Biomechanical Comparison of Vertebroplasty, Kyphoplasty, Vertebrae Stent for Osteoporotic Vertebral Compression Fractures—A Finite Element Analysis
Abstract
1. Introduction
2. Methods
2.1. Models
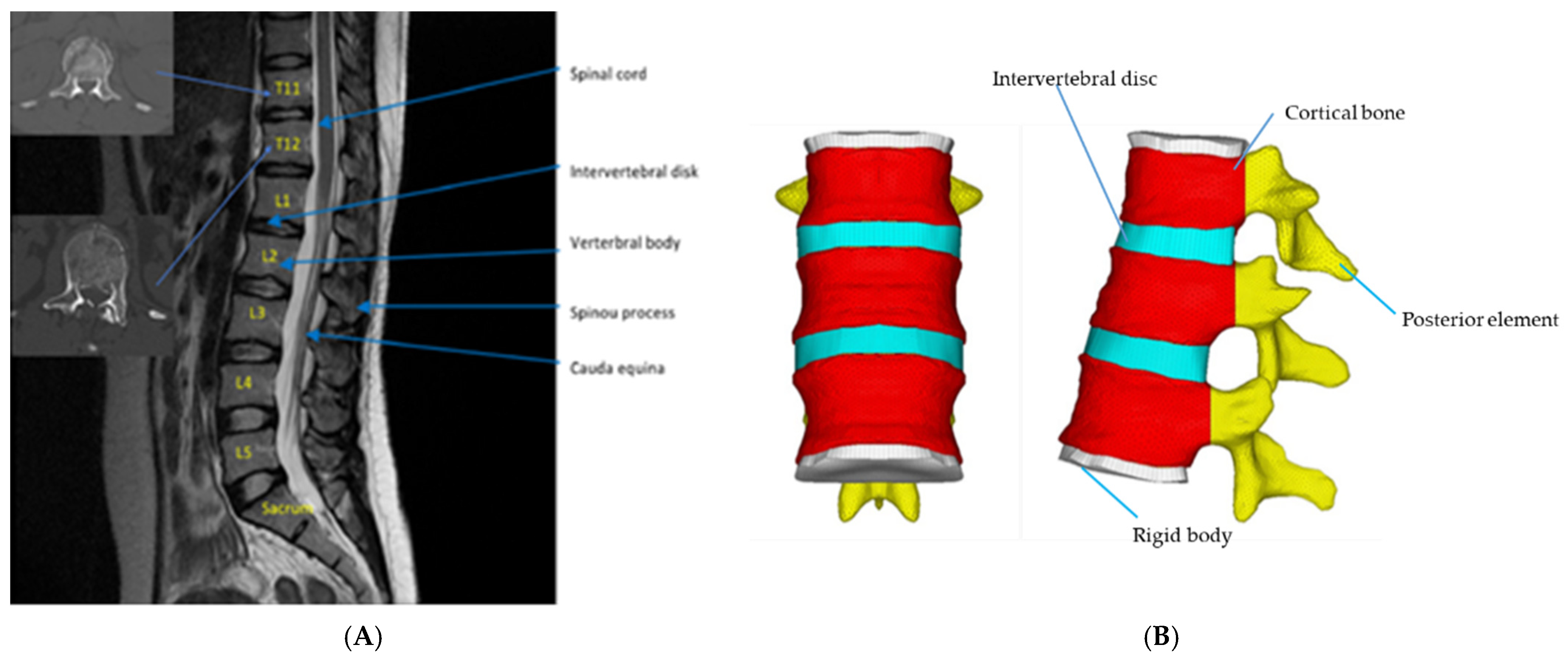
2.1.1. Establishment of an intact normal thoracolumbar spine in the FE model
