione, M.C.; Domenici, E.; Priami, C. A network-based approach to identify deregulated pathways and drug effects in metabolic syndrome. Nat. Commun. 2019, 10, 5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingjan, I.; Linders, P.T.A.; Verboogen, D.R.J.; Revelo, N.H.; Ter Beest, M.; van den Bogaart, G. Endosomal and Phagosomal SNAREs. Physiol. Rev. 2018, 98, 1465–1492. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Al Qarni, A.; Hawwari, A.; Alghanem, A.F.; Ahmed, G. Metabolic Syndrome, Dyslipidemia and Regulation of Lipoprotein Metabolism. Curr. Diabetes Rev. 2018, 14, 427–433. [Google Scholar] [CrossRef] [PubMed]
| Women (n = 1215) | Men (n = 1323) | ||||||
|---|---|---|---|---|---|---|---|
| Normal (n = 773, 63.6%) | iMetS (n = 442, 36.4%) | Normal (n = 775, 58.6%) | iMetS (n = 548, 41.4%) | ||||
| General Characteristics | |||||||
| Age (year) | 46.4 ± 5.9 | 50.5 ± 7.9 | <0.001 | 47.5 ± 6.5 | 48.2 ± 7.3 | 0.062 | |
| Smoker | Ex-smoker | 8 (1.0%) | 4 (0.9%) | 0.504 | 291 (37.5%) | 178 (32.5%) | <0.001 |
| Current smoker | 12 (1.6%) | 11 (2.5%) | 291 (37.5%) | 275 (50.2%) | |||
| Family history of T2DM | Yes | 112 (14.5%) | 80 (18.1%) | 0.097 | 91 (11.7%) | 85 (15.5%) | 0.047 |
| Family history of hypertension | Yes | 155 (20.1%) | 99 (22.4%) | 0.332 | 132 (17.0%) | 132 (24.1%) | 0.002 |
| Job | Blue color | 236 (30.5%) | 129 (29.2%) | 0.363 | 543 (70.1%) | 379 (69.2%) | 0.594 |
| White color | 36 (4.7%) | 14 (3.2%) | 185 (23.9%) | 128 (23.4%) | |||
| Housekeeper | 501 (64.8%) | 299 (67.6%) | 47 (6.1%) | 41 (7.5%) | |||
| Income (KRW) | <1 million | 96 (12.4%) | 88 (19.9%) | 0.005 | 56 (7.2%) | 41 (7.5%) | 0.515 |
| 1–2 | 238 (30.8%) | 133 (30.1%) | 213 (27.5%) | 154 (28.1%) | |||
| 2–4 | 347 (44.9%) | 178 (40.3%) | 388 (50.1%) | 255 (46.5%) | |||
| 4≤ | 92 (11.9%) | 43 (9.7%) | 118 (15.2%) | 98 (17.9%) | |||
| Education | <12 year | 96 (12.4%) | 41 (9.3%) | 0.096 | 258 (33.3%) | 165 (30.1%) | 0.222 |
| Marry Status | Yes | 709 (91.7%) | 388 (87.8%) | 0.026 | 754 (97.3%) | 532 (97.1%) | 0.820 |
| Menopause Status | Yes | 118 (15.3%) | 118 (26.7%) | <0.001 | NA | NA | |
| Body Mass Index (kg/m2) | 23.6 ± 2.5 | 25.8 ± 2.8 | <0.001 | 23.9 ± 2.6 | 25.4 ± 2.5 | <0.001 | |
| Height (cm) | 155.7 ± 5.1 | 154.5 ± 4.9 | <0.001 | 167.8 ± 5.5 | 167.9 ± 5.8 | 0.635 | |
| Weight (kg) | 57.3 ± 6.7 | 61.6 ± 7.2 | <0.001 | 67.4 ± 8.4 | 71.6 ± 9.1 | <0.001 | |
| Alcohol consumption (g/day) | 1.3 ± 4.7 | 1.9 ± 6.4 | 0.072 | 17.0 ± 25.2 | 22.6 ± 30.2 | <0.001 | |
