Force–Time Characteristics of Dynamic and Isometric Muscle Actions: Association with Muscle Architecture in Female Athletes
Abstract
:1. Introduction
2. Materials and Methods
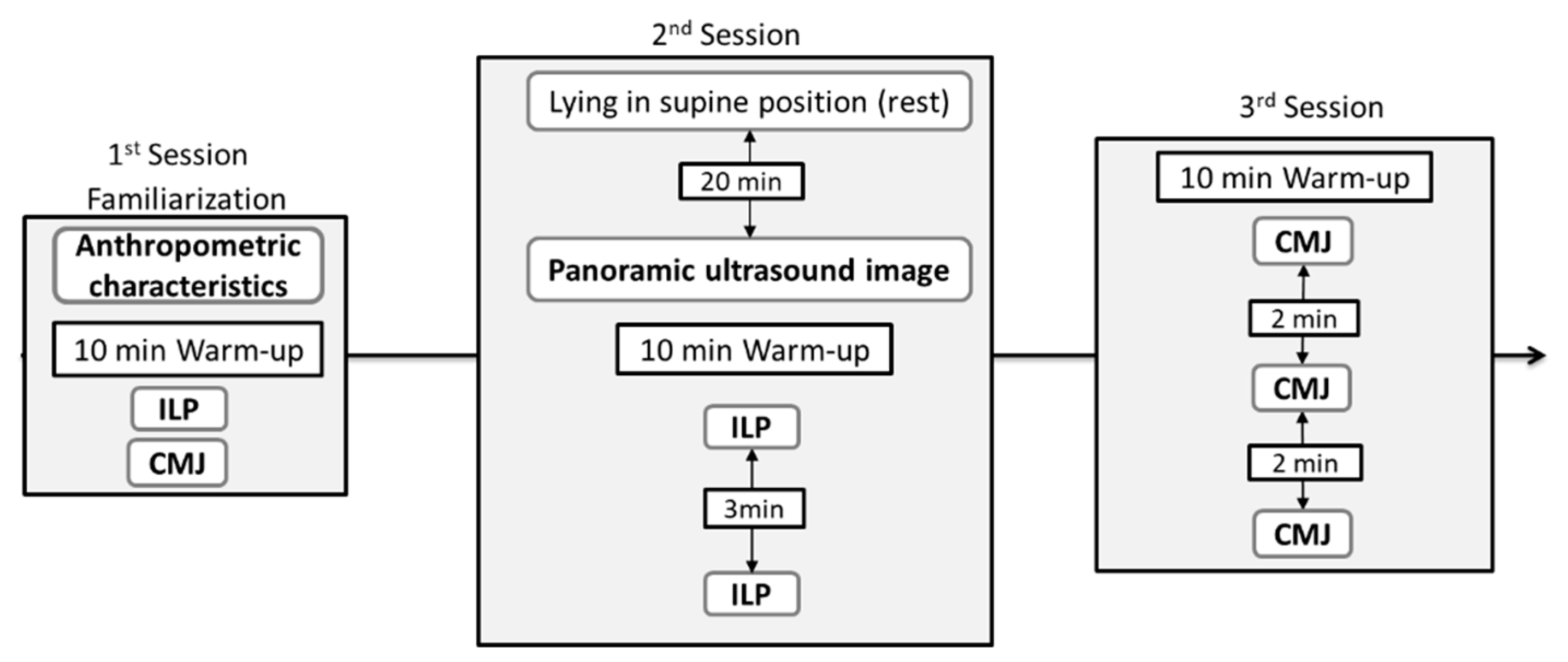
2.1. Experimental Design
2.2. Subjects
2.3. Procedures
2.3.1. Anthropometry
2.3.2. Muscle Architecture and Ultrasound
2.3.3. Isometric Leg Press Performance
2.3.4. Countermovement Jump Performance
2.4. Statistical Analysis
3. Results
3.1. Correlations between Jumping and Isometric Force–Time Parameters
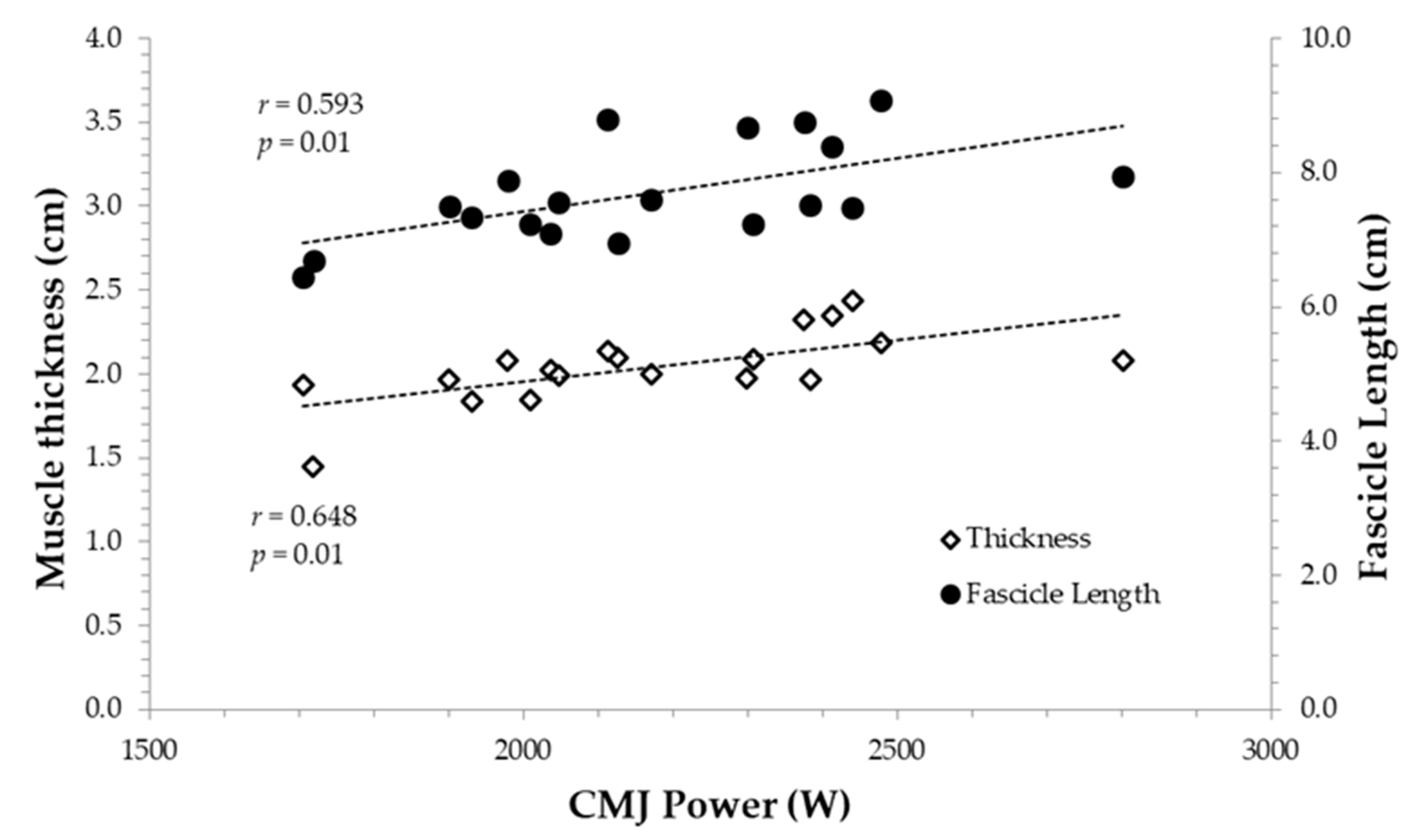
3.2. Correlations between Muscle Architecture and Jumping Parameters
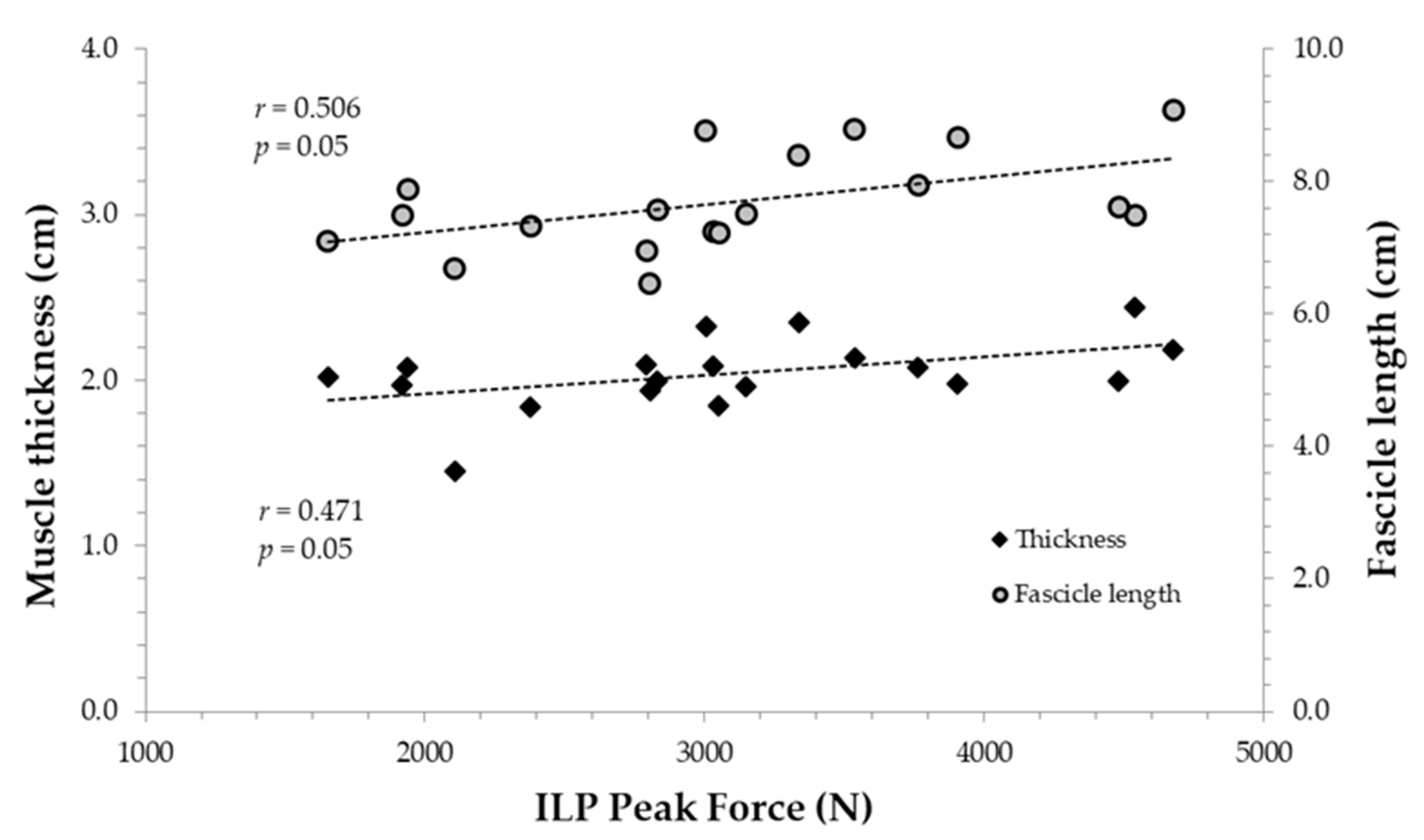
3.3. Correlations between Muscle Architecture and Isometric Parameters
3.4. Stepwise Regression Analysis for Countermovement Jump Power
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suchomel, T.J.; Nimphius, S.; Stone, M.H. The importance of muscular strength in athletic performance. Sports Med. 2016, 46, 1419–1449. [Google Scholar] [CrossRef]
- Stone, M.H.; Sanborn, K.; O’Bryant, H.S.; Hartman, M.; Stone, M.E.; Proulx, C.; Ward, B.; Hruby, J. Maximum strength-power-performance relationships in collegiate throwers. J. Strength Cond. Res. 2003, 17, 739–745. [Google Scholar] [CrossRef]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Aagaard, P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur. J. Appl. Physiol. 2006, 96, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Folland, J.P.; Buckthorpe, M.W.; Hannah, R. Human capacity for explosive force production: Neural and contractile determinants. Scand. J. Med. Sci. Sport. 2014, 24, 894–906. [Google Scholar] [CrossRef]
- Tillin, N.A.; Jimenez-Reyes, P.; Pain, M.T.; Folland, J.P. Neuromuscular performance of explosive power athletes versus untrained individuals. Med. Sci. Sports Exerc. 2010, 42, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.J.; Kent-Braun, J.A.; Ng, A.V.; Miller, R.G.; Dudley, G.A. Muscle fiber type-specific myofibrillar actomyosin Ca2+ ATPase activity in multiple sclerosis. Muscle Nerve 1998, 21, 547–549. [Google Scholar] [CrossRef]