2.1.2. Establishment of T12 Injured Vertebra FE Model
2.2. Material Parameter Setting
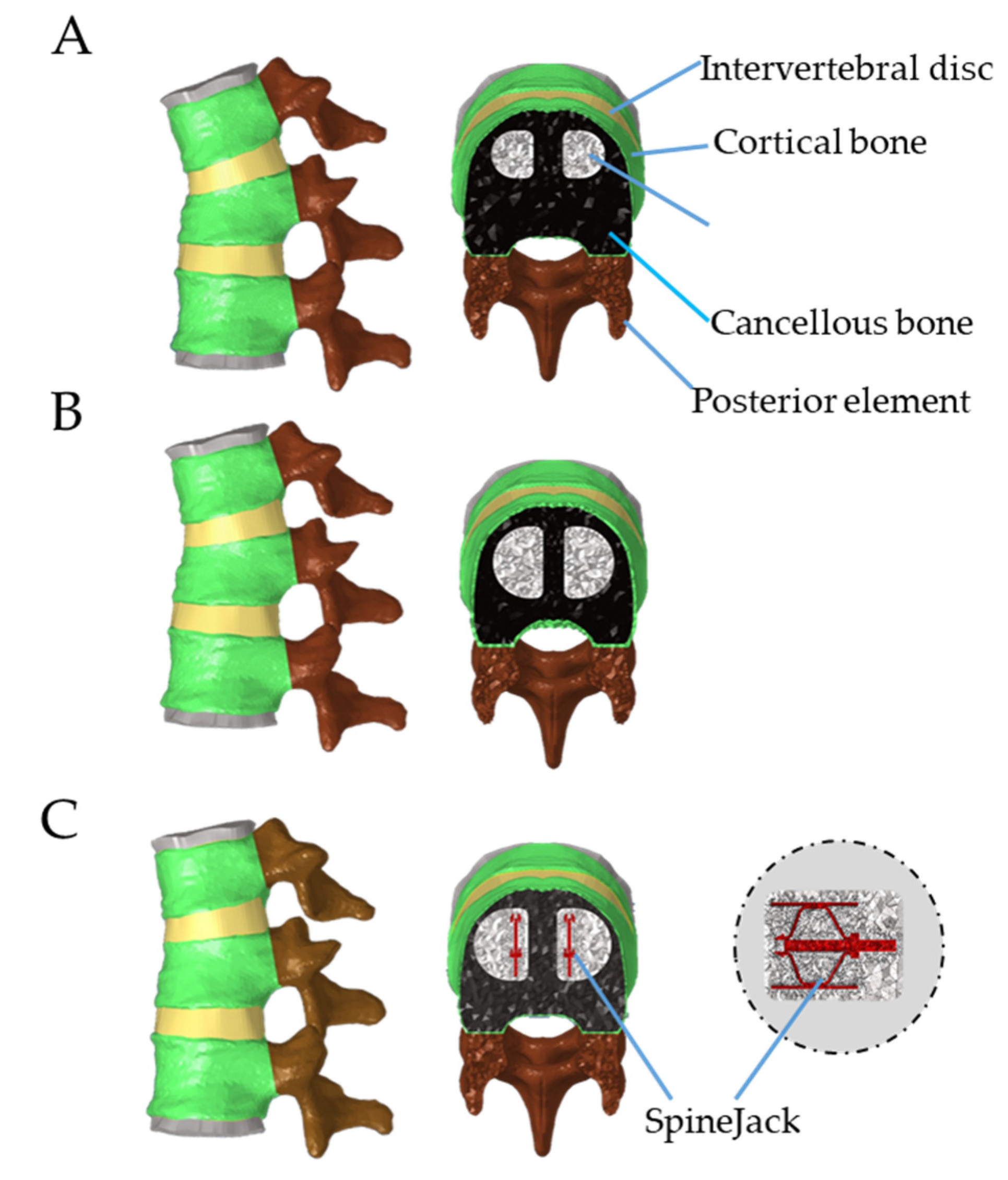
2.2.1. Establishment of VP Model in T12 Compression Fracture
2.2.2. Establishment of BKP Model in T12 Compression Fracture
