Use of Immunofluorescence Technique to Perform a Quantitative Analysis of Masseter Muscle Fibers in Unilateral Posterior Crossbite: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Ethics
2.2. Muscle Biopsies
2.3. Immunofluorescence
2.4. Image Analysis
ImageJ Application
2.5. Statistical Analysis
3. Results
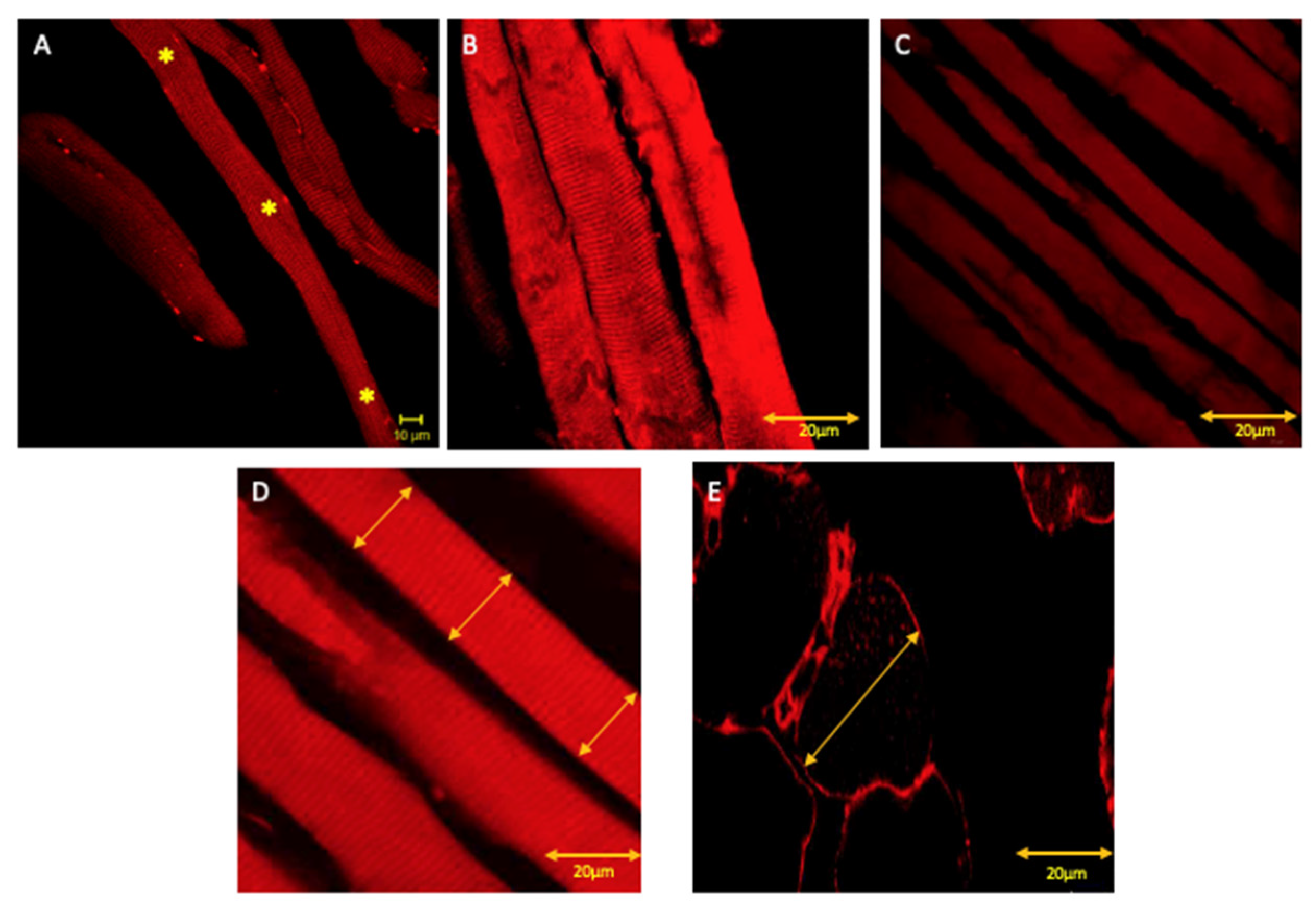
3.1. Immunofluorescence
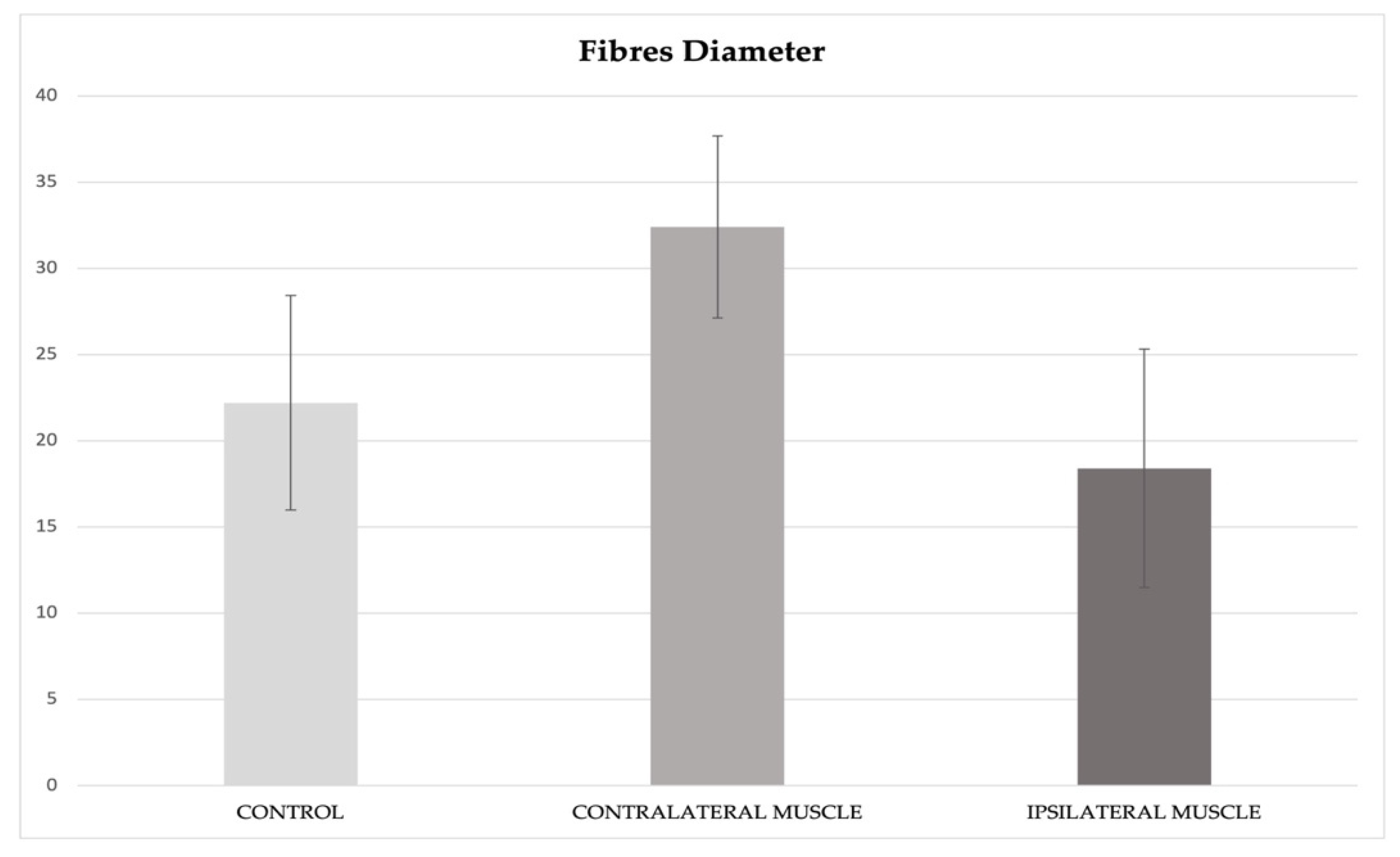
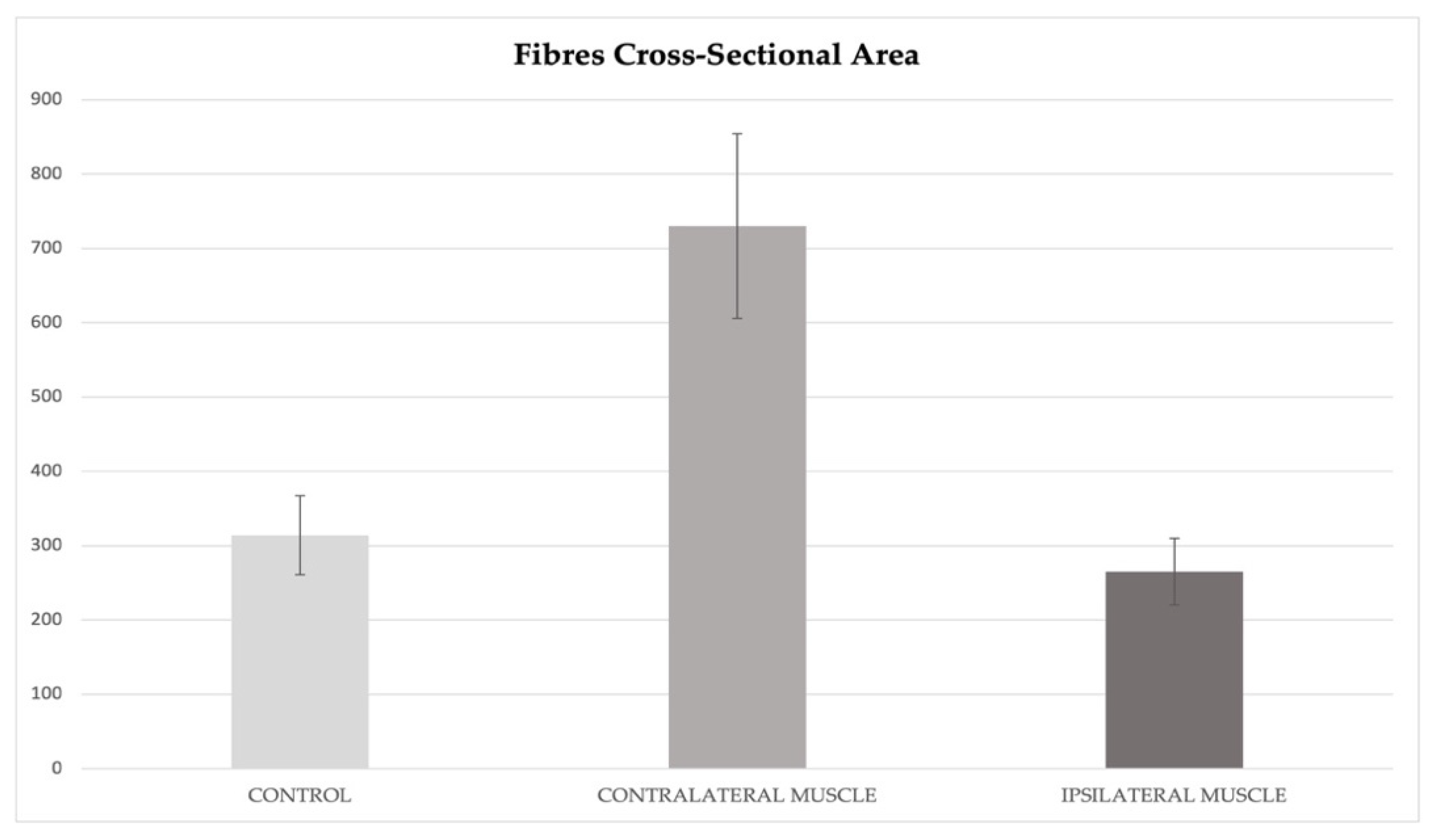
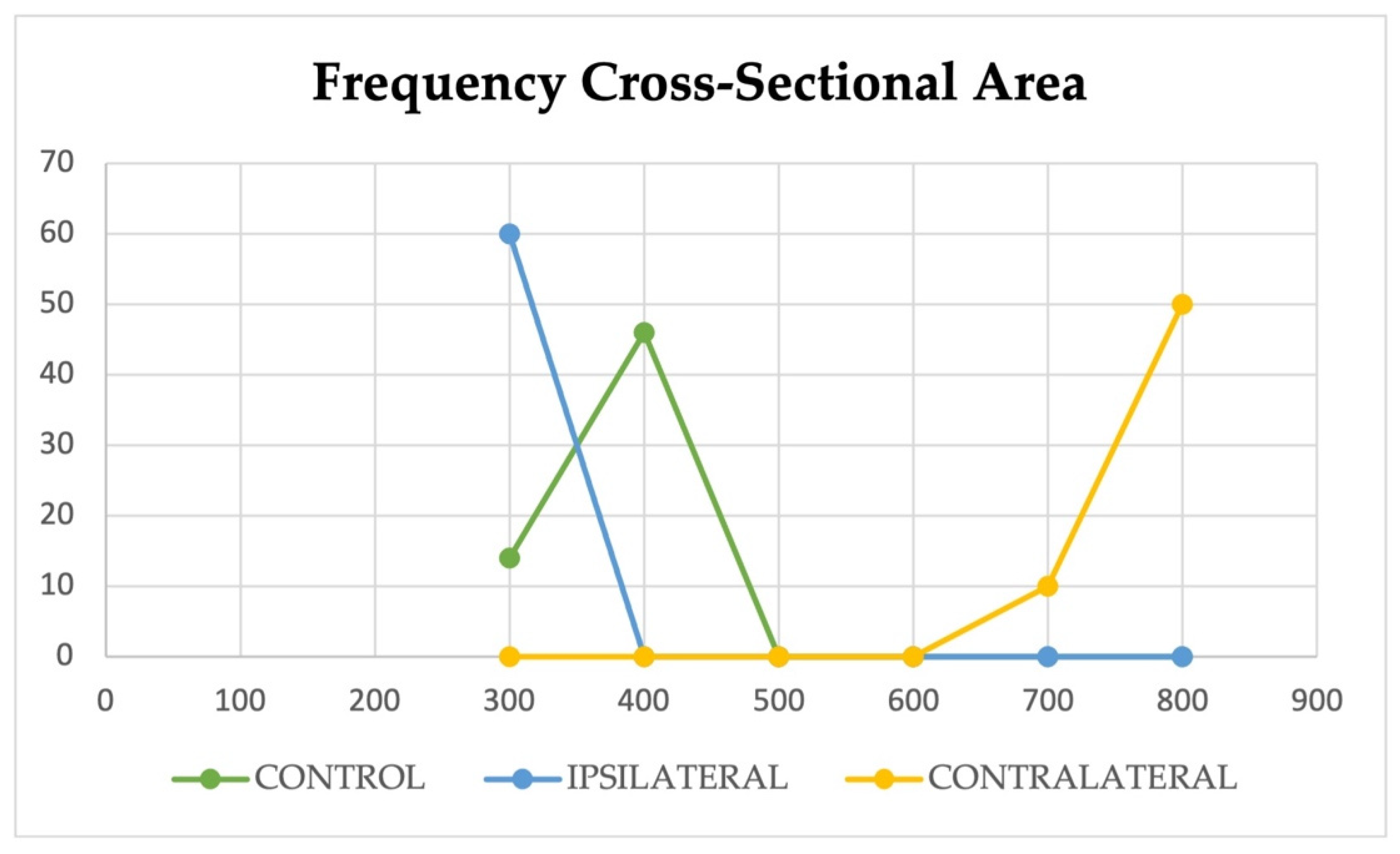
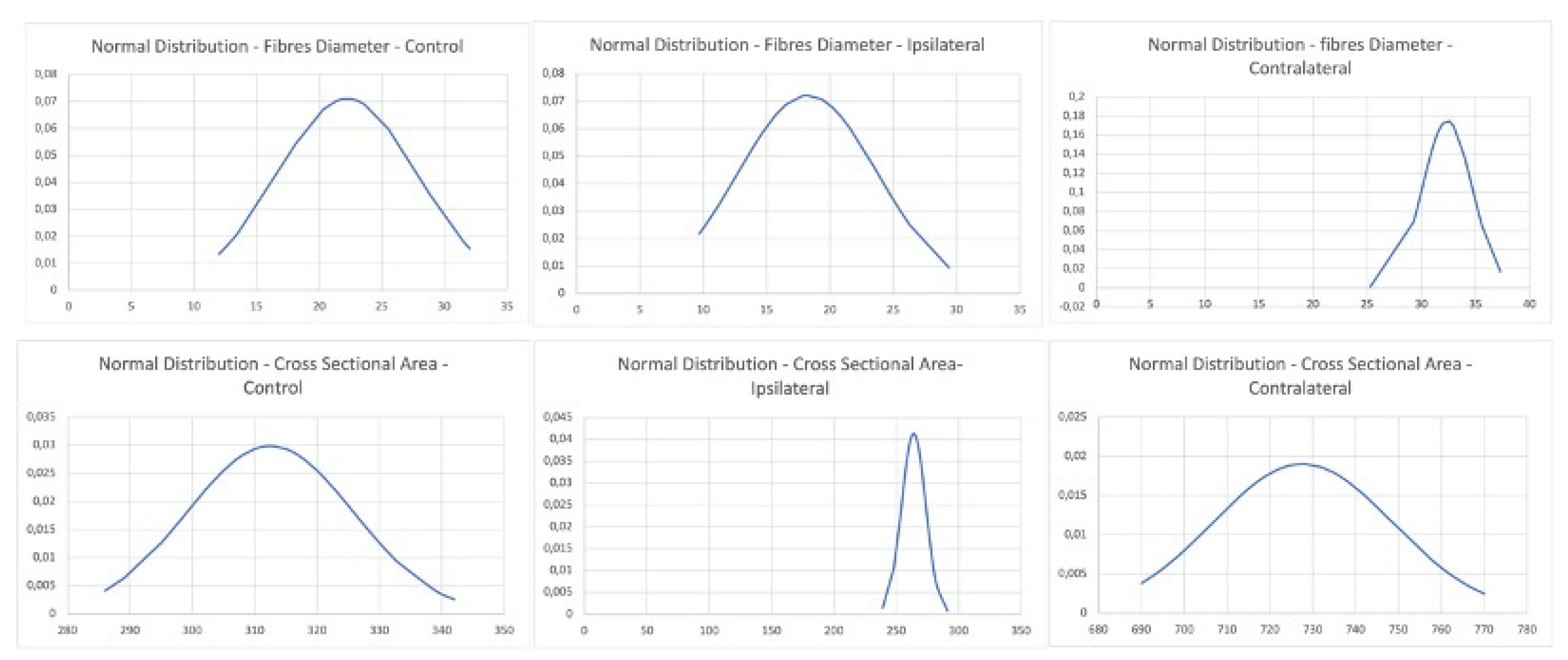
3.2. ImageJ and Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piancino, M.G.; Farina, D.; Talpone, F.; Merlo, A.; Bracco, P. Muscular activation during reverse and non-reverse chewing cycles in unilateral posterior crossbite. Eur. J. Oral Sci. 2009, 117, 122–128. [Google Scholar] [CrossRef]
- Piancino, M.G.; Comino, E.; Talpone, F.; Vallelonga, T.; Frongia, G.; Bracco, P. Reversesequencing chewing patterns evaluation in anterior versus posterior unilateral crossbite patients. Eur. J. Orthod. 2012, 34, 536–541. [Google Scholar] [CrossRef]
- Bani, D.; Bani, T.; Bergamini, M. Morphologic and biochemical changes of the masseter muscles induced by occlusal wear: Studies in a rat model. J. Dent. Res. 1999, 78, 1735–1744. [Google Scholar] [CrossRef]