- Coratella, G.; Longo, S.; Borrelli, M.; Doria, C.; Cè, E.; Esposito, F. Vastus intermedius muscle architecture predicts the late phase of the knee extension rate of force development in recreationally resistance-trained men. J. Sci. Med. Sport 2020, 23, 1100–1104. [Google Scholar] [CrossRef]
- Baker, D. Acute and long-term power responses to power training: Observations on the training of an elite power athlete. Strength Cond. J. 2001, 23, 47–56. [Google Scholar] [CrossRef]
- Thorlund, J.B.; Michalsik, L.B.; Madsen, K.; Aagaard, P. Acute fatigue-induced changes in muscle mechanical properties and neuromuscular activity in elite handball players following a handball match. Scand. J. Med. Sci. Sport. 2008, 18, 462–472. [Google Scholar] [CrossRef]
- Stefanyshyn, D.; Nigg, B. Contribution of the lower extremity joints to mechanical energy in running vertical jumps and running long jumps. J. Sports Sci. 1998, 16, 177–186. [Google Scholar] [CrossRef]
- Taylor, M.J.D.; Beneke, R. Spring Mass Characteristics of the Fastest Men on Earth. Int. J. Sports Med. 2012, 33, 667. [Google Scholar] [CrossRef] [Green Version]
- Markovic, G.; Jaric, S. Is vertical jump height a body size-independent measure of muscle power? J. Sports Sci. 2007, 25, 1355–1363. [Google Scholar] [CrossRef]
- Tillin, N.A.; Pain, M.T.G.; Folland, J. Explosive force production during isometric squats correlates with athletic performance in rugby union players. J. Sports Sci. 2013, 31, 66–76. [Google Scholar] [CrossRef] [PubMed]
- West, D.J.; Owen, N.J.; Jones, M.R.; Bracken, R.M.; Cook, C.J.; Cunningham, D.J.; Shearer, D.A.; Finn, C.V.; Newton, R.U.; Crewther, B.T.; et al. Relationships between force–time characteristics of the isometric midthigh pull and dynamic performance in professional rugby league players. J. Strength Cond. Res. 2011, 25, 3070–3075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuigan, M.R.; Newton, M.J.; Winchester, J.B.; Nelson, A.G. Relationship between isometric and dynamic strength in recreationally trained men. J. Strength Cond. Res. 2010, 24, 2570–2573. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; McLean, B.; Ardagna, J. Relationship between strength qualities and sprinting performance. J. Sports Med. Phys. Fit. 1995, 35, 13–19. [Google Scholar]
