Diversity of Essential Oils and the Respective Hydrolates Obtained from Three Pinus cembra Populations in the Austrian Alps
Abstract
:1. Introduction
- (1)
- To investigate the variability in essential oil composition from three different collection sites;
- (2)
- To take sample from individual trees to get an insight in the chemical variation within populations;
- (3)
- To obtain data on the volatiles in the hydrolates and to learn more about the correlation to their corresponding essential oils.
2. Materials and Methods
2.1. Plant Material
2.2. Steam Distillation
2.3. GC/MS
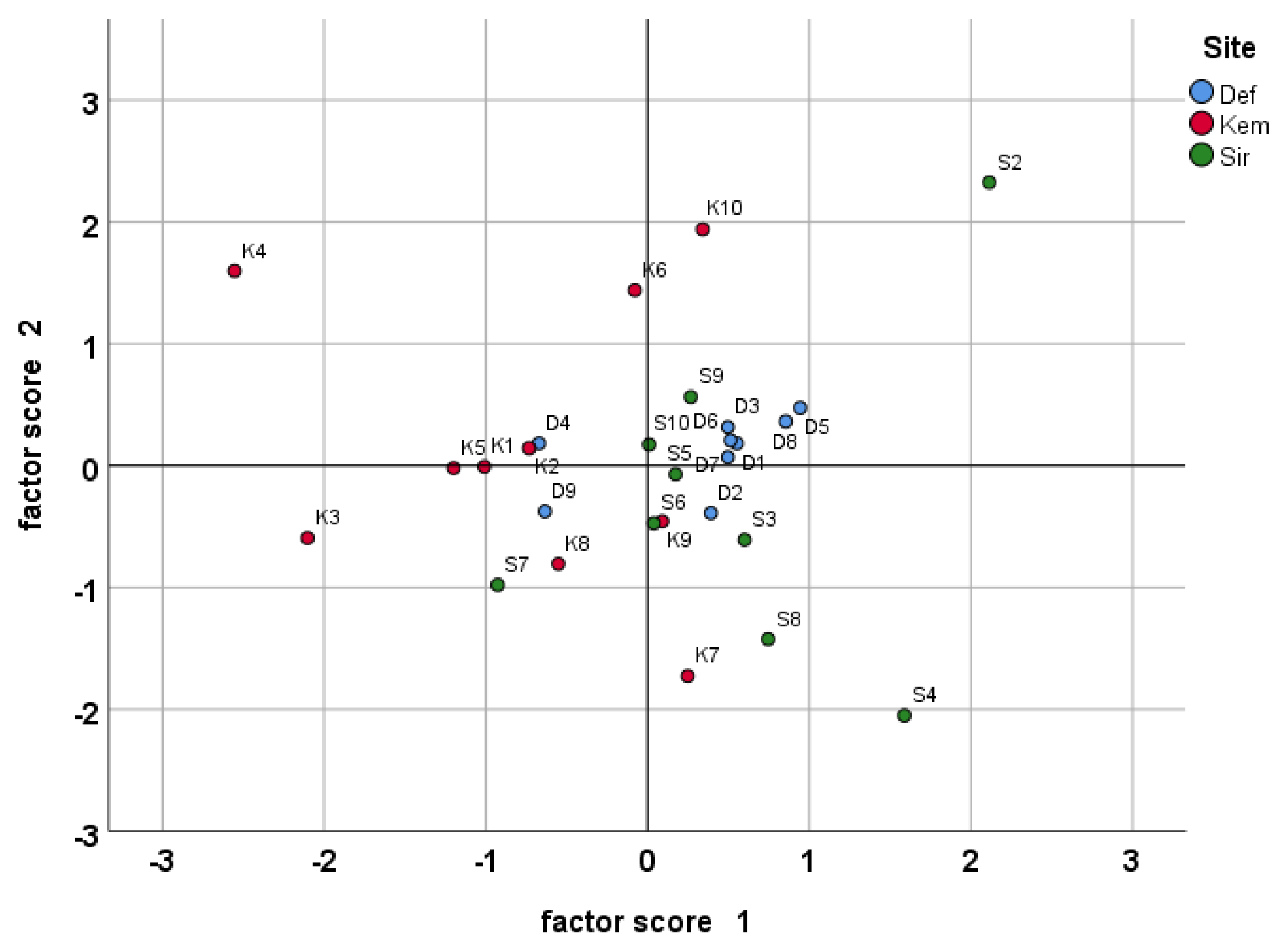
2.4. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Yield
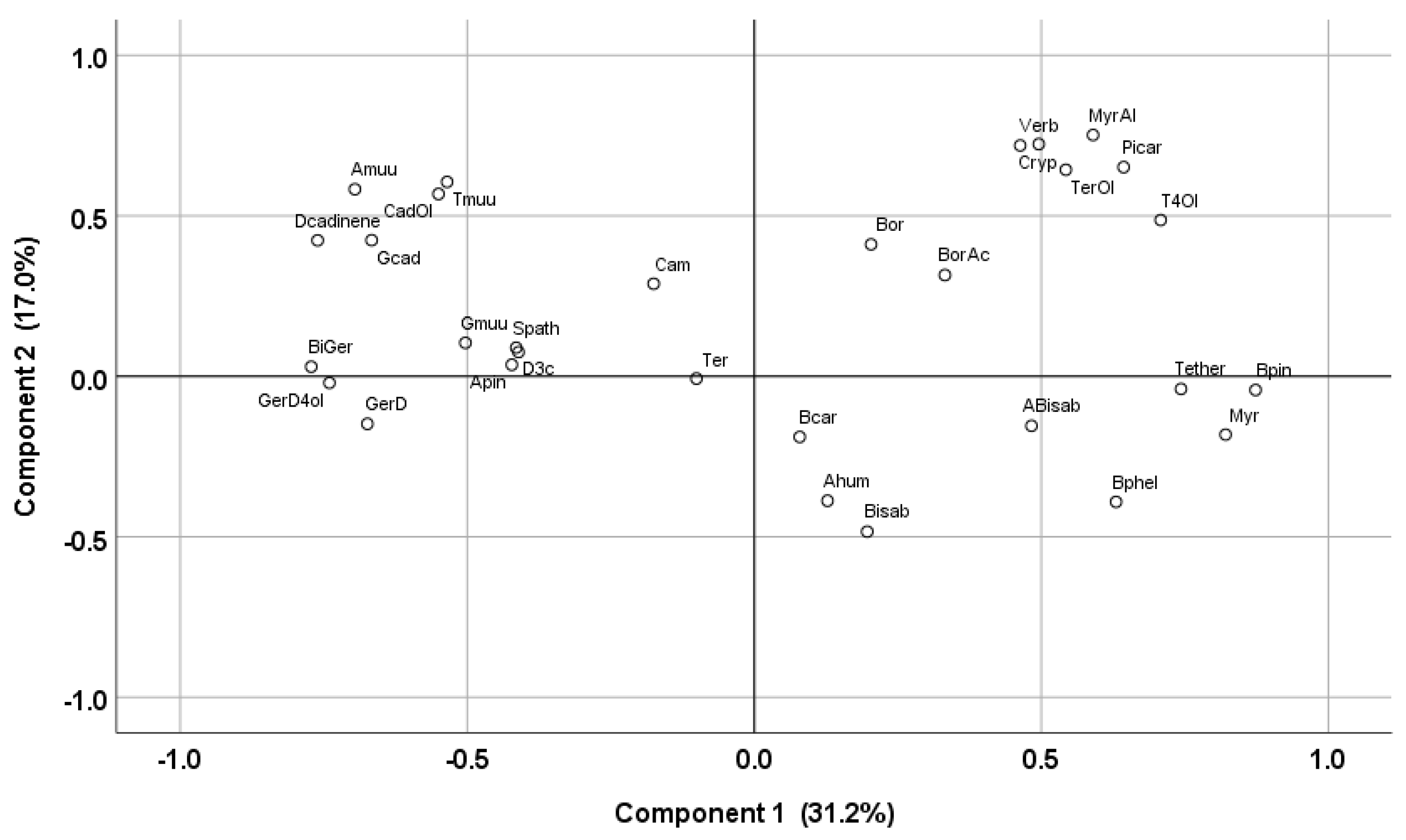
3.2. Essential Oil Composition
3.3. Composition of the Volatile Fractions from the Hydrolates
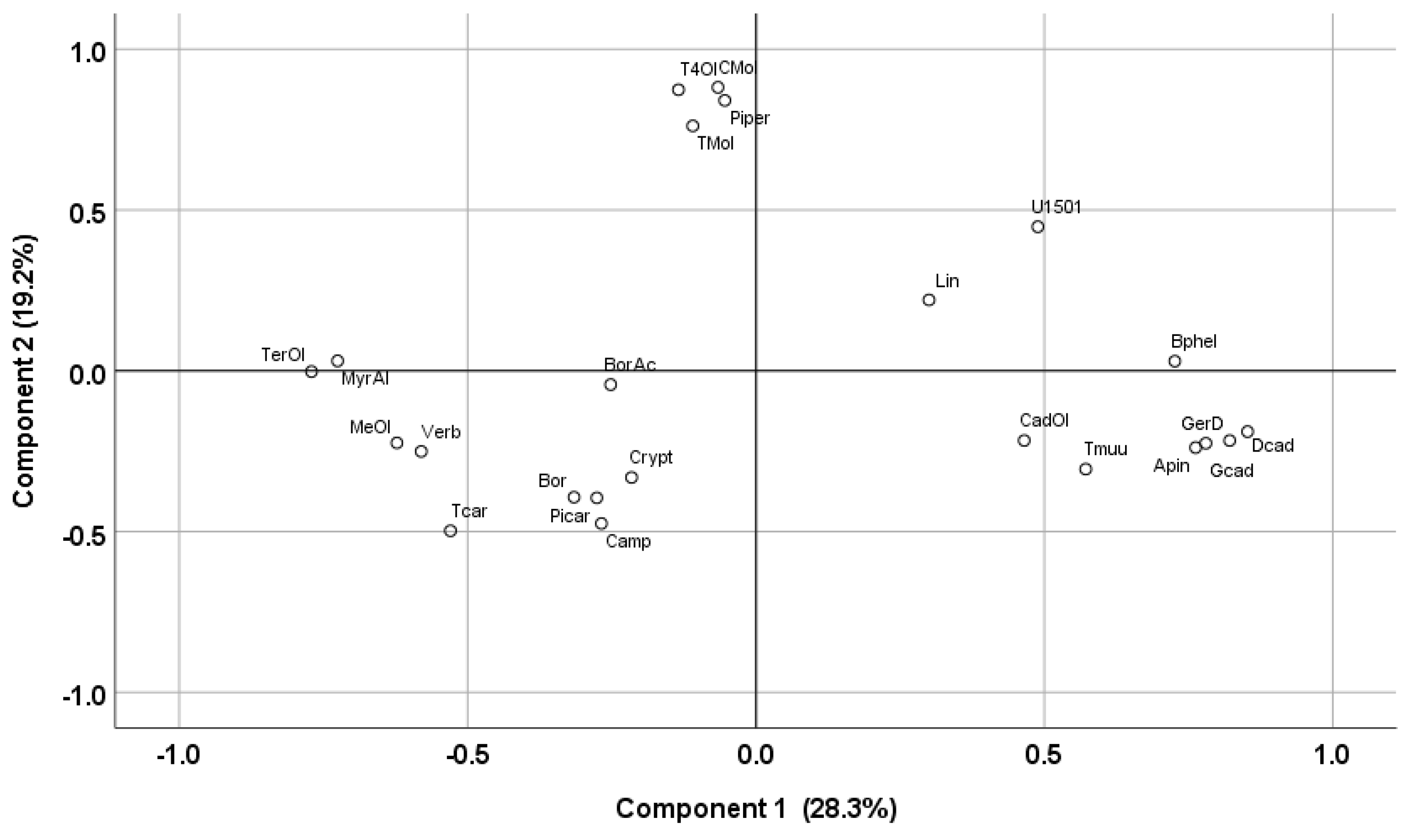
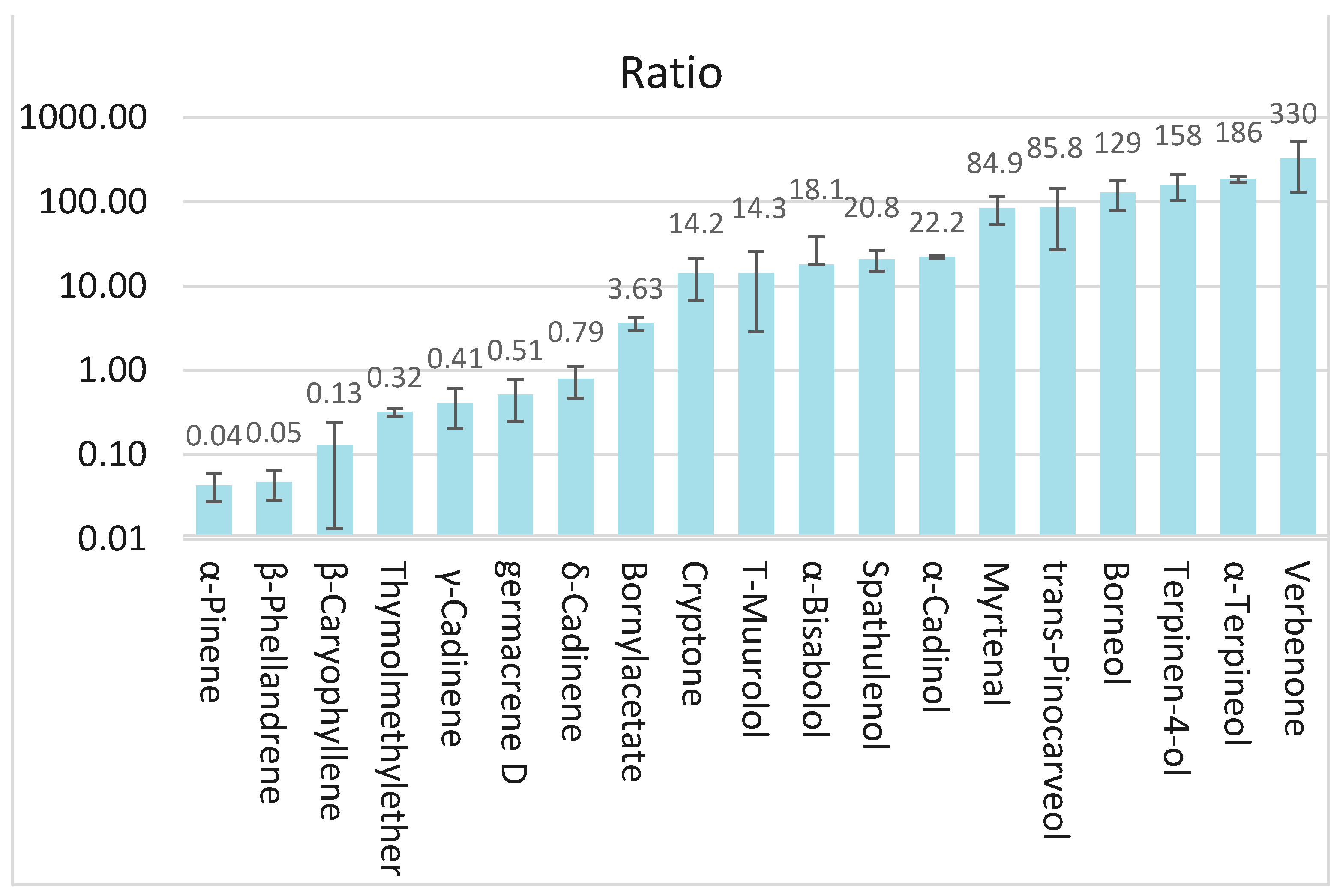
3.4. Comparison of the Essential Oils with the Hydrolate Volatiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schütt, P. Pinus Cembra. In Enzyklopädie der Holzgewächse; Roloff, A., Weissgerber, H., Lang, U.M., Stimm, B., Eds.; Wiley–VCH: Weinheim, Germany, 2014; p. 12. [Google Scholar]
- Caudullo, G.; de Rigo, D. Pinus cembra in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; e01bd9b; pp. 120–121. [Google Scholar]
- Wojnicka-Połtorak, A.; Celinski, K.; Chudzinska, E.; Prus-Głowacki, W.; Niemtur, S. Genetic resources of Pinus cembra L. marginal populations from the Tatra Mountains: Implications for conservation. Biochem. Genet. 2015, 53, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.; Pyttel, P.; Eastaugh, C.S. Impacts of climate change on the establishment, distribution, growth and mortality of Swiss stone pine (Pinus cembra L.). iForest 2009, 3, 82–85. [Google Scholar] [CrossRef]
- Weiss, G.; Ludvig, A.; Zivojinovic, I.; Asamer-Handler, M.; Huber, P. Non-timber innovations: How to innovate in side activities of forestry–Case study Styria, Austria. Austrian J. For. Sci. 2017, 134, 231–250. [Google Scholar]
