Abstract
Streptococcus thermophilus is widely used in dairy fermentation as a starter culture for yogurt and cheese production. Many strains are endowed with potential probiotic properties; however, since they might not survive in adequate amounts after transit through the human gastrointestinal tract, it is advisable to improve cell survivability during this passage. The present study evaluates the use of 2′-fucosyllactose, a prebiotic molecule from human milk, compared with other known molecules, such as gelatin and inulin, to form alginate-based microcapsules to fulfill these requirements. Such microcapsules, obtained by the extrusion technique, were evaluated in terms of encapsulation efficiency, storage stability, gastrointestinal condition resistance, and cell release kinetics. Results reveal that microcapsules made using 2′-fucosyllactose and those with inulin resulted in the most efficient structure to protect S. thermophilus strain TH982 under simulated gastrointestinal conditions (less than 0.45 log CFU/g decrease for both agents). In addition, a prompt and abundant release of encapsulated cells was detected after only 30 min from microcapsules made with sodium alginate plus 2′-fucosyllactose in simulated gastrointestinal fluid (more than 90% of the cells). These encouraging results represent the first report on the effects of 2′-fucosyllactose used as a co-encapsulating agent.
1. Introduction
The genus Streptococcus includes more than 70 species, but to date, only S. thermophilus possesses the GRAS (generally recognized as safe) status due to its long history of safe application in food production [1]. For this reason, this is the only species of the genus used in the food industry and represents the second most important species of industrial lactic acid bacteria (LAB) after Lactococcus lactis and one of the primary starters of yogurt [2,3]. Furthermore, many S. thermophilus strains have several technological properties, such as sugar metabolism, galactose utilization capability, proteolytic activity, and urease activity [4].
Some strains have also shown potential as a probiotic, having revealed various health benefits [5,6]. S. thermophilus is a bacterium of non-human origin and, although known to be sensitive to the acidic gastric conditions, it was able to survive the gastrointestinal tract and moderately capable of adhesion to intestinal epithelial cells [7], thus making it a transient probiotic [7,8]. Nowadays, probiotics are one of the driving forces in the design of functional foods, particularly dairy products; hence it is advisable to improve cell survivability during the gastrointestinal passage. The inclusion of probiotics into microcapsules seems to be a promising solution and also a means to control the release of cells into the target tissues [9].
Several techniques are available for encapsulation, which differ on the nature of their core (content) and intended use [10]. Encapsulation techniques applied to the food industry include spray-drying, fluid bed coating, spray-chilling or spray cooling, extrusion, emulsification, coacervation, co-extrusion, inclusion complexation, liposome entrapment, centrifugal extrusion, encapsulation by a rapid expansion of supercritical fluid (RESS), freeze- or vacuum drying, and nanotechnological approaches [11]. The use of extrusion technology has many advantages for the encapsulation of probiotic cells. It is a simple and inexpensive method using mild operations, and the procedure does not involve any harmful solvent. Moreover, this technique appears to promote probiotic cell viability [12,13,14,15].
Combining alginate with some prebiotic molecules can also enhance cell protection in food systems [10]. Prebiotics form three-dimensional microcrystal networks that interact together, forming small aggregates that contribute to the better protection of the cells [16]. The most frequently used prebiotics in co-encapsulation are oligosaccharides, inulin, and resistant starches [16].
2’-fucosyllactose (2′-FL), is a neutral trisaccharide composed of L-fucose, D-galactose, and D-glucose and is the most abundant oligosaccharide in human milk (HMO), accounting for about 30% of all of HMOs, i.e., 2–3 g/L [17]. This prebiotic molecule has been reported to display numerous health advantages in humans [18,19,20]. Another attractive characteristic of 2′-FL is its simplicity, which facilitates its de novo synthesis using microbial, enzymatic, or chemical methods [21].
In this study, we aimed at evaluating the protective effect of 2’-FL on S. thermophilus TH982 cells, a potential probiotic with the ability to produce high levels of folate [22] compared to some other common co-encapsulating materials in alginate beads.
2. Materials and Methods
2.1. Bacteria and Growth Conditions
S. thermophilus TH982 [23] is part of the collection of the Department of Agronomy, Food, Natural resources, Animals and Environment, University of Padova, Italy. S. thermophilus TH982 was stored at −80 °C in M17 broth (Sigma-Aldrich, St. Louis, MI, USA) containing glycerol (25% v/v) and was activated before each experiment by culturing it in M17 (Sigma-Aldrich) broth at 37 °C for 24 h.
2.2. Encapsulation Procedure
The components used for encapsulation of S. thermophilus TH982 were sodium alginate (S, Sigma-Aldrich), gelatin (G, from bovine skin, Sigma-Aldrich), inulin (I, from chicory, Sigma-Aldrich) and 2′-fucosyllactose (F, Sigma-Aldrich). Fifty milliliters of reactivated cells in M17 broth were centrifuged (Hettich, Westphalia, Germany) at 5500 rpm for 5 min. The bacterial pellets were washed twice with sterile saline solution (0.85%) (Sigma-Aldrich) and added to the encapsulation matrix.
The encapsulating matrix was prepared using the extrusion technique through the combination of sodium alginate with either inulin, gelatin, or 2′-fucosyllactose, each one at a concentration of 2% (w/v). Sodium alginate alone was also used as a control. The different matrices were mixed and added to the S. thermophilus TH982 pellet suspension.