| Leisure physical activity (Met) | 127.3 ± 200.3 | 126.5 ± 194.3 | 0.945 | 124.0 ± 187.9 | 127.1 ± 213.1 | 0.781 | |
| Metabolic Components | |||||||
| Waist circumference (cm) | 73.6 ± 6.2 | 79.5 ± 6.6 | <0.001 | 80.9 ± 6.7 | 85.4 ± 6.6 | <0.001 | |
| HDL-cholesterol (mg/dL) | 54.9 ± 11.9 | 49.8 ± 10.7 | <0.001 | 49.6 ± 10.7 | 44.6 ± 8.9 | <0.001 | |
| Triglycerides (mg/dL) | 100.0 ± 52.0 | 133.1 ± 75.0 | <0.001 | 132.4 ± 70.1 | 188.1 ± 109.2 | <0.001 | |
| Systolic Blood Pressure (mmHg) | 107.0 ± 13.8 | 118.7 ± 15.9 | <0.001 | 114.0 ± 13.8 | 120.3 ± 15.7 | <0.001 | |
| Diastolic Blood Pressure (mmHg) | 70.8 ± 9.0 | 78.2 ± 9.9 | <0.001 | 77.9 ± 10.0 | 82.0 ± 10.8 | <0.001 | |
| Muscle Strength | |||||||
| Hand grip Strength (HGS) | |||||||
| Mean HGS (kg) | 22.0 ± 3.8 | 21.4 ± 3.8 | <0.001 | 35.4 ± 5.5 | 35.0 ± 5.8 | 0.216 | |
| BMI-relative Mean HGS | 0.94 ± 0.19 | 0.84 ± 0.16 | <0.001 | 1.49 ± 0.26 | 1.39 ± 0.24 | <0.001 | |
| Weight-relative Mean HGS | 0.39 ± 0.07 | 0.35 ± 0.06 | <0.001 | 0.53 ± 0.09 | 0.49 ± 0.08 | <0.001 | |
| WC-relative Mean HGS | 0.30 ± 0.06 | 0.27 ± 0.05 | <0.001 | 0.44 ± 0.08 | 0.41 ± 0.07 | <0.001 | |
| Back Muscle Strength (BMS) | |||||||
| Mean BMS (kg) | 42.9 ± 12.3 | 42.5 ± 11.8 | 0.653 | 83.6 ± 19.2 | 84.3 ± 19.2 | 0.536 | |
| BMI-relative Mean BMS | 1.83 ± 0.55 | 1.66 ± 0.46 | <0.001 | 3.52 ± 0.82 | 3.34 ± 0.78 | <0.001 | |
| Weight-relative Mean BMS | 0.76 ± 0.22 | 0.69 ± 0.19 | <0.001 | 1.25 ± 0.29 | 1.19 ± 0.28 | <0.001 | |
| WC-relative Mean BMS | 0.59 ± 0.17 | 0.54 ± 0.15 | <0.001 | 1.04 ± 0.24 | 0.99 ± 0.23 | <0.001 | |
| HGS | BMS | |||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| HR (95% CI) p | HR (95% CI) p * | HR (95% CI) p | HR (95% CI) p * | |
| Women | ||||
| Mean (kg) | 1.176 (1.066–1.296) 0.001 | 1.042 (0.938–1.157) 0.445 | 1.045 (0.951–1.149) 0.355 | 0.932 (0.845–1.028) 0.161 |
| BMI-relative | 1.680 (1.514–1.864) < 0.001 | 1.494 (1.337–1.671) < 0.001 | 1.352 (1.224–1.494) < 0.001 | 1.192 (1.072–1.324) 0.001 |
| Weight-relative | 1.618 (1.461–1.792) < 0.001 | 1.454 (1.306–1.620) < 0.001 | 1.297 (1.175–1.432) < 0.001 | 1.158 (1.044–1.283) 0.005 |
| WC-relative | 1.662 (1.501–1.841) < 0.001 | 1.447 (1.293–1.620) < 0.001 | 1.314 (1.190–1.451) < 0.001 | 1.135 (1.022–1.261) 0.019 |
| Men | ||||
| Mean HGS (kg) | 1.076 (0.984–1.176) 0.107 | 1.078 (0.982–1.182) 0.114 | 0.982 (0.903–1.069) 0.679 | 0.987 (0.905–1.076) 0.766 |