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils; Baser, K.H.C., Buchbauer, G., Eds.; CRC-Press: Boca Raton, FL, USA, 2010; pp. 83–119. [Google Scholar]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Apetrei, C.L.; Spac, A.; Brebu, M.; Tuchilus, C.; Miron, A. Composition, and antioxidant and antimicrobial activities of the essential oils of a full-grown Pinus cembra L. tree from the Calimani Mountains (Romania). J. Serbian Chem. Soc. 2013, 78, 27–37. [Google Scholar] [CrossRef]
- Kubeczka, K.H.; Schultze, W. Biology and chemistry of conifer oils. Flavour Fragr. J. 1987, 2, 137–148. [Google Scholar] [CrossRef]
- Lis, A.; Kalinowska, A.; Krajewska, A.; Mellor, K. Chemical composition of the essential oils from different morphological parts of Pinus cembra L. Chem. Biodivers. 2017, 14, e1600345. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, B.; Todosijević, M.; Ratknić, M.; Đorđević, I.; Stanković, J.; Cvetković, M.; Marin, P.D.; Tešević, V. Terpenes and n-alkanes in needles of Pinus cembra. Nat. Prod. Commun. 2018, 13, 1035–1037. [Google Scholar] [CrossRef] [Green Version]
- Dormont, L.; Roques, A.; Malosse, C. Cone and foliage volatiles emitted by Pinus cembra and some related conifer species. Phytochemistry 1998, 49, 1269–1277. [Google Scholar] [CrossRef]
- Chizzola, R.; Müllner, K. Variability of volatiles in Pinus cembra L. within and between trees from a stand in the Salzburg Alps (Austria) as assessed by essential oil and SPME analysis. Genet. Resour. Crop Evol. 2021, 68, 567–579. [Google Scholar] [CrossRef]
- Silori, G.K.; Kushwaha, N.; Kumar, V. Essential oils from pines: Chemistry and applications. In Essential Oil Research; Malik, S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 275–297. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured: Carol Stream, IL, USA, 2007. [Google Scholar]
