Analysis of Compound Stained Cervical Cell Images Using Multi-Spectral Imaging
Abstract
:1. Introduction
2. Design and Principle
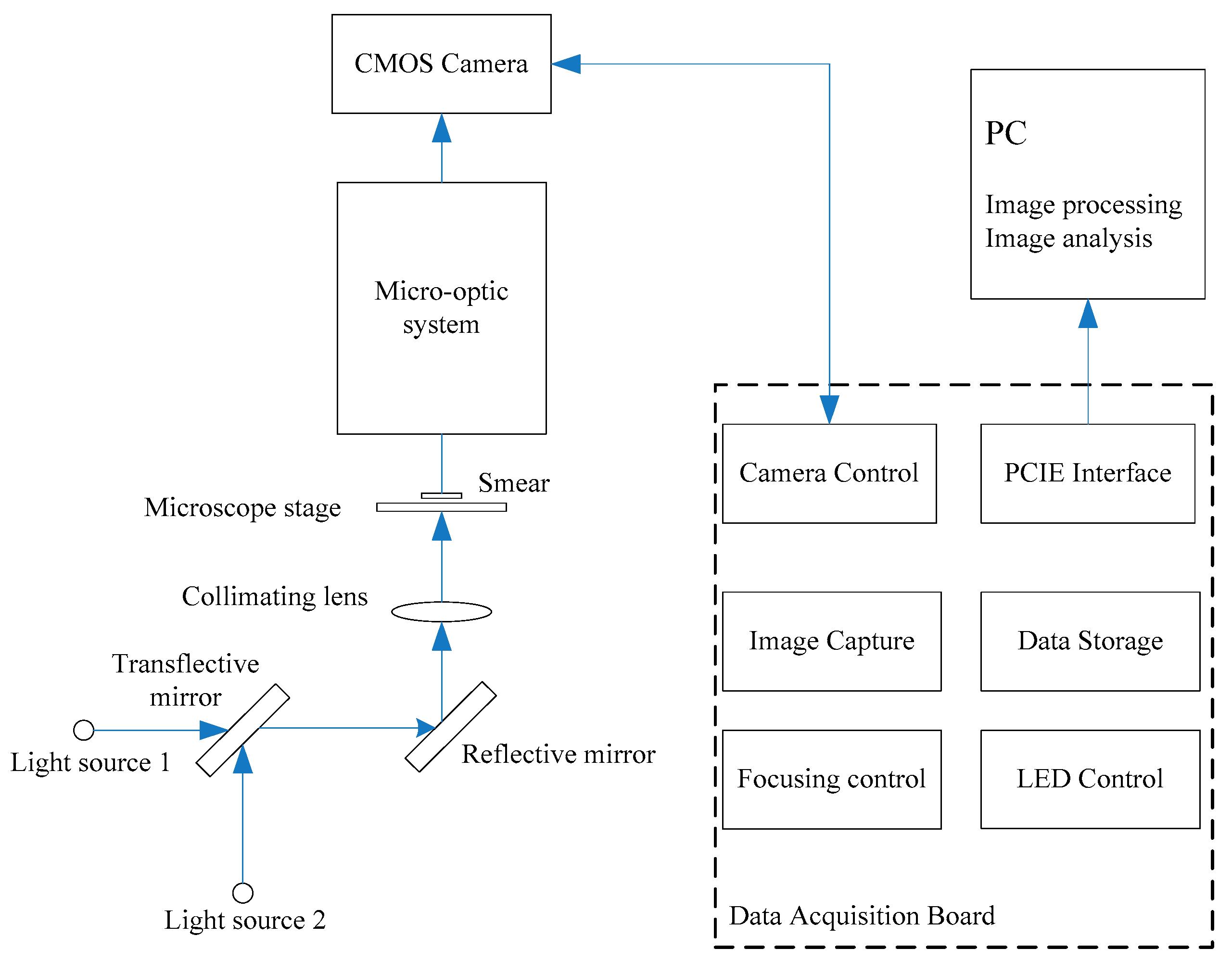
2.1. Design of Microscopic Multi-Spectral Imaging System
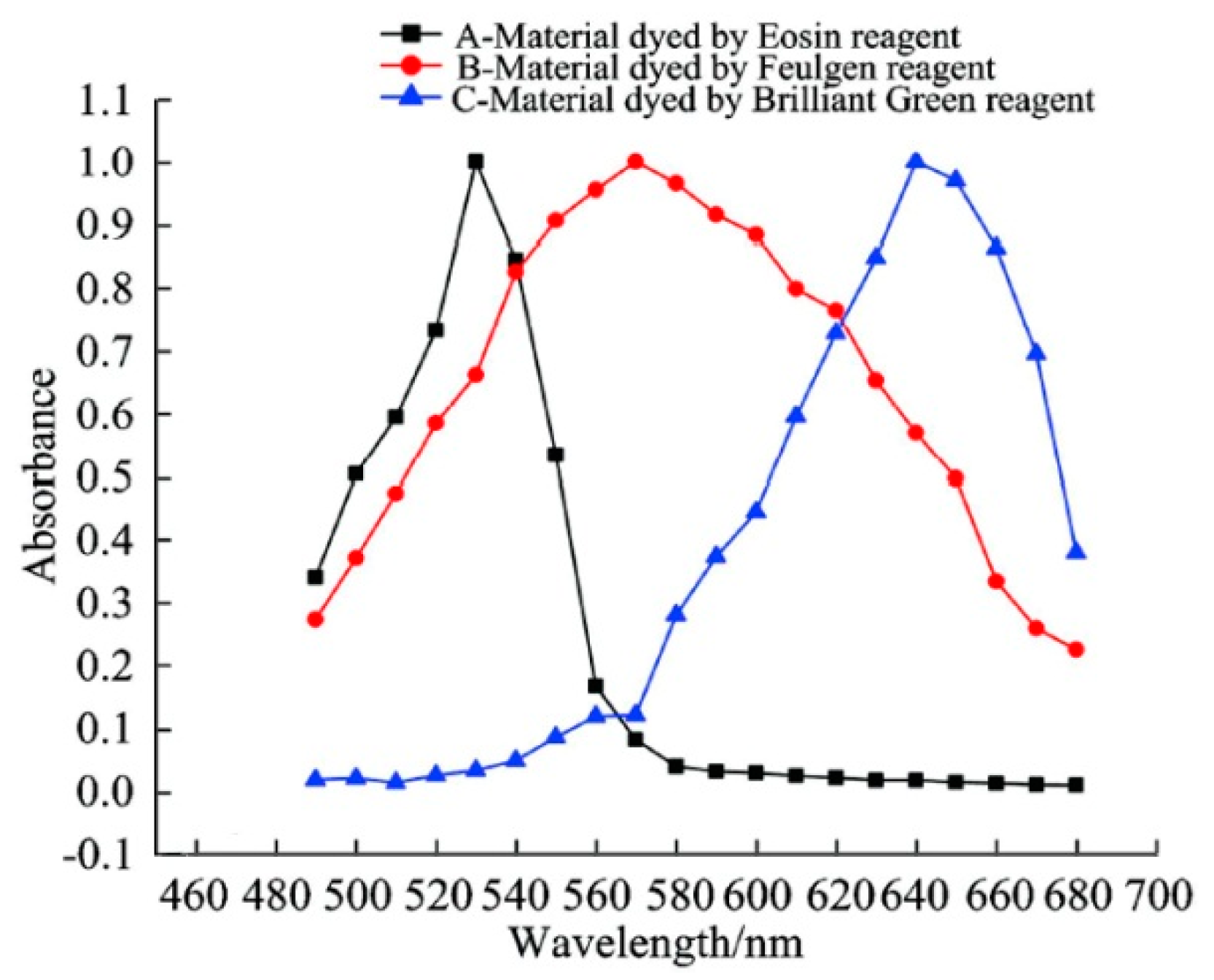
2.2. Multi-Spectral Absorbance Unmixing Model
3. Experiments and Results
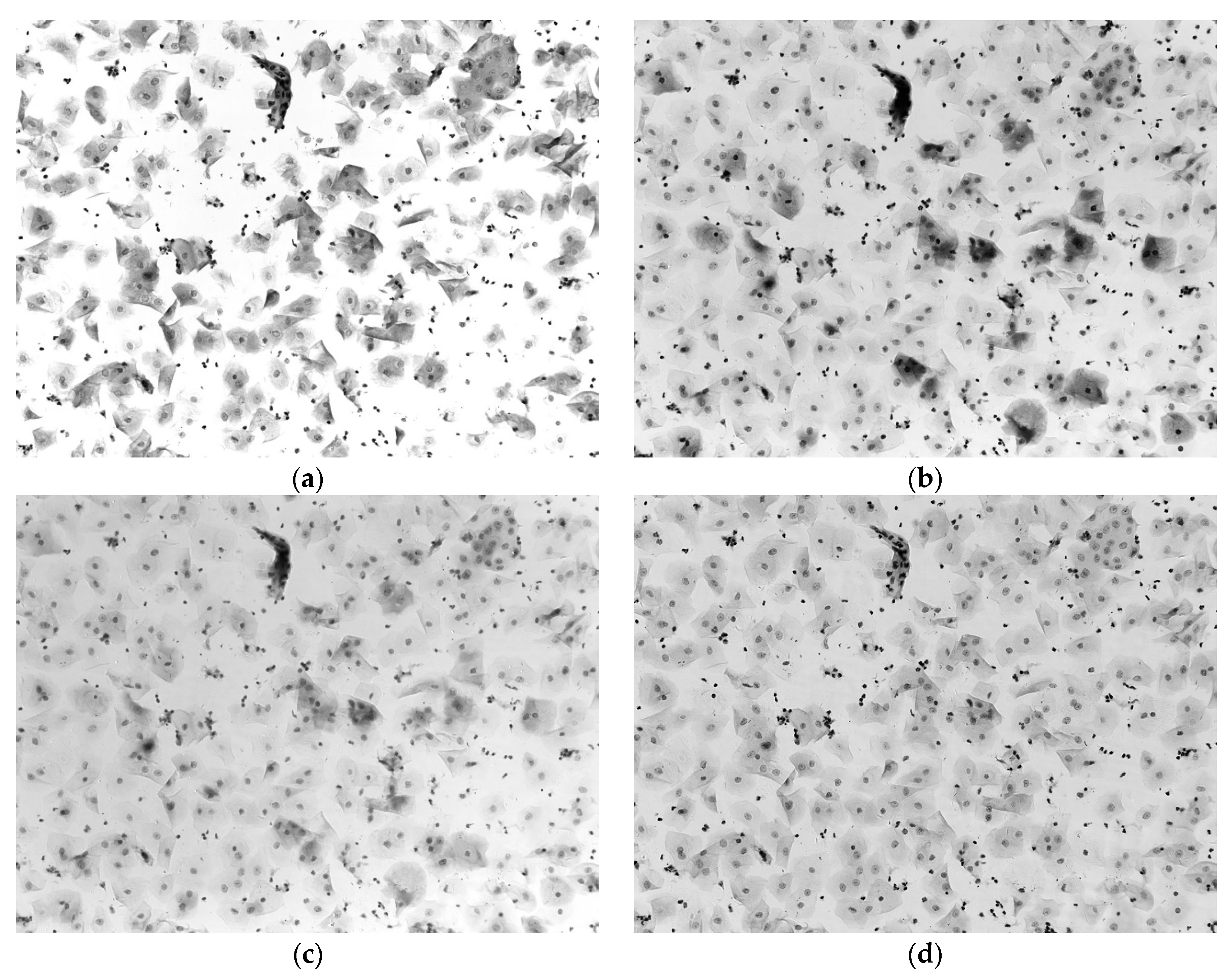
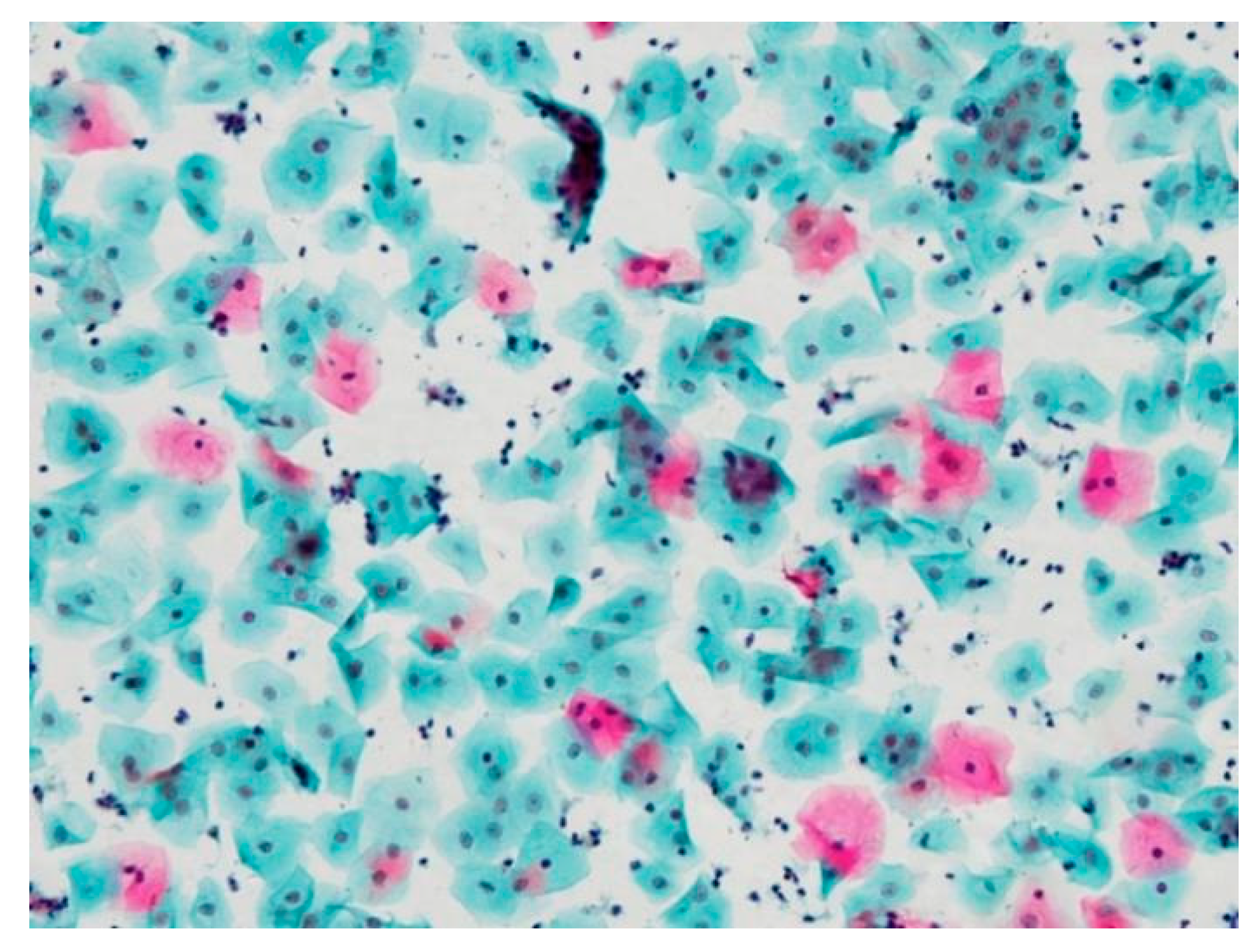
3.1. Pseudo-Color Image Synthesizing and Absorbance Unmixing
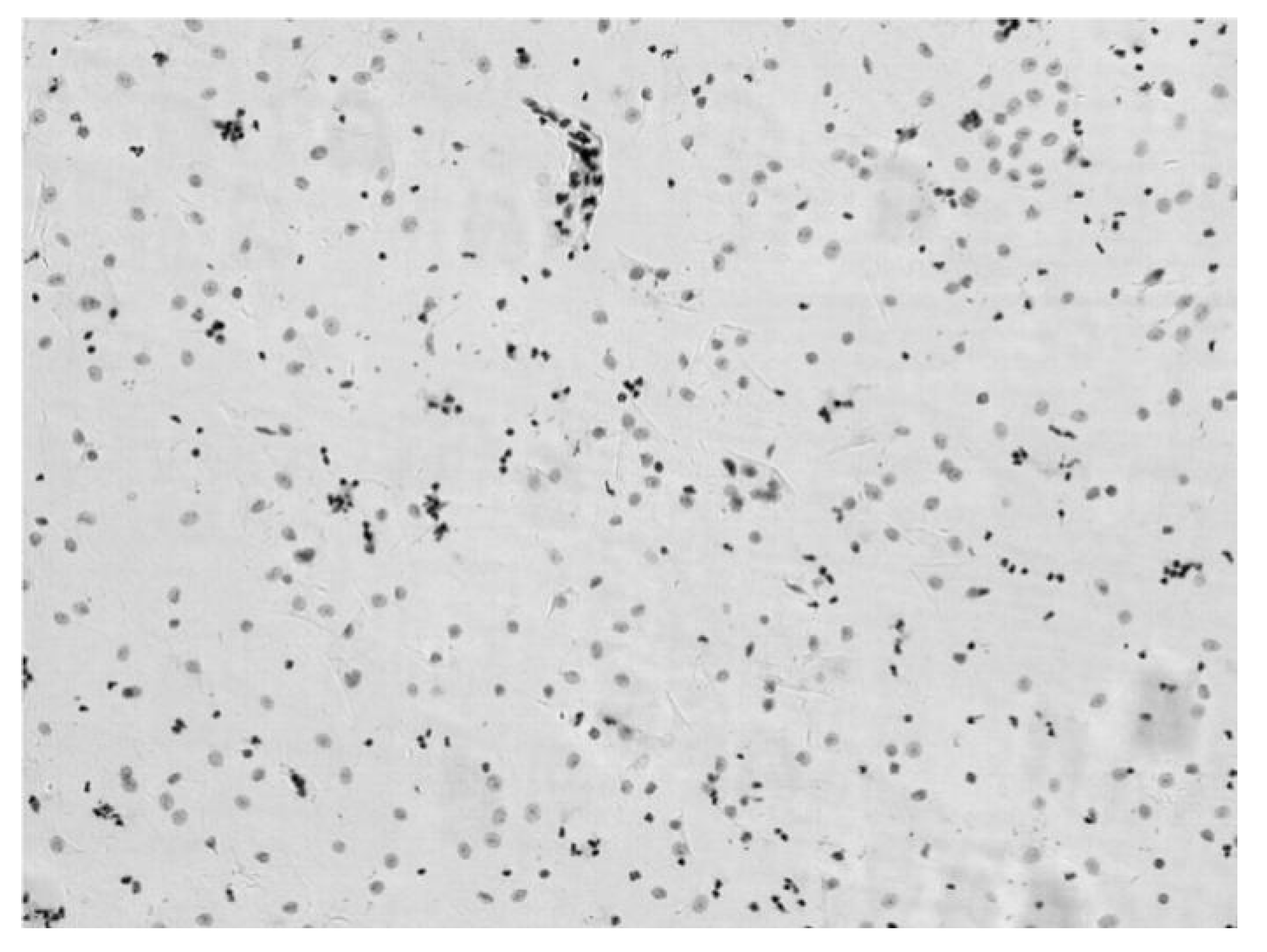
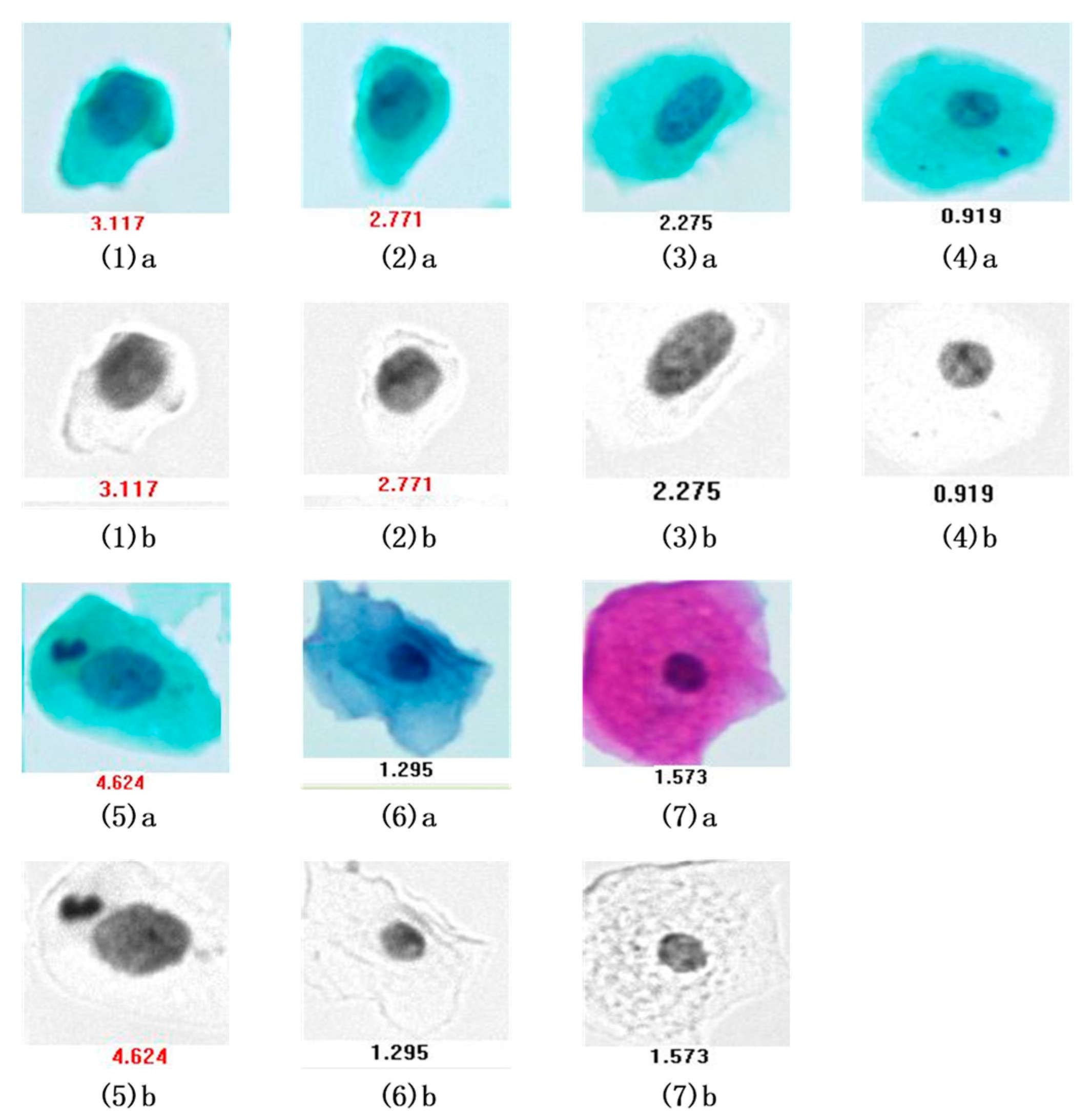
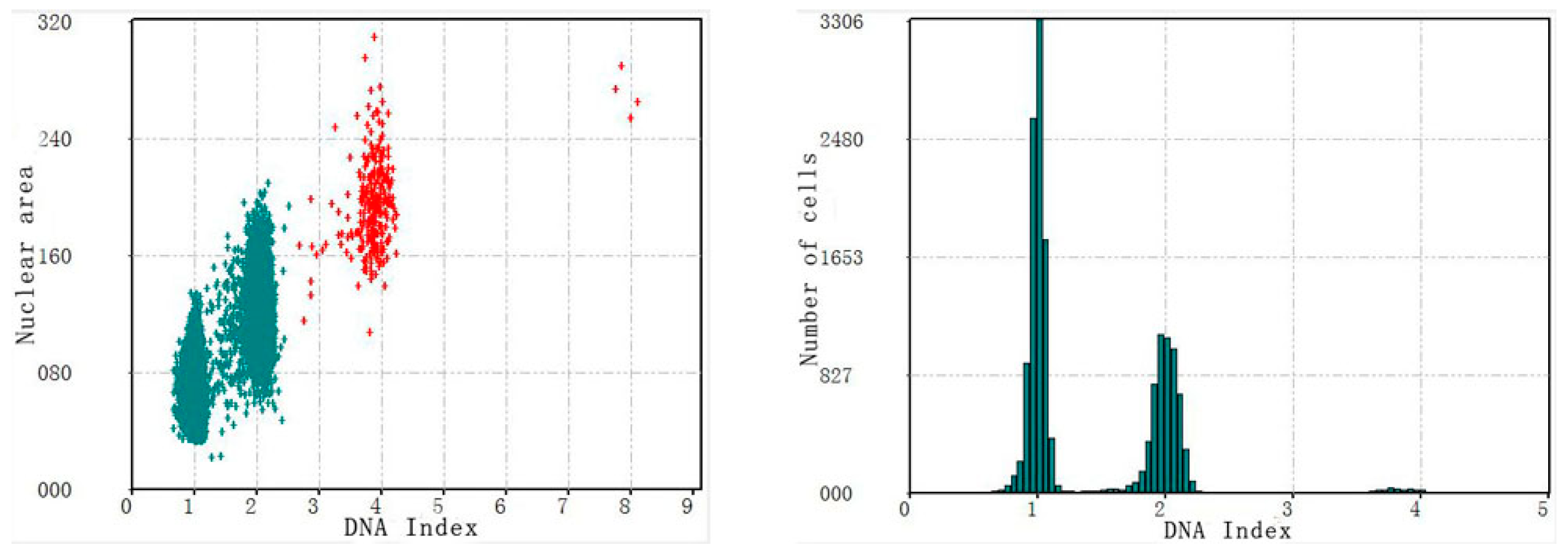
3.2. Verification Test of DNA Quantitative Analysis
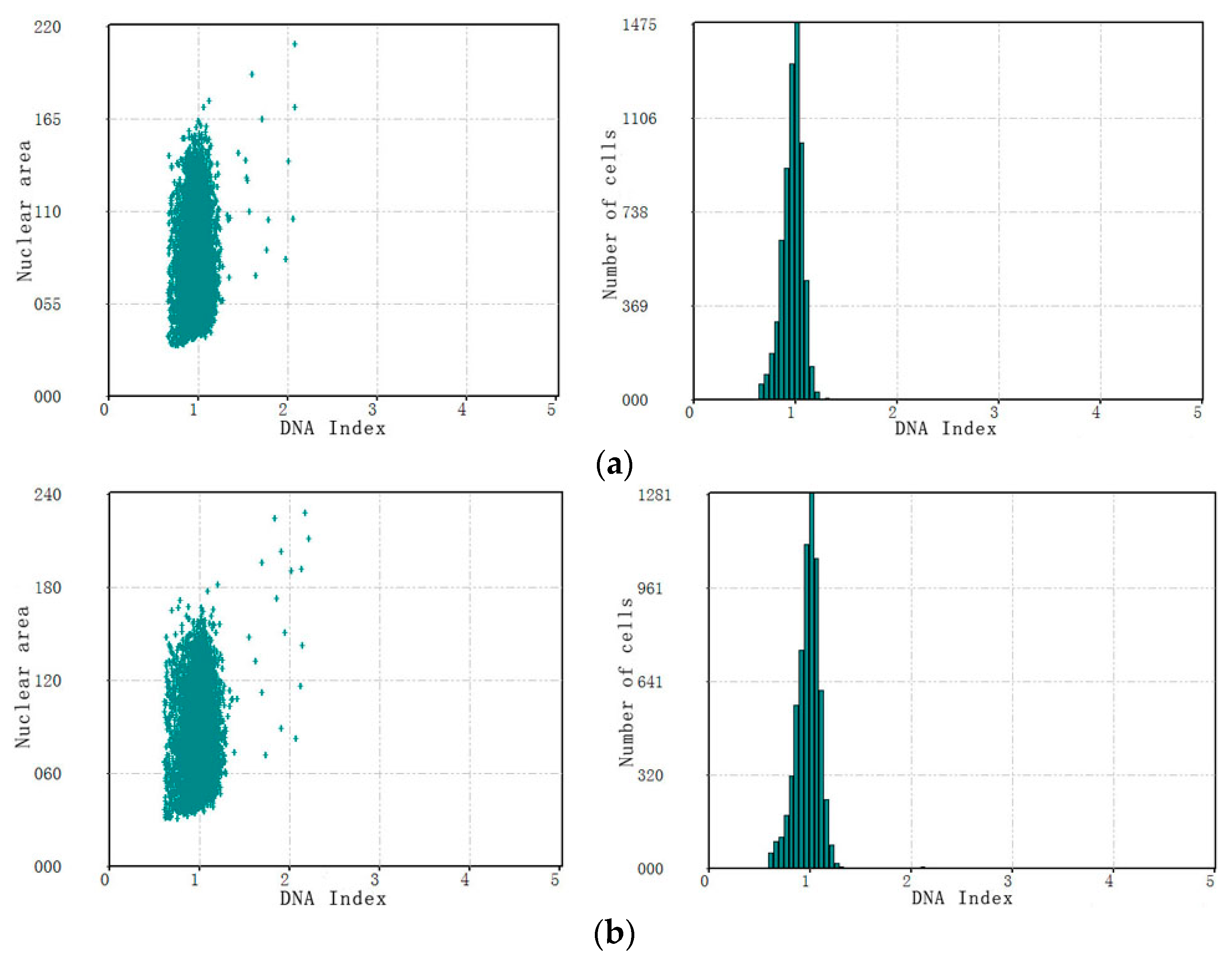
3.3. The Performance of Cervical Cell Screening
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Malagon, T.; Drolet, M.; Boily, M.C.; Laprise, J.F.; Brisson, M. Changing inequalities in cervical cancer: Modeling the impact of vaccine uptake, vaccine herd effects, and cervical cancer screening in the post-vaccination era. Cancer Epidemiol. Biomark. Prev. 2015, 24, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Gupta, S.; Mehrotra, R.; Sodhani, P. Cervical Cancer Screening in Resource Constrained Countries: Current Status and Future Directions. Asian Pac. J. Cancer Prev. 2017, 18, 1461–1467. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Rosa, C.; Patrick, P.; Gabriel, D.; Pierre, V. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J. Clin. Oncol. 2015, 6, 281–290. [Google Scholar]
- Peto, J.; Gilham, C.; Fletcher, O.; Matthews, F.E. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004, 364, 249–256. [Google Scholar] [CrossRef]
- Remmerbach, T.W.; Weidenbach, H.; Pomjanski, N.; Knops, K.; Mathes, S.; Hemprich, A.; Bocking, A. Cytologic and DNA-cytometric early diagnosis of oral cancer. Anal. Cell. Pathol. 2001, 22, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; O’Connor, D.; Prey, M.; Raab, S.; Sherman, M.; Wilbur, D.; Young, N.; et al. The 2001 Bethesda System—Terminology for reporting results of cervical cytology. JAMA J. Am. Med. Assoc. 2002, 287, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- Guillaud, M.; Benedet, J.L.; Cantor, S.B.; Staerkel, G.; Follen, M.; MacAulay, C. DNA ploidy compared with human papilloma virus testing (Hybrid capture-II) and conventional cervical cytology as a primary screening test for cervical high-grade lesions and cancer in 1555 patients with biopsy confirmation. Cancer 2006, 107, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Demirel, D.; Akyurek, N.; Ramzy, I. Diagnostic and prognostic significance of image cytometric DNA ploidy measurement in cytological samples of cervical squamous intraepithelial lesions. Cytopathology 2013, 24, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Grote, H.J.; Nguyen, H.V.Q.; Leick, A.G.; Bocking, A. Identification of progressive cervical epithelial cell abnormalities using DNA image cytometry. Cancer Cytopathol. 2004, 102, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bocking, A.; Nguyen, V.Q. Diagnostic and prognostic use of DNA image cytometry in cervical squamous intraepithelial lesions and invasive carcinoma. Cancer 2004, 102, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Fleskens, S.J.H.M.; Takes, R.P.; Otte-Holler, I.; van Doesburg, L.; Smeets, A.; Speel, E.J.M.; Slootweg, P.J.; Van Der Laak, J.A. Simultaneous assessment of DNA ploidy and biomarker expression in