| BMI-relative | 1.454 (1.324–1.596) < 0.001 | 1.470 (1.335–1.618) < 0.001 | 1.203 (1.106–1.309) < 0.001 | 1.212 (1.113–1.320) < 0.001 |
| Weight-relative | 1.465 (1.338–1.604) < 0.001 | 1.472 (1.342–1.614) < 0.001 | 1.207 (1.110–1.313) < 0.001 | 1.212 (1.114–1.317) < 0.001 |
| WC-relative | 1.422 (1.298–1.559) < 0.001 | 1.431 (1.302–1.574) < 0.001 | 1.187 (1.091–1.292) < 0.001 | 1.190 (1.092–1.297) < 0.001 |
| Muscle Strength | Group | HGS | BMS | ||
|---|---|---|---|---|---|
| Women | Men | Women | Men | ||
| HR (95% CI) p * | HR (95% CI) p * | HR (95% CI) p * | HR (95% CI) p * | ||
| Mean (kg) | Q4 | Reference | Reference | Reference | Reference |
| Q3 | 0.880 (0.669–1.159) 0.363 | 1.032 (0.805–1.323) 0.801 | 1.135 (0.877–1.469) 0.336 | 0.897 (0.706–1.139) 0.372 | |
| Q2 | 0.896 (0.685–1.173) 0.426 | 1.117 (0.875–1.426) 0.375 | 0.907 (0.688–1.197) 0.491 | 1.141 (0.903–1.441) 0.268 | |
| Q1 | 0.897 (0.674–1.194) 0.456 | 1.173 (0.914–1.505) 0.211 | 0.895 (0.676–1.185) 0.439 | 0.968 (0.757–1.238) 0.794 | |
| p value for linear trend | 0.506 | 0.166 | 0.224 | 0.72 | |
| BMI-relative | Q4 | Reference | Reference | Reference | Reference |
| Q3 | 1.801 (1.293–2.511) 0.001 | 1.369 (1.042–1.799) 0.024 | 1.787 (1.334–2.393) < 0.001 | 1.188 (0.921–1.531) 0.185 | |
| Q2 | 2.209 (1.597–3.057) < 0.001 | 1.553 (1.188–2.030) 0.001 | 1.631 (1.213–2.193) 0.001 | 1.551 (1.215–1.980) < 0.001 | |
| Q1 | 2.821 (2.028–3.923) < 0.001 | 2.401 (1.854–3.110) < 0.001 | 1.579 (1.163–2.143) 0.003 | 1.596 (1.247–2.041) < 0.001 | |
| p value for linear trend | <0.001 | <0.001 | 0.009 | <0.001 | |
| Weight-relative | Q4 | Reference | Reference | Reference | Reference |
| Q3 | 1.868 (1.350–2.585) < 0.001 | 1.486 (1.129–1.955) 0.005 | 1.649 (1.236–2.200) 0.001 | 1.193 (0.925–1.538) 0.174 | |
| Q2 | 2.166 (1.572–2.986) < 0.001 | 1.858 (1.420–2.431) < 0.001 | 1.601 (1.197–2.141) 0.002 | 1.484 (1.165–1.890) 0.001 | |
| Q1 | 2.700 (1.962–3.717) < 0.001 | 2.514 (1.940–3.258) < 0.001 | 1.531 (1.134–2.067) 0.005 | 1.642 (1.291–2.089) < 0.001 | |
| p value for linear trend | <0.001 | <0.001 | 0.01 | <0.001 | |
| WC-relative | Q4 | Reference | Reference | Reference | Reference |
| Q3 | 1.694 (1.229–2.335) 0.001 | 1.517 (1.156–1.991) 0.003 | 1.670 (1.258–2.217) < 0.001 | 1.006 (0.780–1.299) 0.962 | |
| Q2 | 2.109 (1.536–2.896) < 0.001 | 1.743 (1.333–2.279) < 0.001 | 1.459 (1.092–1.950) 0.011 | 1.368 (1.076–1.739) 0.011 | |