- McLafferty, F.W. Registry of Mass Spectral Data, 5th ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Hänsel, R.; Sticher, O. Pharmakognosie–Phytopharmazie, 9th ed.; Springer Medizinverlag: Heidelberg, Germany, 2010; p. 1014. [Google Scholar]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus. A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Kännaste, A.; Copolovici, L.; Pazouki, L.; Suhhorutsenko, M.; Niinemets, Ü. Highly variable chemical signatures over short spatial distances among Scots pine (Pinus sylvestris) populations. Tree Physiol. 2013, 33, 374–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keefover-Ring, K.; Linhart, Y.B. Variable chemistry and herbivory of Ponderosa pine cones. Int. J. Plant Sci. 2010, 171, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Jäger, W. Metabolism of terpenoids in animal models and humans. In Handbook of Essential Oils; Baser, K.H.C., Buchbauer, G., Eds.; CRC-Press: Boca Raton, FL, USA, 2010; pp. 209–234. [Google Scholar]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciag, A.; Szoka, L.; Karna, E. Chemical composition and biological activity of Abies alba and A. koreana seed and cone essential oils and characterization of their hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Garneau, F.X.; Collin, G.; Gagnon, H.; Pichette, A. Chemical composition of the yydrosol and the essential oil of three different species of the Pinaceae family: Picea glauca (Moench) Voss., Picea mariana (Mill.)B.S.P., and Abies balsamea (L.) Mill. J. Essent. Oil Bear. Plants 2012, 15, 227–236. [Google Scholar] [CrossRef]
- Garneau, F.-X.; Collin, G.; Gagnon, H. Chemical composition and stability of the hydrosols obtained during essential oil production. II. The case of Picea glauca (Moench) Voss., Solidago puberula Nutt., and Mentha piperita L. Am. J. Essent. Oils Nat. Prod. 2014, 2, 29–35. [Google Scholar]
- St-Gelais, A.; Caron, L.; Collin, G.; Marceau, H.; Pichette, A. Aromas from Quebec. III. Composition of the essential oil and hydrolate of the roots of Anthriscus sylvestris (L.) Hoffm. from Saguenay. J. Essent. Oil Res. 2015, 27, 373–379. [Google Scholar] [CrossRef]
- National Institutes of Health (HIH). PubChem Open Chemistry Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ (accessed on 27 May 2021).