- De Souza Guerra, C.; Pereira, C.L.; Mardegan Issa, J.P.; Galisteu Luiz, K.; Del Bel Guimarães, E.A.; Gerlach, R.F.; Iyomasa, M. Histological, histochemical, and protein changes after induced malocclusion by occlusion alteration of Wistar rats. Biomed. Res. Int. 2014, 2014, 563463. [Google Scholar] [CrossRef]
- Iodice, G.; Danzi, G.; Cimino, R.; Paduano, S.; Michelotti, A. Association between posterior crossbite, skeletal, and muscle asymmetry: A systematic review. Eur. J. Orthod. 2016, 38, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, G.; Vermiglio, G.; Centofanti, A.; Rizzo, G.; Runci, M.; Favaloro, A.; Piancino, M.G.; Bracco, P.; Ramieri, G.; Bianchi, F.; et al. Morphofunctional compensation of masseter muscles in unilateral posterior crossbite patients. Eur. J. Histochem. 2016, 60, 2605. [Google Scholar] [CrossRef]
- Cutroneo, G.; Piancino, M.G.; Ramieri, G.; Bracco, P.; Vita, G.; Isola, G.; Vermiglio, G.; Favaloro, A.; Anastasi, G.; Trimarchi, F. Expression of muscle-specific integrins in masseter muscle fibers during malocclusion disease. Int. J. Mol. Med. 2012, 30, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Vermiglio, G.; Centofanti, A.; Ramieri, G.; Tepedino, M.; Runci Anastasi, M.; Micali, A.G.; Arco, A.; Piancino, M.G. Immunofluorescence Evaluation of Myf5 and MyoD in Masseter Muscle of Unilateral Posterior Crossbite Patients. J. Funct. Morphol. Kinesiol. 2020, 5, 80. [Google Scholar] [CrossRef]