paraffin-embedded tissue sections. Histopathology 2010, 57, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Levenson, R.M.; Mansfield, J.R. Multispectral imaging in biology and medicine: Slices of life. Cytom. Part A 2006, 69, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Carcagni, P.; Della Patria, A.; Fontana, R.; Greco, M.; Mastroianni, M.; Materazzi, M.; Pampaloni, E.; Pezzati, L. Multispectral imaging of paintings by optical scanning. Opt. Lasers Eng. 2007, 45, 360–367. [Google Scholar] [CrossRef]
- Bautista, P.A.; Yagi, Y. Localization of eosinophilic esophagitis from H & E stained images using multispectral imaging. Diagn Pathol. 2011, 6 (Suppl. 1), S2. [Google Scholar]
- Lu, R.F. Multispectral imaging for predicting firmness and soluble solids content of apple fruit. Postharvest Biol. Technol. 2004, 31, 147–157. [Google Scholar] [CrossRef]
- Jakovels, D.; Spigulis, J. 2-D mapping of skin chromophores in the spectral range 500–700 nm. J. Biophotonics 2010, 3, 125–129. [Google Scholar] [CrossRef]
- Basiri, A.; Nabili, M.; Mathews, S.; Libin, A.; Groah, S.; Noordmans, H.J.; Ramella-Roman, J.C. Use of a multi-spectral camera in the characterization of skin wounds. Opt. Express 2010, 18, 3244–3257. [Google Scholar] [CrossRef] [Green Version]