- Kawamori, N.; Rossi, S.J.; Justice, B.D.; Haff, E.E.; Pistilli, E.E.; O’Bryant, H.S.; Stone, M.H.; Haff, G.G. Peak force and rate of force development during isometric and dynamic mid-thigh clean pulls performed at various intensities. J. Strength Cond. Res. 2006, 20, 483–491. [Google Scholar] [CrossRef]
- Marcora, S.; Miller, M.K. The effect of knee angle on the external validity of isometric measures of lower body neuro-muscular function. J. Sports Sci. 2000, 18, 313–319. [Google Scholar] [CrossRef]
- Haff, G.G.; Carlock, J.M.; Hartman, M.J.; Kilgore, J.L. Force-time curve characteristics of dynamic and isometric muscle actions of elite women olympic weightlifters. J. Strength Cond. Res. 2005, 19, 741. [Google Scholar] [CrossRef] [Green Version]
- Haff, G.G.; Stone, M.H.; O’Bryant, H.S.; Harman, E.; Dinan, C.N.; Johnson, R.; Han, K.H. Force-time dependent characteristics of dynamic and isometric muscle actions. J. Strength Cond. Res. 1997, 11, 269–272. [Google Scholar]
- Lum, D.; Haff, G.G.; Barbosa, T.M. The relationship between isometric force-time characteristics and dynamic performance: A systematic review. Sports 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.J.; Gill, N.D.; Zhou, S. Intra-and intermuscular variation in human quadriceps femoris architecture assessed in vivo. J. Anat. 2006, 209, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Harridge, S.D.R.; Bottinelli, R.; Canepari, M.; Pellegrino, M.A.; Reggiani, C.; Esbjörnsson, M.; Saltin, B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch. Eur. J. Physiol. 1996, 432, 913–920. [Google Scholar] [CrossRef]
- Blazevich, A.J.; Coleman, D.R.; Horne, S.; Cannavan, D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur. J. Appl. Physiol. 2009, 105, 869–878. [Google Scholar] [CrossRef]
- Kawakami, Y.; Ichinose, Y.; Kubo, K.; Ito, M.; Imai, M.; Fukunaga, T. Architecture of contracting human muscles and its functional significance. J. Appl. Biomech. 2000, 16, 88–97. [Google Scholar] [CrossRef]