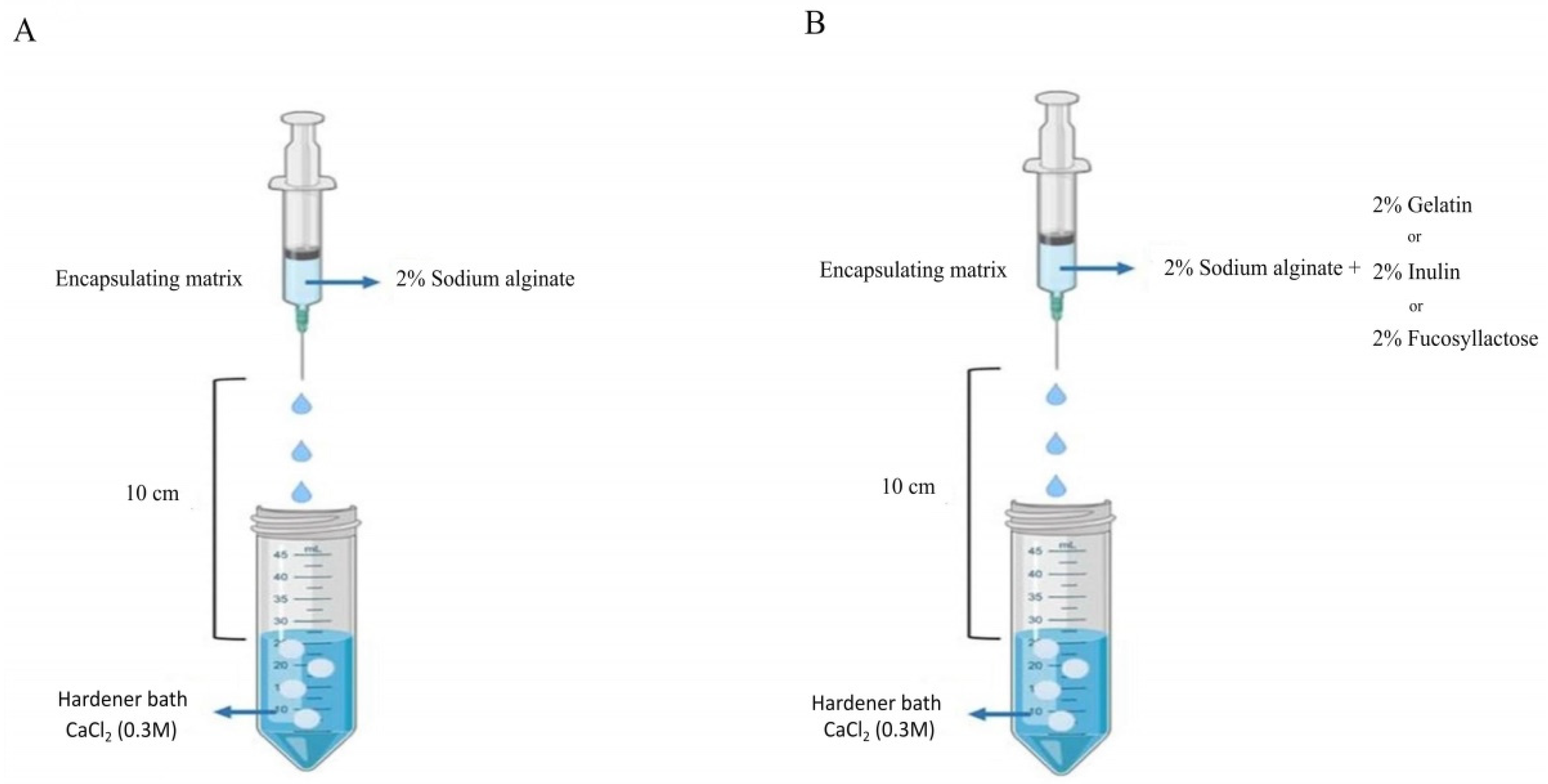
The mixture was then injected using a sterile syringe with a 450 µm needle into sterile 0.3 M CaCl2 (Sigma-Aldrich) hardener solution. The distance from the bottom of the nozzle and the surface of the CaCl2 solution was kept at 10 cm (Figure 1). The microcapsules were left in the hardening solution (CaCl2) for 30 min at room temperature. Finally, capsules were transferred in 0.1% (w/v) sterile peptone water solution and stored at 4 °C until further use. The morphology of the microcapsules was examined using an optical stereomicroscope (Olympus, Tokyo, Japan).
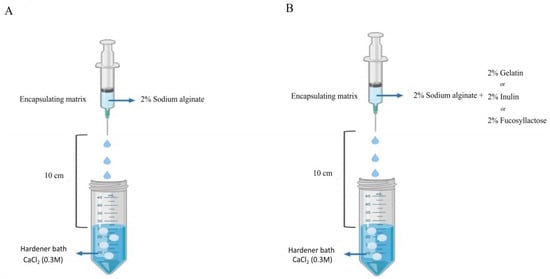
Figure 1.
Scheme of microencapsulation procedure of S. thermophilus TH982 cells using the extrusion technique. (A) The sodium alginate alone as control. (B) Sodium alginate with other agents.
2.3. Encapsulation Efficiency
One milliliter of each type of capsule solution was added to 9 mL of saline solution (0.85%), serially diluted, and plated on M17 agar medium to determine the viable cell concentration in capsule solutions. One gram of each type of capsule was homogenized in 9 mL of 10% (w/v) sterilized sodium citrate by vortexing for 1 min, serially diluted, and plated on M17 to get the number of entrapped cells per g of capsule. The enumeration of viable cells from both the solution and the capsule was done using the micro drop technique by placing 20 µL aliquots on the surface of M17 agar plates and incubating at 37 °C for 48 h. The encapsulation efficiency (EE%) was determined using the equation [24]:
where N1 is the number of viable entrapped cells (log CFU/g) released after the encapsulation procedure and N0 is the number of viable cells (log CFU/mL) added to the mixture before encapsulation.
EE% = (N1/N0) × 100
2.4. Storage Stability in Skimmed Milk
One gram of microcapsules was suspended in 9 mL of 10% (w/v) reconstituted skimmed milk, stored under refrigerated conditions (4 °C) and sampled at different time intervals, namely after 0, 7, 14 and 21 days to evaluate the survival of encapsulated S. thermophilus TH982 [25]. The survival of free (non-encapsulated) bacterial cells was determined by adding 9 mL of 10% (w/v) reconstituted skimmed milk (Sigma-Aldrich) to 1 mL of free cells as well. At each time interval, 1 g aliquot of capsules was aseptically centrifuged at 5500 rpm for 5 min. The supernatant was discarded and the cells were allowed to release in 9 mL of 10% (w/v) sodium citrate by vortexing for 1 min, serially diluted, and plated on M17 agar, followed by incubation at 37 °C for 48 h. The survival was determined as the number of cells recovered during different storage time intervals with respect to the number of initial entrapped cells.
