Evaluation of 3-Methylbutanoic Acid Methyl Ester as a Factor Influencing Flavor Cleanness in Arabica Specialty Coffee
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Green and Roasted Coffee Beans
2.3. Sensorial Evaluation for Chemometric Profiling
2.4. Preparation of Roasted Bean Extracts and GC–MS Analysis
2.5. GC–MS Data Processing, Multivariate Statistical Analysis, and Correlation Plots
2.6. Quantitative Analysis of 3-Methylbutanoic Acid Methyl Ester in Coffee Brews by GC–MS
2.7. Evaluation of the Sensorial Effect of 3-MBM in Coffee Brew
3. Results and Discussion
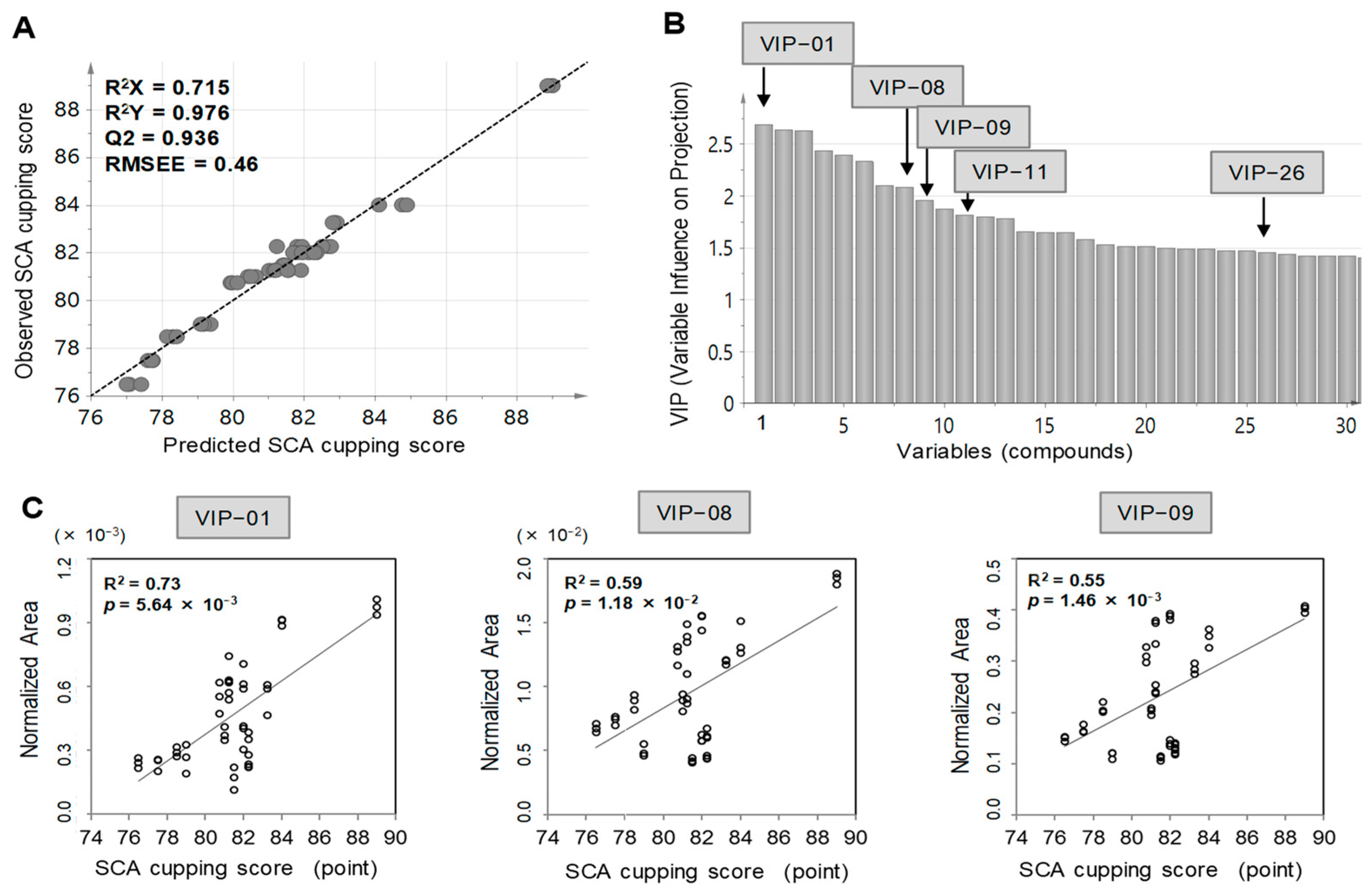
3.1. Identification of the Chemical Compounds Correlated with the SCA Cupping Score Using Chemometric Profiling
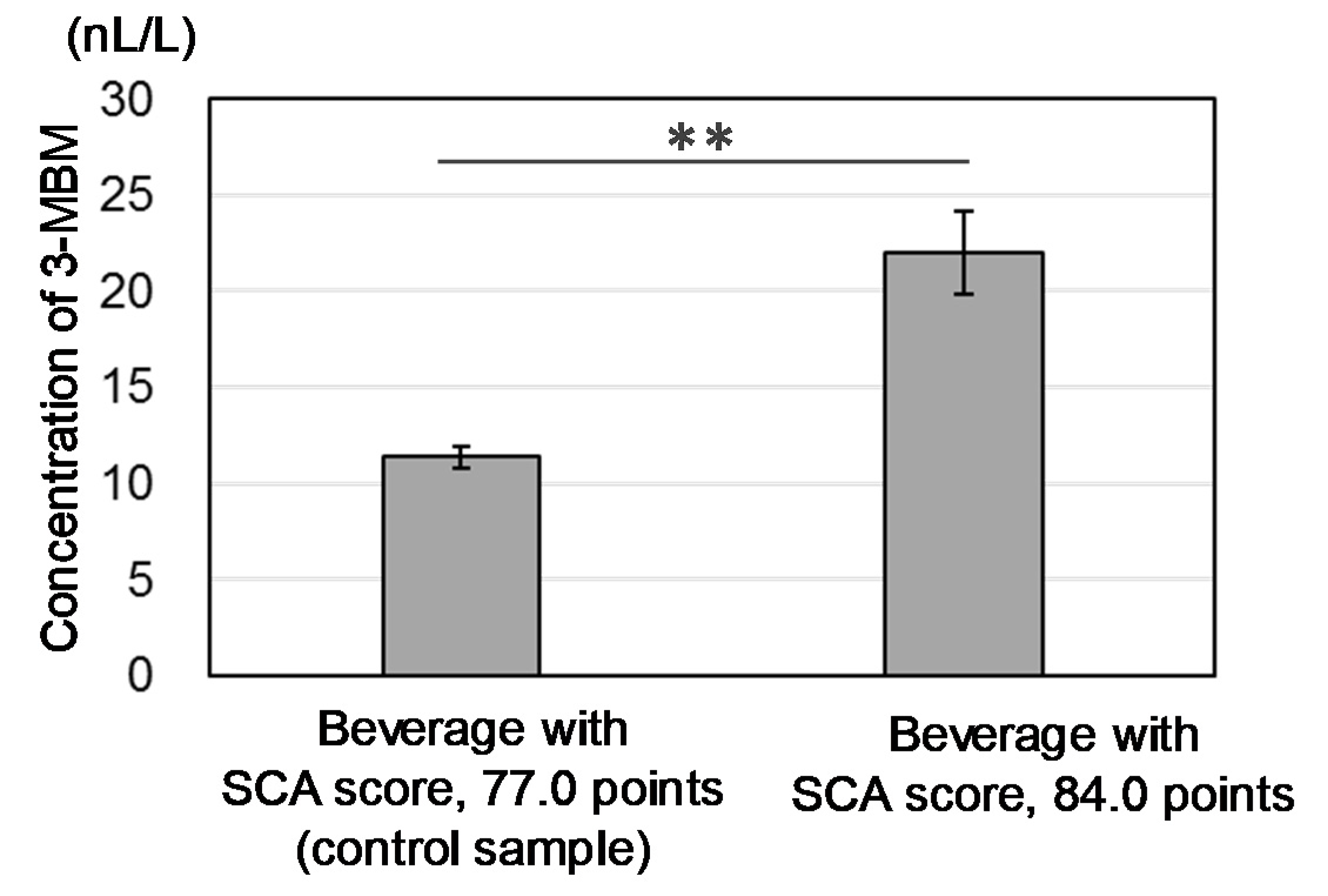
3.2. Validation of the Sensory Effect of MECs in Coffee Brews
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flament, I. Chapter 4, A Historical Survey of Coffee Aroma Research; 4.3, Results; Coffee Flavor Chemistry; John Wiley & Sons, Ltd.: London, UK, 2002; pp. 74–77. [Google Scholar]
- Nijssen, L.M.; Visscher, C.A.; Maarse, H.; Willemsens, L.C.; Boelens, M.H. Volatile Compounds in Food Qualitative and Quantit ative Data, 7th ed.; TNO Nutrition and Food Research Institute: Zeist, The Netherlands, 1996; pp. 72.1–72.23. [Google Scholar]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan coffee brew chemical analysis in comparison to espresso, moka and American brews. Food Res. Int. 2014, 61, 152–160. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onusgu, M.; Iwabuchi, H. Analysis of the headspace volatiles of freshly brewed Arabica coffee using solid-phase microextraction. J. Food Sci. 2007, 72, C388–C396. [Google Scholar] [CrossRef]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace solid-phase microextraction–gas chromatographic–time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Fisk, I.D.; Kettle, A.; Hofmeister, S.; Virdie, A.; Kenny, J.S. Discrimination of roast and ground coffee aroma. Flavour 2012, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Blank, I.; Alina, S.; Grosch, W. Potent odorants of the roasted powder and brew of Arabica coffee. Z. Lebensm. Unters. For. 1992, 195, 239–245. [Google Scholar] [CrossRef]