- Crane, L.M.; Themelis, G.; Pleijhuis, R.G.; Harlaar, N.J.; Sarantopoulos, A.; Arts, H.J.; van der Zee, A.G.; Vasilis, N.; van Dam, G.M. Intraoperative Multispectral Fluorescence Imaging for the Detection of the Sentinel Lymph Node in Cervical Cancer: A Novel Concept. Mol. Imaging Biol. 2010, 13, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Hedley, D.W.; Rugg, C.A.; Gelber, R.D. Association of DNA Index and S-Phase Fraction with Prognosis of Nodes Positive Early Breast-Cancer. Cancer Res. 1987, 47, 4729–4735. [Google Scholar]
- Hiddemann, W.; Schumann, J.; Andreef, M.; Barlogie, B.; Herman, C.J.; Leif, R.C.; Mayall, B.H.; Murphy, R.F.; Sandberg, A.A. Convention on Nomenclature for DNA Cytometry. Cancer Genet. Cytogenet. 1984, 13, 181–183. [Google Scholar] [CrossRef]
- Colditz, G.A.; Crawley, J. DNA Cytometry Testing for Cervical Cancer Screening: Approaches and Reporting Standards for New Technologies. Clin. Cancer Res. 2011, 17, 6971–6972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallioniemi, O.P.; Blanco, G.; Alavaikko, M.; Hietanen, T.; Mattila, J.; Lauslahti, K.; Lehtinen, M.; Koivula, T. Improving the Prognostic Value of DNA Flow-Cytometry in Breast-Cancer by Combining DNA Index and S-Phase Fraction—A Proposed Classification of DNA Histograms in Breast-Cancer. Cancer 1988, 62, 2183–2190. [Google Scholar] [CrossRef]
- Kallioniemi, O.P.; Koivula, T.; Punnonen, R.; Mattila, J.; Lehtinen, M. Prognostic-Significance of DNA Index, Multiploidy, and S-Phase Fraction in Ovarian-Cancer. Cancer 1988, 61, 334–339. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, L.B.; Wu, Q.S. Study of cervical exfoliated cell’s DNA quantitative analysis based on multi-spectral imaging technology. Spectrosc. Spectr. Anal. 2016, 36, 496–501. [Google Scholar]
- Biesterfeld, S.; Beckers, S.; Cadenas, M.D.V.; Schramm, M. Feulgen Staining Remains the Gold Standard for Precise DNA Image Cytometry. Anticancer Res. 2011, 31, 53–58. [Google Scholar]
- Nghiem, V.T.; Davies, K.R.; Beck, J.R.; Follen, M.; MacAulay, C.; Guillaud, M.; Cantor, S.B. Economic evaluation of DNA ploidy analysis vs liquid-based cytology for cervical screening. Br. J. Cancer 2015, 112, 1951–1957. [Google Scholar] [CrossRef] [Green Version]