- Wawrzynczak, K.; Sadowska, B.; Więckowska-Szakiel, M.; Kalemba, D. Composition and antimicrobial activity of Myrica gale L. leaf and flower essential oils and hydrolates. Rec. Nat. Prod. 2020, 15, 35–45. [Google Scholar] [CrossRef]
- Selles, C.; Lemouchi, R.; Dib, M.E.A. Chemical constituents of essential oil and hydrosol extract from Teucrium kabylicum Batt., an endemic species from Algeria. Agric. Conspec. Sci. 2020, 85, 375–377. [Google Scholar]

| KEM | DEF | SIR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RI | Compound | Mean | SD | Max | Mean | SD | Max | Mean | SD | Max |
| Oil yield (% v/w) | 1.29 | 0.27 | 1.6 | 0.24 | 1.66 | 0.42 | ||||
| Monoterpene Hydrocarbons | ||||||||||
| 922 | Tricyclene | 0.1 | <0.05 | 0.2 | 0.2 | <0.05 | 0.2 | 0.2 | 0.1 | 0.2 |
| 931 | α-Thujene | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 | <0.05 | <0.05 | 0.1 |
| 938 | α-Pinene | 36.8 | 6.2 | 44.8 | 37.2 | 2.6 | 42.2 | 36.8 | 5.0 | 43.9 |
| 950 | Camphene | 1.6 | 0.8 | 3.1 | 1.7 | 0.8 | 3.5 | 1.8 | 0.9 | 3.1 |
| 978 | β-Pinene | 7.0 | 2.4 | 10.3 | 8.7 | 1.6 | 10.6 | 9.0 | 2.0 | 11.9 |
| 993 | Myrcene * | 1.2 a | 0.2 | 1.6 | 1.4 ab | 0.1 | 1.6 | 1.4 b | 0.2 | 1.6 |
| 1003 | α-Phellandrene | 0.1 | <0.05 | 0.2 | 0.1 | <0.05 | 0.2 | 0.2 | 0.1 | 0.2 |
| 1009 | δ-3-Carene * | 1.2 ab | 0.6 | 2.7 | 0.5 a | 0.5 | 1.6 | 1.2 b | 0.4 | 1.8 |
| 1018 | α-Terpinene | <0.05 | <0.05 | 0.1 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 |
| 1027 | p-Cymene | 0.1 | 0.05 | 0.2 | 0.1 | 0.05 | 0.1 | 0.1 | 0.1 | 0.2 |
| 1032 | β-Phellandrene ** | 27.5 | 5.4 | 37.8 | 29.8 | 3.0 | 33.5 | 30.0 | 4.8 | 37.2 |
| 1062 | γ-Terpinene | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 |
| 1089 | Terpinolene | 0.5 | 0.3 | 1.2 | 0.4 | 0.2 | 0.9 | 0.6 | 0.3 | 1.0 |
| Sum | 76.3 | 80.3 | 81.3 | |||||||
| Oxidised Monoterpenes | ||||||||||
| 1097 | α-Pinene-epoxide | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 | <0.05 | <0.05 | 0.1 |
| 1123 | trans-Pinene-hydrate | <0.05 | <0.05 | 0.1 | <0.05 | <0.05 | 0.1 | |||
| 1129 | α-Campholenal | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 | |||