2.5. Survivability under Simulated Gastrointestinal Conditions
The simulated gastrointestinal conditions were obtained using basic fluid, gastric fluid, and intestinal fluid. The basic fluid was prepared by dissolving (per liter): 1.12 g potassium chloride (Sigma-Aldrich), 2.0 g sodium chloride (Sigma-Aldrich), 0.11 g calcium chloride (Sigma-Aldrich), and 0.4 g potassium dihydrogen phosphate (Sigma-Aldrich) in saline solution (0.85%). The basic fluid was sterilized by autoclaving at 121 °C for 15 min. The simulated gastric fluid (SGJ) and simulated intestinal fluid (SIJ) were prepared based on a modified method published by Singh et al. [25].
The gastric fluid consisted of 0.01 g/L of swine pepsin (Sigma-Aldrich) and 0.01 g/L of swine mucin (Sigma-Aldrich) added directly to the sterile basic juice. The pH was adjusted to 2.5 with 1 N HCl, and the liquid was filter-sterilized using a 0.22 µm membrane filter (Sigma-Aldrich).
The intestinal fluid contained (per liter of basic fluid): 0.01 g pancreatin (Sigma-Aldrich), 0.08 g Ox-bile extract (Sigma-Aldrich), and 0.01 g lysozyme (Sigma-Aldrich). The pH was adjusted to 7.5 with 1 N NaOH, and the juice was filter sterilized. Both gastric and intestinal fluids were prepared fresh on the day of the experiment.
Overnight bacterial cultures were obtained after subculturing in M17 broth, and capsules were prepared as described in the encapsulation procedure. One gram of each type of capsule was added to 9 mL of SGJ and incubated at 37 °C for 1 h to evaluate the survivability of S. thermophilus TH982 under gastric conditions. For the survival of S. thermophilus TH982 under gastrointestinal conditions, 9 mL of SIJ were added following gastric fluid incubation, and the capsules and free cells with simulated gastrointestinal fluid were left at 37 °C for a further 2 h. The cell viability was evaluated for free and encapsulated S. thermophilus after gastric and gastrointestinal incubations by plating on M17 agar and using the micro drop technique, followed by incubation at 37 °C for 48 h.
2.6. Release Kinetics
The release kinetics from capsules were determined using phosphate solution without enzymes. The release of encapsulated S. thermophilus TH982 was evaluated according to Shi et al. [26] with some modifications. A phosphate solution without the addition of intestinal enzymes (6.8 g/L of K2HPO4, 50 mM) was prepared, the pH was adjusted to 7.5 with 1 N HCl and subsequently sterilized by autoclaving at 121 °C for 15 min. Microcapsules (1 g) from different matrices were added to 9 mL of the abovementioned phosphate solution and incubated at 37 °C with agitation (100 rpm). At predetermined time intervals (0, 30, 60, 90, 120, 150, and 180 min), 100 µL of solution were taken from each capsule type, serially diluted, and plated using the micro drop technique on M17 agar to detect the number of released cells. The number of released cells was determined after 48 h of incubation at 37 °C and expressed as log CFU/mL of K2HPO4 solution.
2.7. Statistical Analysis
All the experiments were performed in triplicate. Data were analyzed using a one-way analysis of variance (ANOVA). Tukey’s test was used as a post-hoc analysis by the GraphPad Prism software (version 7, GraphPad Software, Inc., San Diego, CA, USA). Results were considered significantly different for p values lower than 0.05.
3. Results and Discussion
3.1. Morphology and Encapsulation Efficiency
All capsules appeared globular and irregular in shape with a rough surface and a drop-like shape. This result is probably due to the high surface tension of the used hardening solution (CaCl2) that determines an imperfect sphere formation [27]. However, no surface cavities or fractures were visible. The average size of capsules was 3.5 ± 0.52 (mm) without any significant difference among the different microcapsules types. The capsules with S + G showed a more dense structure, as confirmed by the viscosity of G solution, due to electrostatic interactions between amino groups of G and carboxyl groups of S, which make cells more resistant to enzymatic and acidic hydrolysis [28].
Encapsulation efficiencies (EE%) of the four microcapsule types are reported in Table 1. EE% is a parameter that describes both the survival of viable cells and the efficacy of entrapment of the encapsulation procedure [29]. The yield of S. thermophilus TH982 viable cells co-encapsulated using S + F (96.13%) was significantly higher (p < 0.01) than that of cells encapsulated with S alone (91.07%), used as control, and was similar (not statistically different) to S + I (98.61%). The high EE% obtained with S + F and S + I matrices might be due to the decrease in the porosity of the gel-beads and, consequently, to a reduction in the leakage of entrapped S. thermophilus TH982 cells [30]. Besides, incorporating prebiotics as material for encapsulation may better protect probiotics in food systems and inside the gastrointestinal tract due to mutual cells/prebiotic interactions [31]. By contrast, the number of viable cells encapsulated in S alone and S + G showed no significant difference (EE% 91.07 and 90.50, respectively). The main reason why EE% is normally lower than 100% is linked to cell damage due to detrimental conditions caused by the encapsulation process itself, such as shear stress and the use of concentrated solutions. Furthermore, during the time required for the hardening of capsules, a physical loss of cells can occur in significant numbers [29]. It should also be noted that a dissolution process is required to determine the number of viable cells in the microcapsules, and therefore an incomplete disintegration can underestimate the calculated EE% value. In particular, for the solubilization of S-based capsules, sodium citrate (10% w/v) was used, which is a chelating agent [30] that can remove calcium from the “egg-box” structure, leading to the destabilization of alginate coating and to the effective release of cells [32].

Table 1.
Encapsulation efficiency (EE%) of S. thermophilus TH982 using different encapsulating matrices.