- Muhl, Z.F. Active length-tension relation and the effect of muscle pennation on fiber lengthening. J. Morphol. 1982, 173, 285–292. [Google Scholar] [CrossRef]
- Kawakami, Y.; Abe, T.; Fukunaga, T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J. Appl. Physiol. 1993, 74, 2740–2744. [Google Scholar] [CrossRef]
- Seynnes, O.R.; de Boer, M.; Narici, M.V. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J. Appl. Physiol. 2007, 102, 368–373. [Google Scholar] [CrossRef]
- Sandbakk, O.; Ettema, G.; Leirdal, S.; Holmberg, H.C. Gender differences in the physiological responses and kinematic behaviour of elite sprint cross-country skiers. Eur. J. Appl. Physiol. 2012, 112, 1087–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Gender differences in the viscoelastic properties of tendon structures. Eur. J. Appl. Physiol. 2003, 88, 520–526. [Google Scholar] [CrossRef]
- Abe, T.; DeHoyos, D.V.; Pollock, M.L.; Garzarella, L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur. J. Appl. Physiol. 2000, 81, 174–180. [Google Scholar] [CrossRef]
- Panidi, I.; Bogdanis, G.C.; Gaspari, V.; Spiliopoulou, P.; Donti, A.; Terzis, G.; Donti, O. Gastrocnemius Medialis Architectural Properties in Flexibility Trained and Not Trained Child Female Athletes: A Pilot Study. Sports 2020, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of force development: Physiological and methodological considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef] [Green Version]
- Barker, L.A.; Harry, J.R.; Mercer, J.A. Relationships between countermovement jump ground reaction forces and jump height, reactive strength index, and jump time. J. Strength Cond. Res. 2018, 32, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Moir, G.L.; Getz, A.; Davis, S.E.; Marques, M.; Witmer, C.A. The Inter-Session Reliability of Isometric Force-Time Variables and the Effects of Filtering and Starting Force. J. Hum. Kinet. 2019, 66, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Moir, G.L. Three different methods of calculating vertical jump height from force platform data in men and women. Meas. Phys. Educ. Exerc. Sci. 2008, 12, 207–218. [Google Scholar] [CrossRef]