| 1141 | trans-Pinocarveol | <0.0 | 0.1 | 0.1 | 0.1 | <0.05 | 0.1 | 0.1 | 0.1 | 0.2 |
| 1147 | Camphor | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 | |||
| 1148 | trans-Verbenol | <0.05 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 | <0.05 | 0.1 | 0.2 |
| 1165 | Pinocarvone | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 | <0.05 | <0.05 | 0.1 |
| 1168 | Borneol | <0.05 | <0.05 | 0.1 | <0.05 | <0.05 | 0.1 | 0.1 | 0.1 | 0.2 |
| 1176 | Isopinocamphone | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |||
| 1180 | Terpinen-4-ol | <0.05 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 |
| 1188 | Cryptone | 0.4 | 0.4 | 1.1 | 0.5 | 0.2 | 0.9 | 0.3 | 0.5 | 1.7 |
| 1192 | α-Terpineol | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 |
| 1197 | Myrtenal | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 | 0.1 | 0.1 | 0.2 |
| 1211 | Verbenone | <0.05 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 | <0.05 | 0.1 | 0.2 |
| 1238 | Thymol-methylether | 0.5 | 0.2 | 0.9 | 0.6 | 0.2 | 0.8 | 0.8 | 0.3 | 1.2 |
| 1244 | Cuminic aldehyde | 0.1 | 0.1 | 0.2 | 0.1 | <0.05 | 0.2 | <0.05 | 0.1 | 0.3 |
| 1288 | Bornyl acetate | 0.5 | 0.3 | 1.2 | 0.6 | 0.4 | 1.6 | 0.7 | 0.5 | 1.6 |
| Sum | 1.9 | 2.6 | 2.6 | |||||||
| Sesquiterpene Hydrocarbons | ||||||||||
| 1354 | α-Cubebene | 0.2 | 0.2 | 0.5 | 0.1 | 0.1 | 0.5 | 0.1 | 0.1 | 0.3 |
| 1380 | α-Copaene | 0.2 | <0.05 | 0.2 | 0.1 | <0.05 | 0.2 | 0.1 | <0.05 | 0.2 |
| 1391 | β-Bourbonene | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 | <0.05 | <0.05 | 0.1 |
| 1394 | β-Cubebene | 0.2 | 0.1 | 0.4 | <0.05 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 |
| 1425 | β-Caryophyllene | 0.9 | 0.5 | 2.3 | 1.0 | 0.4 | 1.5 | 1.0 | 0.4 | 1.6 |
| 1446 | Aromadendrene | 0.2 | 0.1 | 0.4 | 0.2 | 0.1 | 0.4 | 0.2 | 0.1 | 0.4 |
| 1460 | α-Humulene | 0.7 | 0.5 | 1.2 | 0.8 | 0.4 | 1.5 | 0.9 | 0.5 | 1.7 |
| 1470 | epi-Bicyclo-sesquiphellandrene | 0.1 | <0.05 | 0.2 | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.2 |
| 1483 | γ-Muurolene * | 0.9 b | 0.3 | 1.4 | 0.3 a | 0.4 | 1.1 | 0.5 ab | 0.2 | 0.8 |