- Czerny, M.; Grosch, W. Potent odorants of raw Arabica coffee. Their changes during roasting. J. Agric. Food Chem. 2000, 48, 868–872. [Google Scholar] [CrossRef]
- Semmelroch, P.; Grosch, W. Studies on character impact odorants of coffee brews. J. Agric. Food Chem. 1996, 44, 537–543. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Identification of odor-active 3-mercapto-3-methylbutyl acetate in volatile fraction of roasted coffee brew isolated by steam distillation under reduced pressure. J. Agric. Food Chem. 2003, 51, 3079–3082. [Google Scholar] [CrossRef] [PubMed]
- Sanz, C.; Czerny, M.; Cid, C.; Schieberle, P. Comparison of potent odorants in a filtered coffee brew and in an instant coffe beverage by aroma extract dilution analysis (AEDA). Eur. Food Res. Technol. 2002, 214, 299–302. [Google Scholar] [CrossRef]
- Specialty Coffee Association. Cupping Protocols. Specialty Coffee Association of America, Santa Ana, CA, USA. Available online: https://sca.coffee/research/protocols-best-practices (accessed on 28 February 2020).
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical Profile and Antioxidant Capacity of Coffee Plant Organs Compared to Green and Roasted Coffee Beans. Antioxidants 2020, 9, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimura, Y.; Kurihara, K.; Ida, M.; Kosaka, R.; Miura, D.; Miura, D.; Wariishi, H.; Maeda-Yamamoto, M.; Nesumi, A.; Saito, T.; et al. Metabolomics-Driven Nutraceutical Evaluation of Diverse Green Tea Cultivars. PLoS ONE 2011, 6, e23426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibutami, E.; Ishii, R.; Harada, S.; Kurihara, A.; Kuwabara, K.; Kato, S.; Iida, M.; Akiyama, M.; Sugiyama, D.; Hirayama, A.; et al. Charged metabolite biomarkers of food intake assessed via plasma metabolomics in a population-based observational study in Japan. PLoS ONE 2021, 16, e0246456. [Google Scholar] [CrossRef]