| Q1 | 2.242 (1.613–3.115) < 0.001 | 2.315 (1.783–3.006) < 0.001 | 1.485 (1.100–2.004) 0.010 | 1.460 (1.144–1.862) 0.002 | |
| p value for linear trend | <0.001 | <0.001 | 0.03 | <0.001 | |
| Standardized Delta Change of HGS | Women | Men | ||
|---|---|---|---|---|
| HR (95% CI) p * | HR (95% CI) p § | HR (95% CI) p * | HR (95% CI) p § | |
| Δ Mean (kg) | 0.971 (0.865–1.091) 0.624 | 0.918 (0.816–1.031) 0.150 | 1.048 (0.946–1.160) 0.370 | 1.058 (0.953–1.176) 0.290 |
| Δ BMI-relative | 1.131 (1.009–1.269) 0.035 | 1.034 (0.916–1.168) 0.585 | 1.173 (1.060–1.297) 0.002 | 1.148 (1.033–1.276) 0.010 |
| Δ Weight-relative | 1.127 (1.003–1.267) 0.045 | 1.025 (0.907–1.159) 0.692 | 1.216 (1.099–1.345) < 0.001 | 1.174 (1.057–1.304) 0.003 |
| Δ WC-relative | 1.160 (1.033–1.302) 0.012 | 1.064 (0.942–1.201) 0.318 | 1.158 (1.046–1.283) 0.005 | 1.160 (1.041–1.291) 0.007 |
| Standardized Delta Change in HGS | Women | Men | |||
|---|---|---|---|---|---|
| HR (95% CI) p * | HR (95% CI) p § | HR (95% CI) p * | HR (95% CI) p § | ||
| Δ Mean (kg) | Q1 | 1.105 (0.799–1.529) 0.545 | 1.181 (0.849–1.643) 0.323 | 1.425 (1.035–1.960) 0.030 | 1.349 (0.973–1.870) 0.073 |
| Q2 | 0.762 (0.536–1.082) 0.128 | 0.749 (0.526–1.068) 0.110 | 1.389 (1.011–1.907) 0.043 | 1.192 (0.864–1.646) 0.285 | |
| Q3 | Reference | Reference | Reference | Reference | |
| Q4 | 0.927 (0.661–1.301) 0.662 | 0.970 (0.689–1.365) 0.861 | 1.333 (0.967–1.837) 0.080 | 1.325 (0.959–1.832) 0.089 | |
| Q5 | 0.864 (0.612–1.221) 0.408 | 0.776 (0.547–1.100) 0.155 | 1.560 (1.136–2.142) 0.006 | 1.500 (1.080–2.085) 0.016 | |
| Δ BMI-relative | Q1 | 0.938 (0.658–1.337) 0.725 | 1.184 (0.825–1.699) 0.359 | 1.057 (0.769–1.454) 0.732 | 1.102 (0.798–1.523) 0.556 |
| Q2 | 1.105 (0.784–1.559) 0.567 | 1.117 (0.789–1.581) 0.533 | 1.082 (0.789–1.485) 0.625 | 1.155 (0.840–1.589) 0.376 | |
| Q3 | Reference | Reference | Reference | Reference | |
| Q4 | 1.417 (1.006–1.997) 0.046 | 1.332 (0.942–1.885) 0.105 | 1.342 (0.986–1.826) 0.061 | 1.335 (0.978–1.821) 0.069 | |
| Q5 | 1.305 (0.913–1.865) 0.144 | 1.258 (0.874–1.811) 0.217 | 1.504 (1.101–2.054) 0.010 | 1.502 (1.091–2.067) 0.013 | |
| Δ Weight-relative | Q1 | 0.842 (0.592–1.197) 0.337 | 1.028 (0.719–1.472) 0.878 | 1.066 (0.775–1.467) 0.693 | 1.157 (0.838–1.598) 0.376 |
| Q2 | 1.005 (0.715–1.413) 0.977 | 0.993 (0.705–1.399) 0.966 | 1.057 (0.768–1.454) 0.734 | 1.132 (0.821–1.560) 0.450 | |
| Q3 | Reference | Reference | Reference | Reference | |