- Carriere, R. The growth of liver parenchymal nuclei and its endocrine regulation. Int. Rev. Cytol. 1969, 25, 201–277. [Google Scholar]
- Styles, J.A. Measurement of Ploidy and Cell-Proliferation in the Rodent Liver. Environ. Health Perspect. 1993, 101, 67–71. [Google Scholar]

| Analysis Method | Cell Types | Quantity | Mean of DI | Standard Deviation | CV |
|---|---|---|---|---|---|
| Feulgen staining method | Diploid cells | 6421 | 0.989 | 0.089 | 9.043 |
| Tetraploid cells | 6 | 1.983 | 0.102 | 5.126 | |
| Aneuploid cells | 15 | 1.473 | 0.154 | 10.427 | |
| High ploidy cells | 0 | 0 | 0 | 0 | |
| Compound staining method of this paper | Diploid cells | 6153 | 1.000 | 0.097 | 9.744 |
| Tetraploid cells | 12 | 2.009 | 0.127 | 6.305 | |
| Aneuploid cells | 29 | 1.351 | 0.141 | 10.433 | |
| High ploidy cells | 0 | 0 | 0 | 0 |
| Analysis Method | Cell Types | Quantity | Mean of DI | Standard Deviation | CV |
|---|---|---|---|---|---|
| Feulgen staining method | Diploid cells | 3517 | 1.034 | 0.092 | 8.918 |
| Tetraploid cells | 243 | 2.032 | 0.128 | 6.276 | |