- Sayers, S.; Harackiewicz, D.; Harman, E.; Fryman, P.; Rosestein, M. Cross-validation of three jump power equations. Med. Sci. Sport. Exerc. 1999, 31, 572–577. [Google Scholar] [CrossRef]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzzo, J.L.; McBride, J.M.; Cormie, P.; McCaulley, G.O. Relationship between countermovement jump performance and multijoint isometric and dynamic tests of strength. J. Strength Cond. Res. 2008, 22, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; Wilson, G.; Byrne, C. Relationship between strength qualities and performance in standing and run-up vertical jumps. J. Sports Med. Phys. Fit. 1999, 39, 285. [Google Scholar]
- Zatsiorsky, V.M.; Kraemer, W.J. Science and Practice of Strength Training, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2006; pp. 155–160. [Google Scholar]
- Secomb, J.L.; Lundgren, L.E.; Farley, O.R.; Tran, T.T.; Nimphius, S.; Sheppard, J.M. Relationships between lower-body muscle structure and lower-body strength, power, and muscle-tendon complex stiffness. J. Strength Cond. Res. 2015, 29, 2221–2228. [Google Scholar] [CrossRef]
- McMahon, J.J.; Stapley, J.T.; Suchomel, T.J.; Comfort, P. Relationships between lower body muscle structure and isometric mid-thigh pull peak force. J. Trainology 2015, 4, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Zaras, N.; Stasinaki, A.N.; Spiliopoulou, P.; Arnaoutis, G.; Hadjicharalambous, M.; Terzis, G. Rate of Force Development, Muscle Architecture, and Performance in Elite Weightlifters. Int. J. Sports Physiol. Perform. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Mangine, G.T.; Fukuda, D.H.; LaMonica, M.B.; Gonzalez, A.M.; Wells, A.J.; Townsend, J.R.; Jajtner, A.R.; Fragala, M.S.; Stout, J.R.; Hoffman, J.R. Influence of gender and muscle architecture asymmetry on jump and sprint performance. J. Sport. Sci. Med. 2014, 13, 904–911. [Google Scholar]
- Alegre, L.M.; Lara, A.J.; Elvira, J.L.L.; Aguado, X. Muscle morphology and jump performance: Gender and intermuscular variability. J. Sports Med. Phys. Fit. 2009, 49, 320. [Google Scholar]