| Q4 | 1.228 (0.872–1.730) 0.240 | 1.131 (0.796–1.606) 0.493 | 1.522 (1.119–2.069) 0.007 | 1.486 (1.089–2.029) 0.013 | |
| Q5 | 1.313 (0.923–1.866) 0.130 | 0.869 (0.612–1.234) 0.433 | 1.531 (1.117–2.099) 0.008 | 1.530 (1.107–2.114) 0.010 | |
| Δ WC-relative | Q1 | 0.719 (0.510–1.015) 0.061 | 0.836 (0.594–1.175) 0.303 | 1.130 (0.824–1.550) 0.448 | 1.044 (0.756–1.443) 0.792 |
| Q2 | 0.812 (0.579–1.139) 0.227 | Reference | 1.100 (0.801–1.510) 0.557 | 1.028 (0.744–1.421) 0.866 | |
| Q3 | Reference | 1.160 (0.831–1.617) 0.383 | Reference | Reference | |
| Q4 | 1.134 (0.817–1.574) 0.452 | 1.007 (0.710–1.428) 0.968 | 1.378 (1.010–1.881) 0.043 | 1.292 (0.944–1.768) 0.109 | |
| Q5 | 1.108 (0.785–1.564) 0.560 | 1.181 (0.849–1.643) 0.323 | 1.524 (1.114–2.085) 0.008 | 1.445 (1.048–1.992) 0.025 | |
| WC | HDL | TG | HTN | DM | |
|---|---|---|---|---|---|
| HR (95% CI) p * | HR (95% CI) p * | HR (95% CI) p * | HR (95% CI) p * | HR (95% CI) p * | |
| Women | |||||
| HGS (kg) | 1.040 (0.913–1.185) 0.554 | 0.902 (0.796–1.021) 0.103 | 0.924 (0.835–1.022) 0.124 | 1.046 (0.940–1.163) 0.409 | 0.818 (0.702–0.953) 0.010 |
| BMI-relative HGS | 0.579 (0.503–0.667) <0.001 | 0.875 (0.773–0.990) 0.034 | 0.829 (0.747–0.919) <0.001 | 0.826 (0.739–0.923) 0.001 | 0.650 (0.552–0.765) <0.001 |
| Weight-relative HGS | 0.530 (0.462–0.608) <0.001 | 0.880 (0.778–0.995) 0.042 | 0.846 (0.765–0.937) 0.001 | 0.833 (0.748–0.928) 0.001 | 0.693 (0.592–0.811) <0.001 |
| WC-relative HGS | 0.647 (0.562–0.745) <0.001 | 0.861 (0.759–0.976) 0.020 | 0.833 (0.748–0.927) 0.001 | 0.841 (0.751–0.942) 0.003 | 0.655 (0.556–0.772) <0.001 |
| BMS (kg) | 1.088 (0.962–1.231) 0.180 | 0.924 (0.819–1.043) 0.202 | 0.957 (0.869–1.054) 0.373 | 1.028 (0.932–1.134) 0.579 | 0.916 (0.792–1.059) 0.235 |
| BMI-relative BMS | 0.740 (0.646–0.849) <0.001 | 0.901 (0.798–1.018) 0.094 | 0.879 (0.797–0.970) 0.010 | 0.893 (0.804–0.991) 0.033 | 0.772 (0.660–0.904) 0.001 |
| Weight-relative BMS | 0.711 (0.621–0.813) <0.001 | 0.907 (0.804–1.024) 0.114 | 0.900 (0.817–0.993) 0.035 | 0.905 (0.817–1.003) 0.056 | 0.820 (0.705–0.955) 0.011 |
| WC-relative BMS | 0.820 (0.717–0.938) 0.004 | 0.900 (0.796–1.018) 0.094 | 0.892 (0.808–0.986) 0.026 | 0.911 (0.820–1.012) 0.082 | 0.800 (0.684–0.935) 0.005 |
| Men | |||||
| HGS (kg) | 1.073 (0.805–1.430) 0.632 | 0.963 (0.875–1.060) 0.441 | 1.007 (0.907–1.119) 0.891 | 0.927 (0.843–1.018) 0.114 | 0.925 (0.824–1.037) 0.182 |