| 1488 | Germacrene D * | 7.4 b | 2.2 | 10.4 | 4.5 a | 2.7 | 9.9 | 4.3 a | 2.3 | 7.4 |
| 1502 | Bicyclogermacrene | 1.8 | 0.6 | 2.7 | 1.5 | 0.3 | 2.1 | 1.3 | 0.4 | 1.8 |
| 1505 | α-Muurolene * | 0.7 b | 0.2 | 1.2 | 0.6 ab | 0.1 | 0.6 | 0.5 a | 0.1 | 0.7 |
| 1513 | β-Bisabolene | 0.3 | 0.3 | 0.9 | 0.6 | 0.2 | 1.0 | 0.7 | 0.3 | 1.2 |
| 1520 | γ-Cadinene * | 1.4 a | 0.3 | 1.7 | 1.2 ab | 0.2 | 1.5 | 1.0 b | 0.3 | 1.4 |
| 1530 | δ-Cadinene | 2.8 | 0.5 | 3.7 | 2.2 | 0.3 | 2.7 | 2.1 | 0.6 | 2.8 |
| 1539 | trans-Cadina-1,4-diene | 0.1 | <0.05 | 0.2 | 0.1 | <0.05 | 0.1 | 0.1 | <0.05 | 0.1 |
| 1545 | α-Cadinene | 0.2 | 0.1 | 0.3 | 0.2 | <0.05 | 0.2 | 0.1 | <0.05 | 0.2 |
| 1548 | cis-α-Bisabolene | 0.1 | 0.1 | 0.2 | <0.05 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 |
| Sum | 18.3 | 13.6 | 13.4 | |||||||
| Oxidised Sesquiterpenes | ||||||||||
| 1583 | Germacrene D-4-ol | 0.4 | 0.2 | 0.8 | 0.4 | 0.2 | 0.9 | 0.2 | 0.1 | 0.3 |
| 1585 | Spathulenol | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 | <0.05 | 0.1 | 0.2 |
| 1591 | Caryophyllene oxide | 0.2 | 0.1 | 0.4 | 0.2 | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 |
| 1650 | τ-Cadinol * | 0.4 b | 0.2 | 0.7 | 0.4 ab | 0.1 | 0.6 | 0.2 a | 0.1 | 0.3 |
| 1663 | α-Cadinol | 0.3 | 0.1 | 0.6 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 |
| 1692 | α-Bisabolol | 0.1 | 0.1 | 0.3 | 0.1 | <0.05 | 0.2 | 0.1 | 0.1 | 0.3 |
| Sum | 1.4 | 1.3 | 0.9 | |||||||
| Other | ||||||||||
| 1199 | Estragol | 0.2 | 0.2 | 0.5 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 |
| Total Sum | 98.0 | 98.0 | 98.3 | |||||||
| RI | KEM | DEF | SIR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Mean | SD | Max | Mean | SD | Max | ||
| Monoterpene Hydrocarbons | ||||||||||
| 933 | α-Pinene * | 2.2 b | 0.7 | 3.2 | 1.4 a | 0.7 | 2.9 | 1.0 a | 0.4 | 1.6 |
| 978 | β-Pinene | 0.2 | 0.2 | 0.6 | ||||||
| 1032 | β-Phellandrene *,** | 1.9 b | 0.9 | 3.8 | 1.2 ab | 0.5 | 2.2 | 0.9 a | 0.5 | 1.9 |
| Sum | 4.4 | 2.6 | 1.9 | |||||||
| Oxidized Monoterpenes | ||||||||||
| 1034 | 1,8-Cineol | 0.5 | 0.2 | 0.9 | 0.4 | 0.3 | 0.8 | 0.7 | 0.5 | 1.3 |