- Bartolomei, S.; Rovai, C.; Lanzoni, I.M.; di Michele, R. Relationships Between Muscle Architecture, Deadlift Performance, and Maximal Isometric Force Produced at the Midthigh and Midshin Pull in Resistance-Trained Individuals. J. Strength Cond. Res. 2019. [Google Scholar] [CrossRef]
- Methenitis, S.K.; Zaras, N.D.; Spengos, K.M.; Stasinaki, A.N.E.; Karampatsos, G.P.; Georgiadis, G.V.; Terzis, G.D. Role of muscle morphology in jumping, sprinting, and throwing performance in participants with different power training duration experience. J. Strength Cond. Res. 2016, 30, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Alegre, L.M.; Aznar, D.; Delgado, T.; Jimenez, F.; Aguado, X. Architectural characteristics of vastus lateralis muscle and jump performance in young men. J. Hum. Mov. Stud. 2005, 48, 109–124. [Google Scholar]
- Earp, J.E.; Kraemer, W.J.; Newton, R.U.; Comstock, B.A.; Fragala, M.S.; Dunn-Lewis, C.; Solomon-Hill, G.; Penwell, Z.R.; Powell, M.D.; Volek, J.S.; et al. Lower-Body Muscle Structure and Its Role in Jump Performance During Squat, Countermovement, and Depth Drop Jumps. J. Strength Cond. Res. 2010, 24, 722–729. [Google Scholar] [CrossRef]
- Ruiz-Cárdenas, J.D.; Rodriguez-Juan, J.J.; Rios-Diaz, J. Relationship between jumping abilities and skeletal muscle architecture of lower limbs in humans: Systematic review and meta-analysis. Hum. Mov. Sci. 2018, 58, 10–20. [Google Scholar] [CrossRef]
- Moreau, N.G.; Falvo, M.J.; Damiano, D.L. Rapid force generation is impaired in cerebral palsy and is related to decreased muscle size and functional mobility. Gait Posture 2012, 35, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Finni, T.; Ikegawa, S.; Komi, P.V. Concentric force enhancement during human movement. Acta Physiol. Scand. 2001, 173, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, B.; Zolotarjova, J. The difference between countermovement and squat jump performances: A review of underlying mechanisms with practical applications. J. Strength Cond. Res. 2017, 31, 2011–2020. [Google Scholar] [CrossRef] [Green Version]