- De Ponte, F.S.; Cutroneo, G.; Falzea, G.; Rizzo, G.; Catalfamo, L.; Favaloro, A.; Vermiglio, G.; Runci, M.; Centofanti, A.; Anastasi, G. Histochemical and morphological aspects of fresh frozen bone: A preliminary study. Eur. J. Histochem. 2016, 60, 2642. [Google Scholar] [CrossRef]
- De Ponte, F.S.; Favaloro, A.; Siniscalchi, E.; Centofanti, A.; Runci, M.; Cutroneo, G.; Catalfamo, L. Sarcoglycans and integrins in bisphosphonate treatment: Immunohistochemical and scanning electron microscopy study. Oncol. Rep. 2013, 30, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- DE Ponte, F.S.; Catalfamo, L.; Micali, G.; Runci, M.; Cutroneo, G.; Vermiglio, G.; Centofanti, A.; Rizzo, G. Effect of bisphosphonates on the mandibular bone and gingival epithelium of rats without tooth extraction. Exp. Ther. Med. 2016, 11, 1678–1684. [Google Scholar] [CrossRef]
- Militi, A.; Cutroneo, G.; Favaloro, A.; Matarese, G.; Di Mauro, D.; Lauritano, F.; Centofanti, A.; Cervino, G.; Nicita, F.; Bramanti, A.; et al. An immunofluorescence study on VEGF and extracellular matrix proteins in human periodontal ligament during tooth movement. Heliyon 2019, 5, e02572. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saitoh, K.; Imamura, R.; Ishii, K.; Negishi, S.; Imamura, R.; Yamaguchi, M.; Kasai, K. Relationship between molar occlusion and masticatory movement in lateral deviation of the mandible. Am. J. Orthod. Dentofacial Orthop. 2017, 151, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Caroccia, F.; Moscagiuri, F.; Falconio, L.; Festa, F.; D’Attilio, M. Early Orthodontic Treatments of Unilateral Posterior Crossbite: A Systematic Review. J. Clin. Med. 2020, 10, 33. [Google Scholar] [CrossRef]
- Lopatienė, K.; Trumpytė, K. Relationship between unilateral posterior crossbite and mandibular asymmetry during late adolescence. Stomatologija 2018, 20, 90–95. [Google Scholar] [PubMed]
- Brizuela, M.; Palla, A.; Dilip, K.N. Posterior Crossbite Review; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Piancino, M.G.; Talpone, F.; Dalmasso, P.; Debernardi, C.; Lewin, A.; Bracco, P. Reversesequencing chewing patterns before and after treatment of children with a unilateral posterior crossbite. Eur. J. Orthod. 2006, 28, 480–484. [Google Scholar] [CrossRef]
- Nie, Q.; Kanno, Z.; Xu, T.; Lin, J.; Soma, K. Clinical study of frontal chewing patterns in various crossbite malocclusions. Am. J. Orthod. Dentofacial. Orthop. 2010, 138, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sever, E.; Marion, L.; Ovsenik, M. Relationship between masticatory cycle morphology and unilateral crossbite in the primary dentition. Eur. J. Orthod. 2011, 33, 620–627. [Google Scholar] [CrossRef]