| 1088 | Fenchone | 0.2 | 0.1 | 0.5 | 0.2 | 0.1 | 0.3 | 0.3 | 0.2 | 0.6 |
| 1100 | Linalool | 0.6 | 1.1 | 3.7 | 0.2 | 0.4 | 1.3 | 0.2 | 0.4 | 1.2 |
| 1112 | exo-Fenchol | 0.8 | 0.2 | 1.1 | 0.9 | 0.1 | 1.1 | 0.9 | 0.1 | 1.0 |
| 1120 | cis-p-Menth-2-en-1-ol | 3.7 | 1.6 | 5.5 | 4.7 | 1.6 | 7.6 | 4.2 | 1.4 | 6.3 |
| 1126 | α-Campholenal | 0.3 | 0.1 | 0.6 | 0.3 | 0.2 | 0.6 | 0.3 | 0.2 | 0.5 |
| 1137 | trans-Pinocarveol | 2.4 | 1.0 | 4.5 | 3.6 | 3.4 | 10.7 | 2.9 | 2.0 | 7.2 |
| 1139 | tr.-p-Menth-2-en-1-ol | 0.7 | 0.4 | 1.4 | 1.5 | 1.2 | 3.3 | 1.4 | 0.7 | 2.2 |
| 1143 | Camphor | 0.7 | 0.8 | 2.3 | 0.6 | 0.4 | 1.5 | 0.8 | 0.6 | 2.2 |
| 1145 | trans-Verbenol | 1.0 | 0.5 | 1.6 | 0.9 | 0.2 | 1.3 | 0.9 | 0.4 | 1.4 |
| 1147 | Camphene Hydrate | 0.6 | 0.4 | 1.3 | 0.6 | 0.4 | 1.3 | 0.4 | 0.2 | 0.8 |
| 1160 | trans-Pinocamphone | 0.4 | 0.3 | 0.9 | 0.6 | 0.5 | 1.6 | 0.3 | 0.1 | 0.5 |
| 1162 | Pinocarvone | 0.3 | 0.1 | 0.5 | 0.2 | 0.3 | 0.8 | 0.2 | 0.1 | 0.4 |
| 1165 | Borneol | 3.1 | 1.6 | 6.1 | 3.5 | 1.1 | 5.5 | 3.6 | 1.5 | 6.8 |
| 1167 | Mentha-1,5-dien-8-ol | 1.5 | 0.4 | 2.1 | 1.8 | 0.8 | 3.3 | 2.3 | 0.9 | 3.5 |
| 1173 | cis-Pinocamphone | 0.6 | 0.2 | 0.9 | 0.6 | 0.3 | 1.1 | 0.6 | 0.2 | 1.1 |
| 1177 | Terpinen-4-ol | 6.3 | 1.2 | 7.7 | 7.0 | 2.1 | 10.6 | 7.8 | 1.2 | 10.3 |
| 1183 | p-Cymen-8-ol | 0.4 | 0.2 | 0.7 | 0.4 | 0.3 | 1.2 | |||
| 1186 | Cryptone | 4.0 | 1.8 | 6.1 | 3.2 | 1.6 | 5.0 | 3.0 | 1.7 | 5.1 |
| 1190 | α-Terpineol * | 27.7 a | 5.4 | 34.6 | 33.1 ab | 4.7 | 39.3 | 34.1 b | 4.1 | 40.5 |
| 1196 | Myrtenal | 3.5 | 1.3 | 5.3 | 3.8 | 0.9 | 5.1 | 4.1 | 0.8 | 5.6 |
| 1200 | trans-Piperol | 0.2 | 0.2 | 0.7 | 0.3 | 0.2 | 0.7 | 0.2 | 0.1 | 0.4 |
| 1209 | Verbenone | 6.1 | 1.6 | 8.5 | 6.3 | 2.1 | 9.5 | 7.0 | 1.1 | 8.5 |
| 1220 | trans-Carveol | 1.2 | 0.3 | 1.7 | 1.1 | 0.4 | 1.5 | 1.4 | 0.5 | 2.2 |
| 1230 | cis-p-Mentha-1(7),8-dien-2-ol | 0.8 | 0.5 | 1.8 | 0.6 | 0.4 | 1.4 | 0.4 | 0.2 | 1.0 |
| 1236 | Thymol-Methylether | 0.2 | 0.1 | 0.5 | 0.2 | 0.1 | 0.3 | 0.2 | 0.2 | 0.6 |
| 1246 | Carvotanacetone | 0.5 | 0.2 | 0.7 | 0.4 | 0.3 | 0.9 | 0.5 | 0.3 | 0.8 |