- Alkner, B.A.; Tesch, P.A.; Berg, H.E. Quadriceps EMG/force relationship in knee extension and leg press. Med. Sci. Sports Exerc. 2000, 32, 459–463. [Google Scholar] [CrossRef]
- Wilson, G.; Murphy, A. The efficacy of isokinetic, isometric and vertical jump tests in exercise science. Aust. J. Sci. Med. Sport 1995, 27, 20–24. [Google Scholar]

| Mean | Sd | |
|---|---|---|
| Fascicle length (m) | 0.077 | 0.007 |
| Pennation angle (ο) | 15.528 | 1.435 |
| Thickness (m) | 0.020 | 0.002 |
| ILP-PF (N) | 3099.805 | 871.435 |
| ILP-Average time to PF (s) | 1.438 | 0.751 |
| ILP-RFD50 ms (N × s−1) | 7635.278 | 4479.910 |
| ILP-RFD100 ms (N × s−1) | 10,430.095 | 4021.079 |
| ILP-RFD150 ms (N × s−1) | 10,080.549 | 3128.380 |
| ILP-RFD200 ms (N × s−1) | 9009.969 | 2605.143 |
| ILP-RFD250 ms (N × s−1) | 7988.025 | 2207.825 |
| ILP-ARFD (N × s−1) | 2736.056 | 2344.847 |
| CMJ height (m) | 0.232 | 0.027 |
| CMJ power (W) | 2170.279 | 271.258 |
| CMJ-PF (N) | 1452.654 | 137.890 |
| CMJ-average time to PF (s) | 0.243 | 0.056 |
| CMJ-RFD50 ms (N × s−1) | 2515.491 | 1360.157 |
| CMJ-RFD100 ms (N × s−1) | 4324.661 | 2739.108 |
| CMJ-RFD150 ms (N × s−1) | 5369.340 | 2744.600 |
| CMJ-ARFD (N × s−1) | 3767.976 | 3055.164 |
| ILP-PF | ILP-RFD50 ms | ILP-RFD100 ms | ILP-RFD150 ms | ILP-RFD200 ms | ILP-RFD250 ms | ILP-ARFD | |
|---|---|---|---|---|---|---|---|
| CMJ height | 0.338 | 0.022 | −0.026 | 0.020 | 0.045 | 0.055 | −0.070 |
| CMJ power | 0.658 ** | 0.181 | 0.177 | 0.295 | 0.305 | 0.293 | −0.063 |
| CMJ-PF | 0.140 | 0.297 | 0.368 | 0.371 | 0.348 | 0.328 | 0.477 * |
| CMJ-RFD50 ms | 0.087 | 0.384 | 0.399 | 0.433 | 0.423 | 0.397 | 0.399 |
| CMJ-RFD100 ms | 0.085 | 0.502 * | 0.533 * | 0.505 * | 0.499 * | 0.477 * | 0.520 * |
| CMJ-RFD150 ms | 0.002 | 0.533 * | 0.561 * | 0.498 * | 0.468 * | 0.425 | 0.447 |
| CMJ-ARFD | −0.133 | 0.074 | 0.276 | 0.315 | 0.320 | 0.343 | 0.625 ** |
| Fascicle Length | Pennation Angle | Thickness | |
|---|---|---|---|
| CMJ height | 0.307 | 0.330 | 0.523 * |
| CMJ-PF | 0.423 | −0.085 | 0.317 |
| CMJ-RFD50 ms | 0.369 | −0.297 | 0.072 |
| CMJ-RFD100 ms | 0.365 | −0.145 | 0.128 |
| CMJ-RFD150 ms | 0.295 | −0.097 | 0.114 |
| CMJ-ARFD | −0.073 | 0.068 | −0.012 |