3.2. Storage Stability in Skimmed Milk
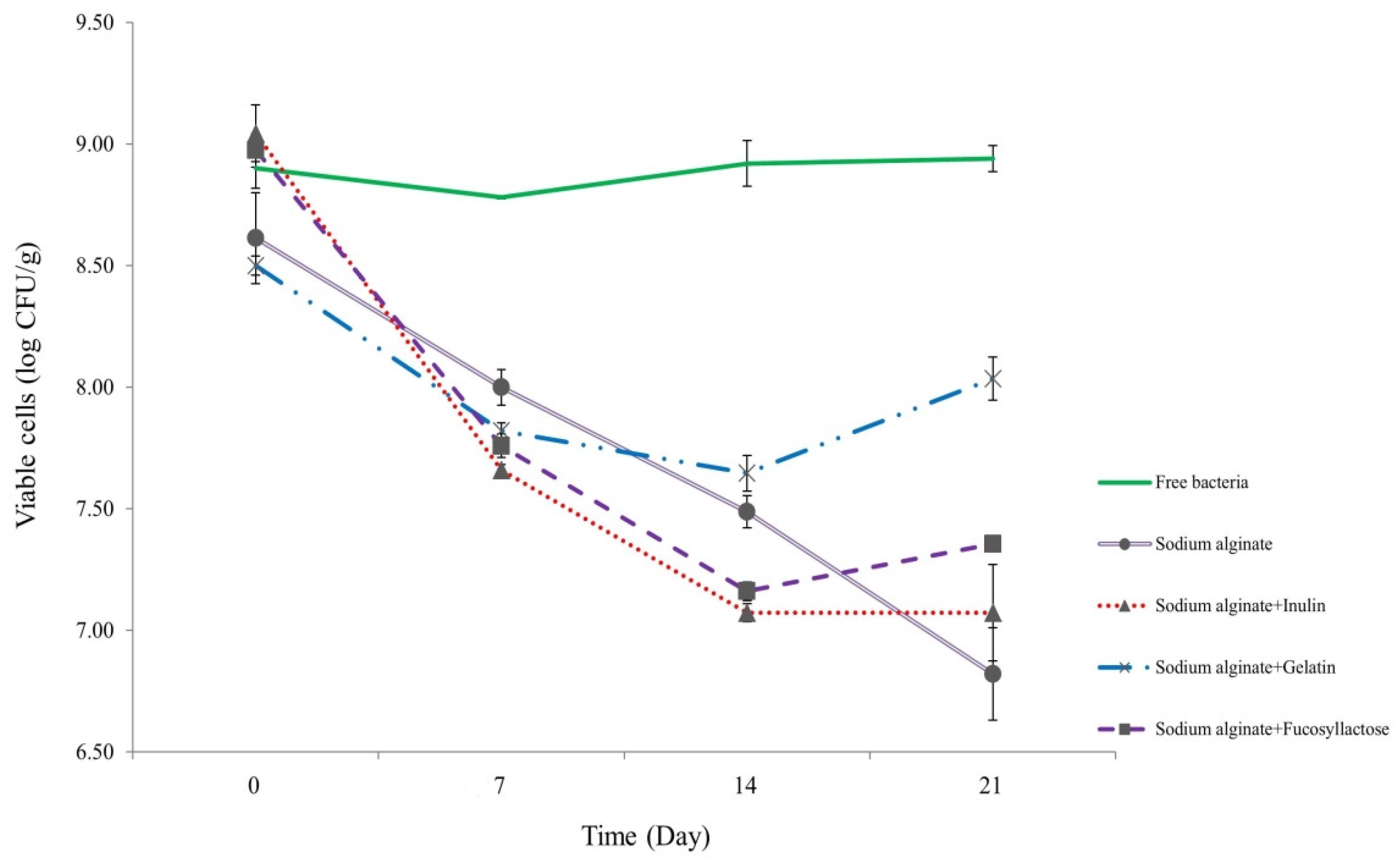
In order to test the viability of S. thermophilus cells inside capsules during prolonged storage, we maintained them in skimmed milk at refrigeration conditions (4 °C) for 21 days (Figure 2). At the end of the period, cell viability showed 0.46, 1.62, 1.79, and 1.97 log decrease for S. thermophilus TH982 cells encapsulated with S + G, S + F, S, and S + I, respectively. Capsules containing G as a co-encapsulating agent showed a better performance after 21 days with respect to other co-encapsulating agents. However, data show that free cells had the highest viability level throughout the storage period since there was no significant difference between initial (8.90 ± 0.08 log CFU/mL; day0) and final (8.94 ± 0.05 log CFU/mL; day21) values.
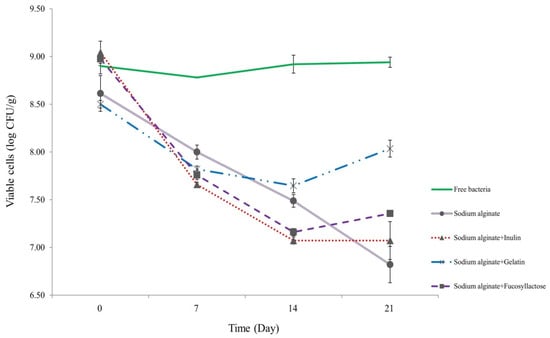
Figure 2.
Survivability of encapsulated and free S. thermophilus TH982 cells during 21 days of storage in skimmed milk at 4 °C.
Interestingly, S, S + I, and S + F capsules revealed a similar, not significantly different, viability reduction (p < 0.05). Unlike some studies that indicate an increase in survivability during refrigerated storage conditions, which could be linked to the difference in species/strain used. S. thermophilus is frequently used in the production of different dairy products, and it is highly adapted to the dairy environment. It was reported that encapsulation improved the stability of L. plantarum during storage [33], and encapsulation of bacteria in alginate was found to improve survivability when compared to unprotected cell counts stored in skimmed milk for 24 h [14]. Since FAO and the International Dairy Federation (IDF) recommend that the minimum content of probiotic cells should be 107 CFU/g of product at the time of consumption [34], the four encapsulating agents used in this study were efficient in maintaining the required viability of S. thermophilus TH982 after refrigerated storage in skimmed milk. The high numbers suggested by IDF have been proposed to compensate for the possible numerical reduction of probiotic organisms during passage through the human stomach and intestine. The cells must remain viable throughout the projected shelf-life period of a product so that when consumed, the product contains a sufficient number of viable cells.
The viability of probiotic bacteria in food products is affected by many intrinsic and extrinsic aspects such as dissolved oxygen and oxygen permeation through the package, post acidification in fermented products (lactic and acetic acids), pH, storage and incubation temperature, duration of fermentation, production of hydrogen peroxide due to bacterial metabolism, and processing conditions [35,36]. Moreover, the specific strains used, the interaction between species, availability of nutrients, and growth promoters and inhibitors can affect viable cells’ survivability [14].
The great survivability of free S. thermophilus TH982 cells in skimmed milk is probably due to the fact that S. thermophilus is a species highly adapted to grow on lactose as its energy and carbon source, that is internalized by lactose permease (LacS) and hydrolyzed to glucose and galactose by β-galactosidase (LacZ) [37]. It is likely that entrapment of S. thermophilus TH982 cells into capsules of different matrices probably led to a reduced diffusion of lactose inside the capsule layer or membrane, limiting the possibility of its use by the entrapped cells [35].