| 1256 | Piperitone | 1.7 | 0.9 | 3.6 | 3.7 | 2.6 | 8.2 | 2.7 | 1.4 | 5.9 |
| 1261 | trans-Myrtanol | 0.3 | 0.3 | 0.9 | 0.2 | 0.1 | 0.4 | 0.3 | 0.3 | 0.9 |
| 1287 | Bornyl Acetate | 1.5 | 1.1 | 4.0 | 1.9 | 1.7 | 6.0 | 2.2 | 1.4 | 5.2 |
| Sum | 71.5 | 82.3 | 84.4 | |||||||
| Sesquiterpene Hydrocarbons | ||||||||||
| 1423 | β-Caryophyllene | 0.3 | 0.5 | 1.3 | 0.2 | 0.2 | 0.5 | |||
| 1485 | Germacrene-D * | 5.6 b | 4.9 | 16.5 | 1.4 a | 1.9 | 6.3 | 1.3 a | 1.6 | 4.6 |
| 1504 | α-Muurolene | 0.3 | 0.2 | 0.6 | ||||||
| 1519 | γ-Cadinene | 0.9 | 1.0 | 3.6 | 0.4 | 0.5 | 1.4 | 0.3 | 0.3 | 0.8 |
| 1527 | δ-Cadinene | 3.1 | 2.7 | 10.3 | 1.5 | 1.2 | 3.9 | 1.0 | 1.0 | 2.9 |
| Sum | 10.2 | 3.4 | 2.5 | |||||||
| Oxidized Sesquiterpenes | ||||||||||
| 1584 | Spathulenol | 0.4 | 0.2 | 0.7 | 0.3 | 0.2 | 0.5 | 0.2 | 0.2 | 0.6 |
| 1634 | cis-Cadin-4-en-7-ol | 0.3 | 0.1 | 0.5 | 0.1 | 0.1 | 0.3 | 0.2 | 0.1 | 0.4 |
| 1648 | τ-Muurolol * | 2.9 b | 0.8 | 4.2 | 2.4 ab | 0.9 | 4.2 | 1.8 a | 0.8 | 2.9 |
| 1652 | Torreyol | 0.6 | 0.3 | 1.3 | 0.5 | 0.2 | 1.0 | 0.5 | 0.2 | 0.9 |
| 1661 | α-Cadinol * | 5.4 b | 2.1 | 8.3 | 4.5 ab | 1.7 | 8.2 | 3. a1 | 1.2 | 4.7 |
| 1690 | α-Bisabolol | 0.3 | 0.5 | 1.4 | 0.6 | 0.3 | 1.3 | 0.8 | 0.6 | 1.7 |
| Sum | 9.9 | 8.5 | 6.5 | |||||||
| Total Sum | 96.0 | 96.8 | 95.4 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chizzola, R.; Billiani, F.; Singer, S.; Novak, J. Diversity of Essential Oils and the Respective Hydrolates Obtained from Three Pinus cembra Populations in the Austrian Alps. Appl. Sci. 2021, 11, 5686. https://doi.org/10.3390/app11125686
Chizzola R, Billiani F, Singer S, Novak J. Diversity of Essential Oils and the Respective Hydrolates Obtained from Three Pinus cembra Populations in the Austrian Alps. Applied Sciences. 2021; 11(12):5686. https://doi.org/10.3390/app11125686
Chicago/Turabian StyleChizzola, Remigius, Felix Billiani, Stefan Singer, and Johannes Novak. 2021. "Diversity of Essential Oils and the Respective Hydrolates Obtained from Three Pinus cembra Populations in the Austrian Alps" Applied Sciences 11, no. 12: 5686. https://doi.org/10.3390/app11125686