| Fascicle Length | Pennation Angle | Thickness | |
|---|---|---|---|
| ILP-RFD50 ms | 0.584 ** | −0.240 | 0.252 |
| ILP-RFD100 ms | 0.606 ** | −0.117 | 0.290 |
| ILP-RFD150 ms | 0.646 ** | −0.063 | 0.347 |
| ILP-RFD200 ms | 0.612 ** | 0.018 | 0.375 |
| ILP-RFD250 ms | 0.565 * | 0.036 | 0.364 |
| ILP-ARFD | 0.368 | −0.234 | 0.105 |
| 95%CI | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Β | SE | Beta | T | Sig | Lower | Upper |
| Fascicle length | 76.654 | 76.987 | 0.204 | 0.996 | 0.335 | −87.349 | 240.748 |
| ILP-PF | 0.121 | 0.061 | 0.389 | 1.993 | 0.065 | −0.008 | 0.250 |
| Thickness | 460.320 | 260.931 | 0.354 | 1.764 | 0.098 | −95.841 | 1016.481 |
| ILP-PF | 0.141 | 0.774 | 0.454 | 2.475 | 0.025 | 0.20 | 0.263 |
| Thickness | 564.804 | 238.836 | 0.434 | 2.365 | 0.031 | 58.495 | 1071.112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspari, V.; Bogdanis, G.C.; Panidi, I.; Giannakopoulou, G.; Terzis, G.; Kotsala, H.; Donti, A.; Donti, O. Force–Time Characteristics of Dynamic and Isometric Muscle Actions: Association with Muscle Architecture in Female Athletes. Appl. Sci. 2021, 11, 5272. https://doi.org/10.3390/app11115272
Gaspari V, Bogdanis GC, Panidi I, Giannakopoulou G, Terzis G, Kotsala H, Donti A, Donti O. Force–Time Characteristics of Dynamic and Isometric Muscle Actions: Association with Muscle Architecture in Female Athletes. Applied Sciences. 2021; 11(11):5272. https://doi.org/10.3390/app11115272
Chicago/Turabian StyleGaspari, Vasiliki, Gregory C. Bogdanis, Ioli Panidi, Georgia Giannakopoulou, Gerasimos Terzis, Helen Kotsala, Anastasia Donti, and Olyvia Donti. 2021. "Force–Time Characteristics of Dynamic and Isometric Muscle Actions: Association with Muscle Architecture in Female Athletes" Applied Sciences 11, no. 11: 5272. https://doi.org/10.3390/app11115272
APA StyleGaspari, V., Bogdanis, G. C., Panidi, I., Giannakopoulou, G., Terzis, G., Kotsala, H., Donti, A., & Donti, O. (2021). Force–Time Characteristics of Dynamic and Isometric Muscle Actions: Association with Muscle Architecture in Female Athletes. Applied Sciences, 11(11), 5272. https://doi.org/10.3390/app11115272