| BMI-relative HGS | 0.416 (0.302–0.572) <0.001 | 0.813 (0.739–0.894) <0.001 | 0.870 (0.787–0.962) 0.007 | 0.793 (0.723–0.869) <0.001 | 0.689 (0.612–0.775) <0.001 |
| Weight-relative HGS | 0.322 (0.237–0.437) <0.001 | 0.826 (0.754–0.905) <0.001 | 0.861 (0.781–0.949) 0.003 | 0.780 (0.712–0.854) <0.001 | 0.691 (0.617–0.774) <0.001 |
| WC-relative HGS | 0.448 (0.329–0.610) <0.001 | 0.826 (0.751–0.909) <0.001 | 0.863 (0.780–0.954) 0.004 | 0.812 (0.740–0.891) <0.001 | 0.700 (0.622–0.787) <0.001 |
| BMS (kg) | 1.268 (0.967–1.663) 0.086 | 1.054 (0.963–1.153) 0.251 | 1.015 (0.919–1.120) 0.772 | 0.942 (0.861–1.031) 0.196 | 1.093 (0.982–1.216) 0.103 |
| BMI-relative BMS | 0.665 (0.502–0.882) 0.005 | 0.941 (0.862–1.027) 0.174 | 0.924 (0.840–1.015) 0.100 | 0.847 (0.776–0.923) <0.001 | 0.890 (0.802–0.988) 0.028 |
| Weight-relative BMS | 0.560 (0.421–0.745) <0.001 | 0.954 (0.876–1.040) 0.290 | 0.917 (0.836–1.005) 0.065 | 0.841 (0.771–0.917) <0.001 | 0.894 (0.807–0.991) 0.033 |
| WC-relative BMS | 0.698 (0.527–0.925) 0.012 | 0.953 (0.872–1.041) 0.287 | 0.916 (0.832–1.008) 0.071 | 0.859 (0.787–0.938) 0.001 | 0.904 (0.813–1.004) 0.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.J.; Lee, S.K.; Shin, C. Relative Hand Grip and Back Muscle Strength, but Not Mean Muscle Strength, as Risk Factors for Incident Metabolic Syndrome and Its Metabolic Components: 16 Years of Follow-Up in a Population-Based Cohort Study. Appl. Sci. 2021, 11, 5198. https://doi.org/10.3390/app11115198
Jeon YJ, Lee SK, Shin C. Relative Hand Grip and Back Muscle Strength, but Not Mean Muscle Strength, as Risk Factors for Incident Metabolic Syndrome and Its Metabolic Components: 16 Years of Follow-Up in a Population-Based Cohort Study. Applied Sciences. 2021; 11(11):5198. https://doi.org/10.3390/app11115198
Chicago/Turabian StyleJeon, Yoo Jeong, Seung Ku Lee, and Chol Shin. 2021. "Relative Hand Grip and Back Muscle Strength, but Not Mean Muscle Strength, as Risk Factors for Incident Metabolic Syndrome and Its Metabolic Components: 16 Years of Follow-Up in a Population-Based Cohort Study" Applied Sciences 11, no. 11: 5198. https://doi.org/10.3390/app11115198
APA StyleJeon, Y. J., Lee, S. K., & Shin, C. (2021). Relative Hand Grip and Back Muscle Strength, but Not Mean Muscle Strength, as Risk Factors for Incident Metabolic Syndrome and Its Metabolic Components: 16 Years of Follow-Up in a Population-Based Cohort Study. Applied Sciences, 11(11), 5198. https://doi.org/10.3390/app11115198