| Aneuploid cells | 86 | 1.353 | 0.134 | 9.934 | |
| High ploidy cells | 32 | 3.424 | 0.429 | 12.544 | |
| Compound staining method of this paper | Diploid cells | 3780 | 1.026 | 0.094 | 9.111 |
| Tetraploid cells | 210 | 2.039 | 0.141 | 6.921 | |
| Aneuploid cells | 90 | 1.377 | 0.152 | 11.063 | |
| High ploidy cells | 30 | 3.440 | 0.463 | 13.462 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, R.; Zeng, L.; Yi, F. Analysis of Compound Stained Cervical Cell Images Using Multi-Spectral Imaging. Appl. Sci. 2021, 11, 5628. https://doi.org/10.3390/app11125628
Fang R, Zeng L, Yi F. Analysis of Compound Stained Cervical Cell Images Using Multi-Spectral Imaging. Applied Sciences. 2021; 11(12):5628. https://doi.org/10.3390/app11125628
Chicago/Turabian StyleFang, Run, Libo Zeng, and Fan Yi. 2021. "Analysis of Compound Stained Cervical Cell Images Using Multi-Spectral Imaging" Applied Sciences 11, no. 12: 5628. https://doi.org/10.3390/app11125628
APA StyleFang, R., Zeng, L., & Yi, F. (2021). Analysis of Compound Stained Cervical Cell Images Using Multi-Spectral Imaging. Applied Sciences, 11(12), 5628. https://doi.org/10.3390/app11125628