- Fujimura, Y.; Kawano, C.; Maeda-Murayama, A.; Nakamura, A.; Koike-Miki, A.; Yukihira, D.; Hayakawa, E.; Ishii, T.; Tachibana, H.; Wariishi, H.; et al. A Chemometrics-driven strategy for the bioactivity evaluation of complex multicomponent systems and the effective selection of bioactivity-predictive chemical combinations. Sci. Rep. 2017, 7, 2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Furihata, K.; Koda, M.; Hu, F.; Kato, R.; Miyakawa, T.; Tanokura, M. 13C NMR-based metabolomics for the classification of green coffee beans according to variety and origin. J. Agric. Food Chem. 2012, 60, 10118–10125. [Google Scholar] [CrossRef] [PubMed]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Discrimination between Arabica and Robusta green coffee using visible micro Raman spectroscopy and chemometric analysis. Food Chem. 2011, 126, 1443–1448. [Google Scholar] [CrossRef]
- Santos, J.R.; Sarraguca, M.C.; Rangel, A.O.S.S.; Lopes, J.A. Evaluation of green coffee beans quality using near infrared spectroscopy: A quantitative approach. Food Chem. 2012, 135, 1828–1835. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Grebby, S.; Fisk, I.D. Non-destructive analysis of sucrose, caffeine and trigonelline on single green coffee beans by hyperspectral imaging. Food Res. Int. 2018, 106, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Whitworth, M.B.; Grebby, S.; Fisk, I.D. Rapid prediction of single green coffee bean moisture and lipid content by hyperspectral imaging. J. Food Eng. 2018, 227, 18–29. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Serra, F.; Reniero, F.; Héberger, K. Botanical and geographical characterization of green coffee (Coffea arabica and Coffea canephora): Chemometric evaluation of phenolic and methylxanthine contents. J. Agric. Food Chem. 2009, 57, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Choi, W.; Park, J.H.; Lim, J.; Kwon, S.W. Determination of coffee origins by integrated metabolomics approach of combining multiple analytical data. Food Chem. 2010, 121, 1260–1268. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, K.; Setoyama, H.; Seta, H.; Fujimura, Y.; Miura, D.; Wariishi, H.; Nagai, C.; Nakahara, K. Identification of 3-methylbutanoyl glycosides in green Coffea arabica beans as causative determinants for the quality of coffee flavors. J. Agric. Food Chem. 2015, 63, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Maeztu, L.; Sanz, C.; Andueza, S.; De Peña, M.P.; Bello, J.; Cid, C. Characterization of Espresso Coffee Aroma by Static Headspace GC–MS and Sensory Flavor Profile. J. Agric. Food Chem. 2001, 49, 5437–5444. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, M.; Sopelana, P.; de Peña, M.P.; Cid, C. Application of multivariate analysis to the effects of additives on chemical and sensory quality of stored coffee brew. J. Agric. Food Chem. 2008, 56, 11845–11853. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Rocchetti, G.; Braceschi, G.P.; Odello, L.; Bertuzzi, T.; Trevisan, M.; Lucini, L. Identification of markers of sensory quality in ground coffee: An untargeted metabolomics approach. Metabolomics 2020, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Bressanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee aroma: Chemometric comparison of the chemical information provided by three different samplings combined with GC–MS to describe the sensory properties in cup. Food Chem. 2017, 214, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Automated Mass Spectral Deconvolution and Identification System (AMDIS). Available online: http://chemdata.nist.gov/mass-spc/amdis (accessed on 28 February 2020).
- Davies, T. The new automated mass spectrometry deconvolution and identification system (AMDIS). Spectrosc. Eur. 1998, 10, 24–27. [Google Scholar]
- Stein, S.E. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J. Am. Soc. Mass Spectrom. 1999, 10, 770–781. [Google Scholar] [CrossRef] [Green Version]
- NIST 11 Mass Spectral Library. Available online: https://chemdata.nist.gov/ (accessed on 28 February 2020).