3.3. Survivability under Simulated Gastrointestinal Conditions
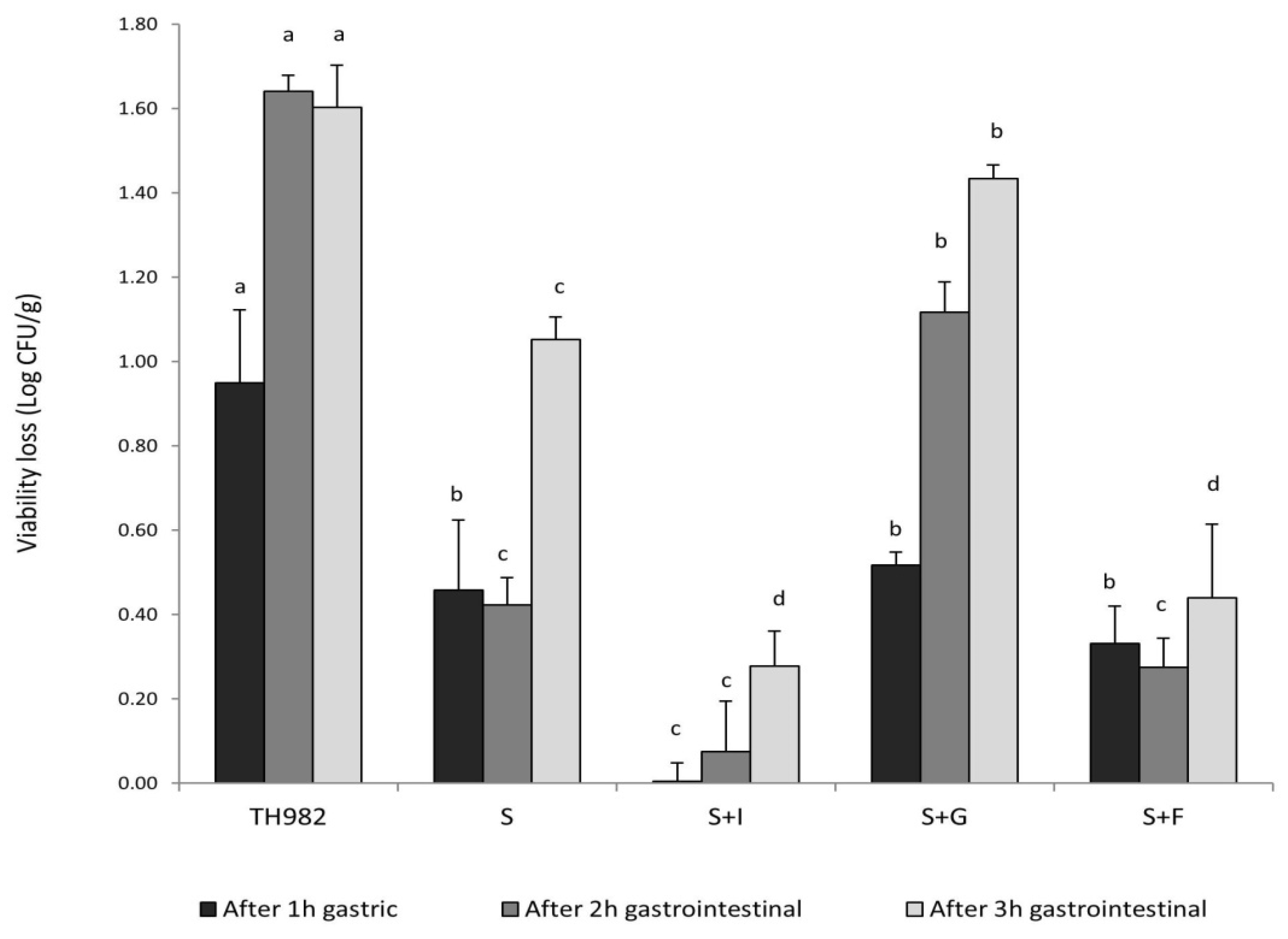
The survival of probiotics under gastrointestinal conditions represents a significant issue for their effectiveness, and microencapsulation could represent a valid method to provide significant protection [15]. The survival of free and encapsulated S. thermophilus TH982 cells after 1, 2, and 3 h of incubation in simulated gastrointestinal conditions are presented in Figure 3. After 1 h of gastric fluid incubation, the highest reduction of viable cells was found, as expected, for free S. thermophilus TH982, as the initial number of 10.40 ± 0.10 log CFU/mL was diminished by 0.95 log CFU/mL. A notable decrease in viability after 3 h of gastrointestinal conditions was confirmed for free S. thermophilus TH982 cells, reduced by approximately 1.60-log CFU/mL, dropping to 8.79 ± 0.02 log CFU/mL. By contrast, for cells encapsulated with S alone, only 1.05 log CFU/g reduction of viable cells was observed, implying that microencapsulation provided protection compared to the free cells. This protection offered by sodium alginate might be related to the establishment of a hydrogel barrier through an external layer of sodium alginate that has retarded the permeation of simulated fluids into the capsules and thus the interaction with the probiotic cells [38].
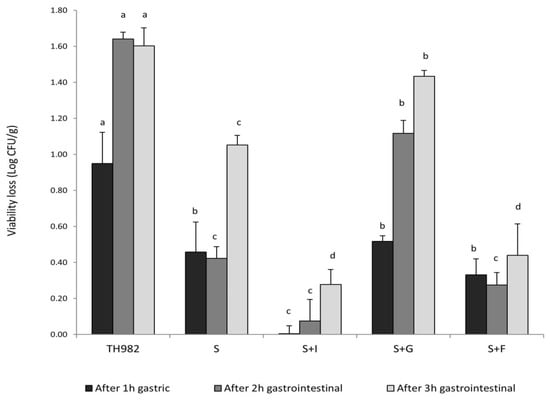
Figure 3.
Survivability of encapsulated and free S. thermophilus TH982 cells during exposure to simulated gastrointestinal conditions. Different letters indicate significant differences within the same incubation step (p < 0.05) among different encapsulating matrix types.