- Zammit, P.; Heslop, L.; Hudon, L.; Rosenblatt, J.D.; Tajbakhsh, S.; Buckingham, M.; Beauchamp, J.R.; Partridge, A. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell. Res. 2002, 281, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef]
- Bruschetta, D.; Anastasi, G.; Andronaco, V.; Cascio, F.; Rizzo, G.; Di Mauro, D.; Bonanno, L.; Izzo, V.; Buda, D.; Vermiglio, G.; et al. Human calf muscles changes after strength training as revealed by diffusion tensor imaging. J. Sports Med. Phys. Fit. 2019, 59, 853–860. [Google Scholar] [CrossRef]
- Cutroneo, G.; Centofanti, A.; Speciale, F.; Rizzo, G.; Favaloro, A.; Santoro, G.; Bruschetta, D.; Milardi, D.; Micali, A.; Di Mauro, D.; et al. Sarcoglycan complex in masseter and sternocleidomastoid muscles of baboons: An immunohistochemical study. Eur. J. Histochem. 2015, 59, 2509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raoul, G.; Rowlerson, A.; Sciote, J.; Codaccioni, E.; Stevens, L.; Maurage, C.A.; Duhamel, A.; Ferri, J. Masseter myosin heavy chain composition varies with mandibular asymmetry. J. Craniofac. Surg. 2011, 22, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Ventura Spagnolo, E.; Mondello, C.; Di