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikstr, C.; Wold, S. Multi- and Megavariate Data Analysis. Part I: Basic Principles and Applications; Umetrics: Umea, Sweden, 2006; p. 425. [Google Scholar]
- Wiklund, S.; Johansson, E.; Sjostrom, L.; Mellerowicz, E.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Grosch, W. Flavour of coffee. A review. Nahrung Food 1998, 42, 344–350. [Google Scholar] [CrossRef]
- Grosch, W. Chemistry III: Volatile compounds. In Coffee—Recent Developments; Clarke, R.J., Vitzthum, O.G., Eds.; Blackwell Science Ltd.: London, UK, 2001; pp. 67–89. [Google Scholar]
- Kerler, J.; Poisson, L. Understanding coffee aroma for product development. New Food Mag. 2011, 14, 39–43. [Google Scholar]
- Flament, I. Chapter 5, The Individual Constituents: Structure, Nomenclature, Origin, Chemical and Organoleptic Properties; 5.6, Esters; Coffee Flavor Chemistry; John Wiley & Sons, Ltd.: London, UK, 2002; pp. 169–181. [Google Scholar]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry Cultivars using GC–Olfactometry, GC–MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Tsuda, A.; Bai, J.; Fujishita, N.; Chachin, N. Characteristic pattern of aroma ester formation from banana, melon, and strawberry with reference to the substrate specificity of ester synthetase and alcohol contents in pulp. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Schieberle, H.; Hofmann, T. Evaluation of the character impact odorants in fresh strawberry juice by quantitative measurements and sensory studies on model mixtures. J. Agric. Food Chem. 1997, 45, 227–232. [Google Scholar] [CrossRef]
- Lindinger, C.; Pollien, P.; de Vos, R.C.; Tikunov, Y.; Hageman, J.A.; Lambot, C.; Fumeaux, R.; Voiro-Baliguet, E.; Blank, I. Identification of ethyl formate as a quality marker of the fermented off-note in coffee by a nontargeted chemometric approach. J. Agric. Food Chem. 2009, 57, 9972–9978. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, Y.; Xiao, Z. Evaluation of perceptual interaction between ester aroma components in Langjiu by GC–MS, GC–O, sensory analysis, and vector model. Foods 2020, 9, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, A.; Chen, L.; Niu, Y.; Zue, J.; Zhang, J.; Deng, J. Evalutation of the interaction between esters and sulfur compounds in pineapple using Feller’s additive model, OAV, and odor activity coefficient. Food Anal. Methods 2021. [Google Scholar] [CrossRef]
- Miyazawa, T.; Gallagher, M.; Preti, G.; Wise, P.M. Synergistic Mixture Interactions in Detection of Perithreshold Odors by Humans. Chem. Sens. 2008, 33, 363–369. [Google Scholar] [CrossRef]
- Miyazawa, T.; Gallagher, M.; Preti, G.; Wise, P.M. The Impact of Sub-threshold Carboxylic Acids on the Odor Intesity of Supra- threshold Flavor Compounds. Chem. Percept. 2008, 1, 163–167. [Google Scholar] [CrossRef]
- Miyazawa, T.; Gallagher, M.; Preti, G.; Wise, P.M. Odor detection of mixtures of homologous carboxylic acids and coffee aroma compounds by humans. J. Agric. Food Chem. 2009, 57, 9895–9901. [Google Scholar] [CrossRef]
- Miyazawa, T.; Gallagher, M.; Preti, G.; Wise, P.M. Psychometric functions for ternary odor mixtures and their unmixed components. Chem. Sens. 2009, 34, 753–761. [Google Scholar] [CrossRef] [Green Version]

| SCA Cupping Score (Point) | |
|---|---|
| 89 | 89.0 |
| 88 | |
| 87 | |
| 86 | |
| 85 | |
| 84 | 84.0 |
| 83 | 83.25 |
| 82 | 82.0, 82.0, 82.25, 82.5 |
| 81 | 81.0, 81.25, 81.25, 81.5 |
| 80 | 80.75 |
| 79 | 79.0 |
| 78 | 78.5 |
| 77 | 77.5 |
| 76 | 76.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasa, K.; Seta, H.; Matsuo, Y.; Nakahara, K. Evaluation of 3-Methylbutanoic Acid Methyl Ester as a Factor Influencing Flavor Cleanness in Arabica Specialty Coffee. Appl. Sci. 2021, 11, 5413. https://doi.org/10.3390/app11125413
Iwasa K, Seta H, Matsuo Y, Nakahara K. Evaluation of 3-Methylbutanoic Acid Methyl Ester as a Factor Influencing Flavor Cleanness in Arabica Specialty Coffee. Applied Sciences. 2021; 11(12):5413. https://doi.org/10.3390/app11125413
Chicago/Turabian StyleIwasa, Keiko, Harumichi Seta, Yoshihide Matsuo, and Koichi Nakahara. 2021. "Evaluation of 3-Methylbutanoic Acid Methyl Ester as a Factor Influencing Flavor Cleanness in Arabica Specialty Coffee" Applied Sciences 11, no. 12: 5413. https://doi.org/10.3390/app11125413