S + G capsules showed low survivability during gastric environment; indeed, the reduction of viable cells was higher (0.52 log CFU/g decrease) than encapsulated cells with S + I (no reduction detected) after 1 h of gastric condition (pH 2.5). This fact might be due to a variation of pH, which changes the gelatin charge of amino and carboxyl groups so that the modification of cross-links and structure of the chain could influence the swelling behavior of gelatin capsules [39].
On the other side, an interesting outcome was given by the two types of prebiotics used as a co-encapsulating agent with sodium alginate. After 3 h of exposure to gastrointestinal conditions, S + I and S + F revealed the highest cell survivability without any significant difference, leading to a low cell reduction (less than 0.45 log CFU/g decrease for both agents). Prebiotics, such as 2′-FL and inulin, which could provide good protection and even promote cell proliferation, appeared to contribute to the growth of S. thermophilus TH982. In addition, oligosaccharides are difficult to decompose by enzymes in digestive fluid but can be metabolized by beneficial bacteria in the colon [30]. It has been further demonstrated that oligosaccharides contained in human milk have an extraordinary resistance to hydrolysis by digestive enzymes of the small intestine [40]. The combination of calcium alginate with prebiotics such as inulin improves the viability of probiotics and facilitates the formation of an integrated structure of capsules. Researchers found better protection of Lactobacillus casei and Bifidobacterium bifidum cells in coated capsules with prebiotics such as inulin after the gastrointestinal transit. The addition of prebiotics could be synergistic in gelling, and as a result, may help to maintain and improve the degree of protection of the bacterial cells [41].
Lastly, the free cell suffered more from the gastric conditions than that during the 3-h gastrointestinal transition. In contrast, encapsulated bacteria evidenced greater reductions during gastrointestinal transit than under gastric conditions alone. This behavior may be due to the fact that alginate is stable in low-pH solutions: the ionotropic alginate gel formed by Ca2+ cross-linking of carboxylate groups is insoluble at low pH, but exposure to neutral pH or higher solubilize the alginate, causing swelling [42].
3.4. Release Kinetics
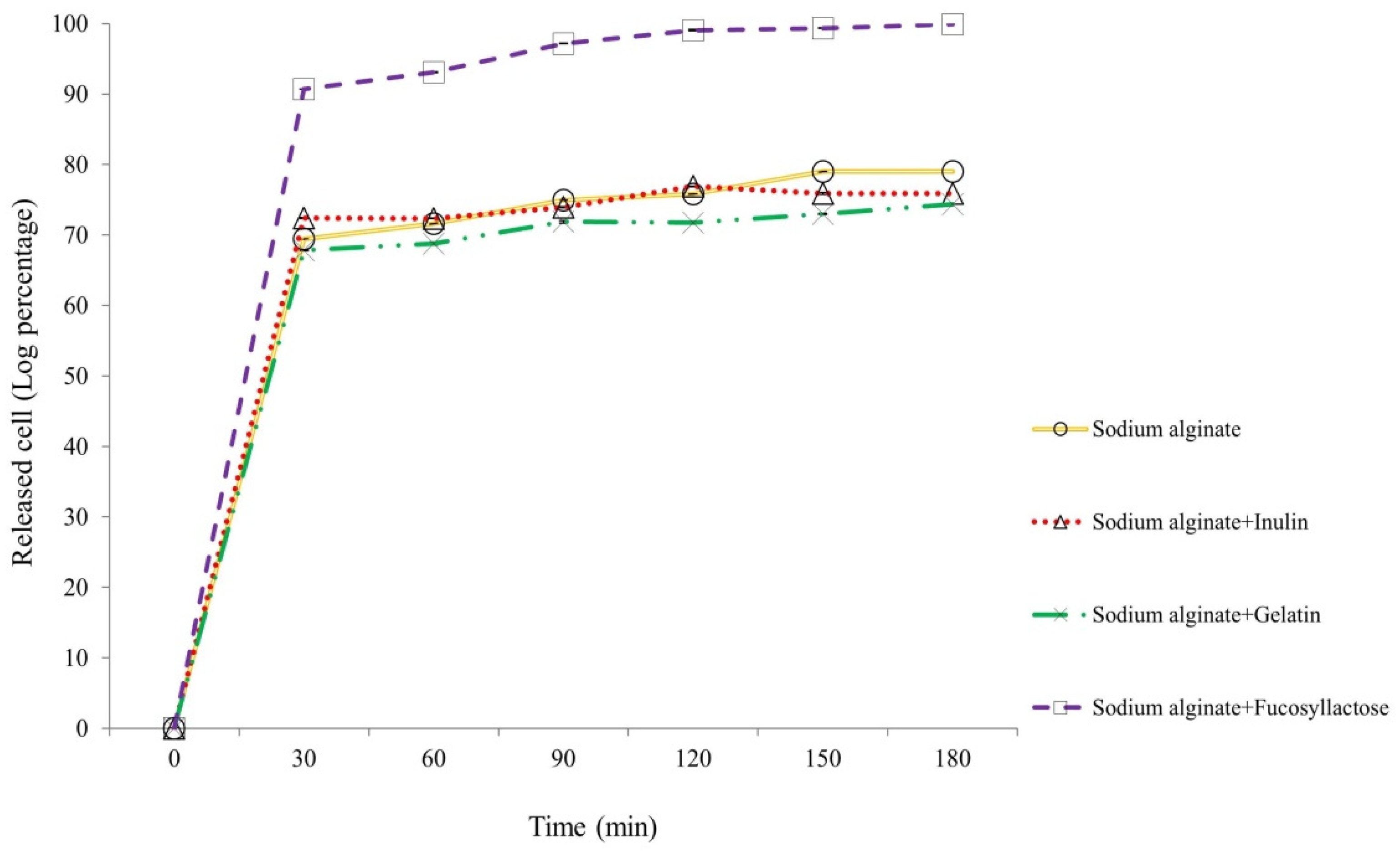
The release of encapsulated S. thermophilus TH982 after incubation in simulated intestinal fluid was evaluated after 30, 60, 90, 120, 150, and 180 min, as reported in Figure 4. After 30 min of agitation at 100 rpm at 37 °C in 50 mM K2HPO4, a great release of cells encapsulated with S + F was detected. Considering the initial number of cells entrapped in this type of capsules (9.73 ± 0.04 log CFU/g of capsules), the number of cells released in the simulated intestinal fluid after 30 min was 8.83 ± 0.04 log CFU/mL of K2HPO4, representing a very high value compared with the other types of encapsulating matrices after 180 min, which resulted about 2-log lower. This result confirms what was visible during the experiment: after 30 min, there was an evident solubilization of S + F capsules, a characteristic not displayed by the others (S, S + I, and S + G). Moreover, the number of released cells suggests that once liberated, S. thermophilus TH982 can withstand well simulated intestinal conditions, since after 180 min the number of cells released from S + F capsules was 9.72 ± 0.05 log CFU/mL, which means 99.89% of the initial entrapped cells.