Mauro, D.; Vermiglio, G.; Asmundo, A.; Filippini, E.; Alibrandi, A.; Rizzo, G. Analysis on sarcoglycans expression as markers of septic cardiomyopathy in sepsis-related death. Int. J. Legal Med. 2018, 132, 1685–1692. [Google Scholar] [CrossRef]
- Arco, A.; Favaloro, A.; Gioffrè, M.; Santoro, G.; Speciale, F.; Vermiglio, G.; Cutroneo, G. Sarcoglycans in the normal and pathological breast tissue of humans: An immunohistochemical and molecular study. Cells Tissues Organs 2012, 195, 550–562. [Google Scholar] [CrossRef]
- Ringqvist, M. Size and distribution of histochemical fibre types in masseter muscle of adults with different states of occlusion. J. Neurol. Sci. 1974, 22, 429–438. [Google Scholar] [CrossRef]
- Sciote, J.J.; Horton, M.J.; Rowlerson, A.M.; Ferri, J.; Close, J.M.; Raoul, G. Human masseter muscle fiber type properties, skeletal malocclusions, and muscle growth factor expression. J. Oral Maxillofac. Surg. 2012, 70, 440–448. [Google Scholar] [CrossRef]
- Sciote, J.J.; Raoul, G.; Ferri, J.; Close, J.; Horton, M.J.; Rowlerson, A. Masseter function and skeletal malocclusion. Rev. Stomatol. Chir. Maxillofac. Chir. Orale 2013, 114, 79. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermiglio, G.; Piancino, M.; Runci Anastasi, M.; Picciolo, G.; Centofanti, A.; Santoro, G.; Malandrino, M.; Cutroneo, G.; Anastasi, G. Use of Immunofluorescence Technique to Perform a Quantitative Analysis of Masseter Muscle Fibers in Unilateral Posterior Crossbite: A Pilot Study. Appl. Sci. 2021, 11, 5350. https://doi.org/10.3390/app11125350