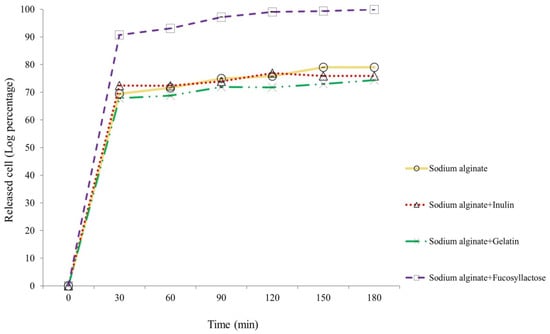
Figure 4.
Release kinetics of entrapped S. thermophilus TH982 cells from different capsule formulations during incubation in a simulated intestinal environment.
It is essential to ensure that the capsules give protection through the simulated gastrointestinal passage and ensure that the encapsulation matrix allows a release of viable and metabolically active cells in the intestine [30,43]. The release of the cells from microcapsules in the colon is essential for the growth and possible colonization of probiotics [30,44]. In the absence of this property, the entrapped organisms and the capsules will be washed out from the body without exerting a significant beneficial effect [45]. Many studies investigated the release of encapsulated probiotics in simulated intestinal fluid; however, to the best of our knowledge, there is no similar outcome from any encapsulating agent reported in the literature to date [25,30,43]. Sabikhi et al. [45] reported the release of encapsulated L. acidophilus in alginate-starch microspheres using a simulated colonic fluid with the same formulation (50mM KH2PO4, pH 7.4 ± 0.2 during 2.5 h). They found that the cell count after 150 min of incubation was 7.45 log CFU/mL, suggesting that all the microencapsulated cells were released at that time (initial number of 7.47 log CFU/g of capsule). Although 150 min is about the time needed for intestinal transit of microcapsules, they did not find a significant release after 30 min [45]. In another study, a fully released L. plantarum encapsulated with sodium alginate and sodium alginate with inulin in a 60-min exposure to simulated intestinal fluid was reported. From the results, it was concluded that the release mechanism was probably due to the replacing of calcium ions in the encapsulation matrices. Also, capsules with inulin showed a faster release rate during the first 20 min, which could have been induced by the addition of inulin in sodium alginate affecting the binding of calcium ion [33].
A possible mechanism that could be involved in the fast release of S. thermophilus TH982 cells encapsulated with sodium alginate and 2′-fucosyllactose could be that this small soluble milk glycan used as a co-encapsulating agent in this experiment is formed by fucose linked to the two positions of β-Gal residues of lactose; hence no electrostatic repulsions may occur with the negatively charged carboxyl groups of sodium alginate structure. Therefore, the complete release of the cells is probably due to the vigorous interaction of the prebiotic structure with the divalent cations (Ca2+) of the sodium alginate network, resulting in disintegration of the “egg-box” structure in K2HPO4. This process is well known and recognized to explain the inulin capsules releasing behavior in simulated intestinal fluid, a prebiotic commonly used as encapsulating material [33].
4. Conclusions
This study represents the first report among the available literature on the use of 2′-fucosyllactose, a trisaccharide from human milk, used as a prebiotic agent in microencapsulation of S. thermophilus cells. The alginate-based microcapsules showed interesting features, including the capability to protect bacterial cells from harsh simulated gastrointestinal conditions. Compared to other molecules used in microencapsulation together with alginate, such as gelatin and inulin, 2′-fucosyllactose evidenced an extremely quick (30 min) and abundant release of bacterial cells from the capsules inside a simulated intestinal fluid. These results encourage further studies aimed at testing these properties under in vivo conditions.
Author Contributions
S.P.: investigation, data curation, and writing–original draft. G.G.: investigation and writing–original draft. A.T.: conceptualization, data curation, and writing–original draft. A.G.: funding acquisition, writing–review, and editing. V.C.: funding acquisition, writing–review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded in part by the Ministero dell’Università e della Ricerca Scientifica (MIUR, Dotazione Ordinaria Ricerca, DOR).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Tarrah, A.; Noal, V.; Giaretta, S.; Treu, L.; Duarte, V.D.S.; Corich, V.; Giacomini, A. Effect of different initial pH on the growth of Streptococcus macedonicus and Streptococcus thermophilus strains. Int. Dairy J. 2018, 86, 65–68. [Google Scholar] [CrossRef]
- Tarrah, A.; Noal, V.; Treu, L.; Giaretta, S.; Duarte, V.D.S.; Corich, V.; Giacomini, A. Short communication: Comparison of growth kinetics at different temperatures of Streptococcus macedonicus and Streptococcus thermophilus strains of dairy origin. J. Dairy Sci. 2018, 101, 7812–7816. [Google Scholar] [CrossRef] [PubMed]
- Giaretta, S.; Treu, L.; Vendramin, V.; Duarte, V.D.S.; Tarrah, A.; Campanaro, S.; Corich, V.; Giacomini, A. Comparative Transcriptomic Analysis of Streptococcus thermophilus TH1436 and TH1477 Showing Different Capability in the Use of Galactose. Front. Microbiol. 2018, 9, 1765. [Google Scholar] [CrossRef] [Green Version]
- Tarrah, A.; Treu, L.; Giaretta, S.; Duarte, V.; Corich, V.; Giacomini, A. Differences in Carbohydrates Utilization and Antibiotic Resistance Between Streptococcus macedonicus and Streptococcus thermophilus Strains Isolated from Dairy Products in Italy. Curr. Microbiol. 2018, 75, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Mater, D.D.; Bretigny, L.; Firmesse, O.; Flores, M.J.; Mogenet, A.; Bresson, J.L.; Corthier, G. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol. Lett. 2005, 250, 185–187. [Google Scholar]
- Sybesma, W.; Starrenburg, M.; Tijsseling, L.; Hoefnagel, M.H.N.; Hugenholtz, J. Effects of Cultivation Conditions on Folate Production by Lactic Acid Bacteria. Appl. Environ. Microbiol. 2003, 69, 4542–4548. [Google Scholar] [CrossRef] [Green Version]
- Iyer, R.; Tomar, S.; Maheswari, T.U.; Singh, R. Streptococcus thermophilus strains: Multifunctional lactic acid bacteria. Int. Dairy J. 2010, 20, 133–141. [Google Scholar] [CrossRef]