Vermiglio G, Piancino M, Runci Anastasi M, Picciolo G, Centofanti A, Santoro G, Malandrino M, Cutroneo G, Anastasi G. Use of Immunofluorescence Technique to Perform a Quantitative Analysis of Masseter Muscle Fibers in Unilateral Posterior Crossbite: A Pilot Study. Applied Sciences. 2021; 11(12):5350. https://doi.org/10.3390/app11125350
Chicago/Turabian StyleVermiglio, Giovanna, Mariagrazia Piancino, Michele Runci Anastasi, Giacomo Picciolo, Antonio Centofanti, Giuseppe Santoro, Mariachiara Malandrino, Giuseppina Cutroneo, and Giuseppe Anastasi. 2021. "Use of Immunofluorescence Technique to Perform a Quantitative Analysis of Masseter Muscle Fibers in Unilateral Posterior Crossbite: A Pilot Study" Applied Sciences 11, no. 12: 5350. https://doi.org/10.3390/app11125350
APA StyleVermiglio, G., Piancino, M., Runci Anastasi, M., Picciolo, G., Centofanti, A., Santoro, G., Malandrino, M., Cutroneo, G., & Anastasi, G. (2021). Use of Immunofluorescence Technique to Perform a Quantitative Analysis of Masseter Muscle Fibers in Unilateral Posterior Crossbite: A Pilot Study. Applied Sciences, 11(12), 5350. https://doi.org/10.3390/app11125350