- Collins, J.; Thornton, G.; Sullivan, G. Selection of Probiotic Strains for Human Applications. Int. Dairy J. 1998, 8, 487–490. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- Timilsena, Y.P.; Haque, A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V. Technology and potential applications of probiotic encapsulation in fermented milk products. J. Food Sci. Technol. 2015, 52, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and Methods of Microencapsulation of Probiotic Microorganisms; National Institute of Genetic Engineering and Biotechnology: Tehran, Iran, 2007. [Google Scholar]
- Rokka, S.; Rantamäki, P. Protecting probiotic bacteria by microencapsulation: Challenges for industrial applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Etchepare, M.D.A.; Barin, J.S.; Cichoski, A.J.; Jacob-Lopes, E.; Wagner, R.; Fries, L.L.M.; De Menezes, C.R. Microencapsulation of probiotics using sodium alginate. Ciência Rural 2015, 45, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Luna, M.S.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [Green Version]
- Elison, E.; Vigsnaes, L.K.; Krogsgaard, L.R.; Rasmussen, J.; Sørensen, N.; McConnell, B.; Hennet, T.; Sommer, M.O.A.; Bytzer, P. Oral supplementation of healthy adults with 2′-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br. J. Nutr. 2016, 116, 1356–1368. [Google Scholar] [CrossRef] [Green Version]
- Salli, K.; Anglenius, H.; Hirvonen, J.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Tiihonen, K.; Maukonen, J.; Ouwehand, A.C. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Coulet, M.; Phothirath, P.; Allais, L.; Schilter, B. Pre-clinical safety evaluation of the synthetic human milk, nature-identical, oligosaccharide 2′-O-Fucosyllactose (2′FL). Regul. Toxicol. Pharmacol. 2014, 68, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A.; De Castilhos, J.; Rossi, R.C.; Duarte, V.D.S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In vitro Probiotic Potential and Anti-cancer Activity of Newly Isolated Folate-Producing Streptococcus thermophilus Strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef]
- Treu, L.; Vendramin, V.; Bovo, B.; Campanaro, S.; Corich, V.; Giacomini, A. Genome Sequences of Four Italian Streptococcus thermophilus Strains of Dairy Origin. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavarri, M.; Maranon, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M.D.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, D.; Chauhan, R.; Goel, G. Skimmed Milk-Based Encapsulation for Enhanced Stability and Viability of Lactobacillus gastricus BTM 7 Under Simulated Gastrointestinal Conditions. Probiotics Antimicrob. Proteins 2019, 11, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-E.; Li, Z.-H.; Zhang, Z.-L.; Zhang, T.-T.; Yu, W.-M.; Zhou, M.-L.; Tang, Z.-X. Encapsulation of Lactobacillus bulgaricus in carrageenan-locust bean gum coated milk microspheres with double layer structure. LWT 2013, 54, 147–151. [Google Scholar] [CrossRef]
- El-Shafei, K.; Abdallah, N.A.; Tawfik, N.F.; El-Sayed, H.S.; Mahmoud, M. Effect of different microencapsulating materials on survivability of Streptococcus thermophilus under simulated food processing and gastrointestinal conditions. Sciences 2018, 8, 259–271. [Google Scholar]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of Probiotic Cells for Food Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Liao, N.; Luo, B.; Gao, J.; Li, X.; Zhao, Z.; Zhang, Y.; Ni, Y.; Tian, F. Oligosaccharides as co-encapsulating agents: Effect on oral Lactobacillus fermentum survival in a simulated gastrointestinal tract. Biotechnol. Lett. 2019, 41, 263–272. [Google Scholar] [CrossRef]
- Sathyabama, S.; Kumar, M.R.; Devi, P.B.; Vijayabharathi, R.; Priyadharisini, V.B. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Annan, N.; Borza, A.; Hansen, L.T. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res. Int. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Xu, H.; Aguilar, Z.P.; Wei, H. Effect of skim milk coated inulin-alginate encapsulation beads on viability and gene expression of Lactobacillus plantarum during freeze-drying. LWT 2016, 68, 8–13. [Google Scholar] [CrossRef]
- Codex Standard for Fermented Milks. Alimentarius Commission No CODEX Stan 243-2003. 11p. Available online: www.fao.org/input/download/standards/400/CXS_243e.pdf (accessed on 28 March 2019).
- Shah, N. Probiotic Bacteria: Selective Enumeration and Survival in Dairy Foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.; Shah, N. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Res. Int. 2006, 39, 203–211. [Google Scholar] [CrossRef]
- Hols, P.; Hancy, F.; Fontaine, L.; Grossiord, B.; Prozzi, D.; Leblond-Bourget, N.; Decaris, B.; Bolotin, A.; Delorme, C.; Duskoehrlich, S. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 2005, 29, 435–463. [Google Scholar] [CrossRef]
- Jiménez-Pranteda, M.L.; Poncelet, D.; Nader-Macías, M.E.; Arcos, A.; Aguilera, M.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A. Stability of lactobacilli encapsulated in various microbial polymers. J. Biosci. Bioeng. 2012, 113, 179–184. [Google Scholar] [CrossRef]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi Zanjani, M.A.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. 2014, 13, 843–852. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Lee, Y.; Ji, Y.R.; Lee, S.; Choi, M.-J.; Cho, Y. Microencapsulation of Probiotic Lactobacillus acidophilus KBL409 by Extrusion Technology to Enhance Survival under Simulated Intestinal and Freeze-Drying Conditions. J. Microbiol. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-Y.; Zheng, W.; Dong, Q.-Y.; Li, Z.-H.; Shi, L.-E.; Tang, Z.-X. Activity of encapsulated Lactobacillus bulgaricus in alginate-whey protein microspheres. Braz. Arch. Biol. Technol. 2014, 57, 736–741. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of Microencapsulated Lactobacillus acidophilus LA1 to Processing Treatments and Simulated Gut Conditions. Food Bioprocess Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).