2.2.3. Establishment of VS Model with Bone Cement Augmentation in T12 Compression Fracture
2.3. Loading and Boundary Condition Settings
3. Results
3.1. Maximum Bone Cement Stress
3.2. Maximum Adjacent Segment Interfacial Stress Distribution
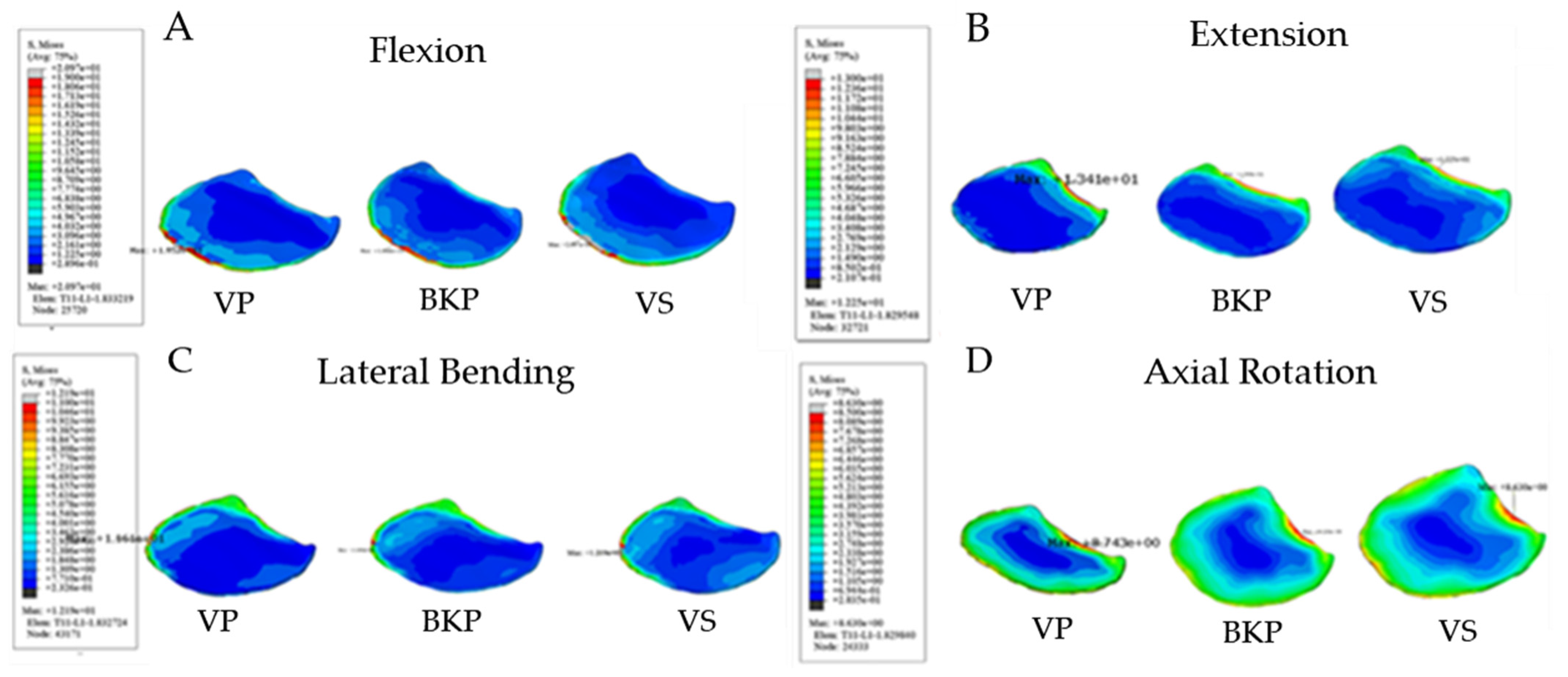
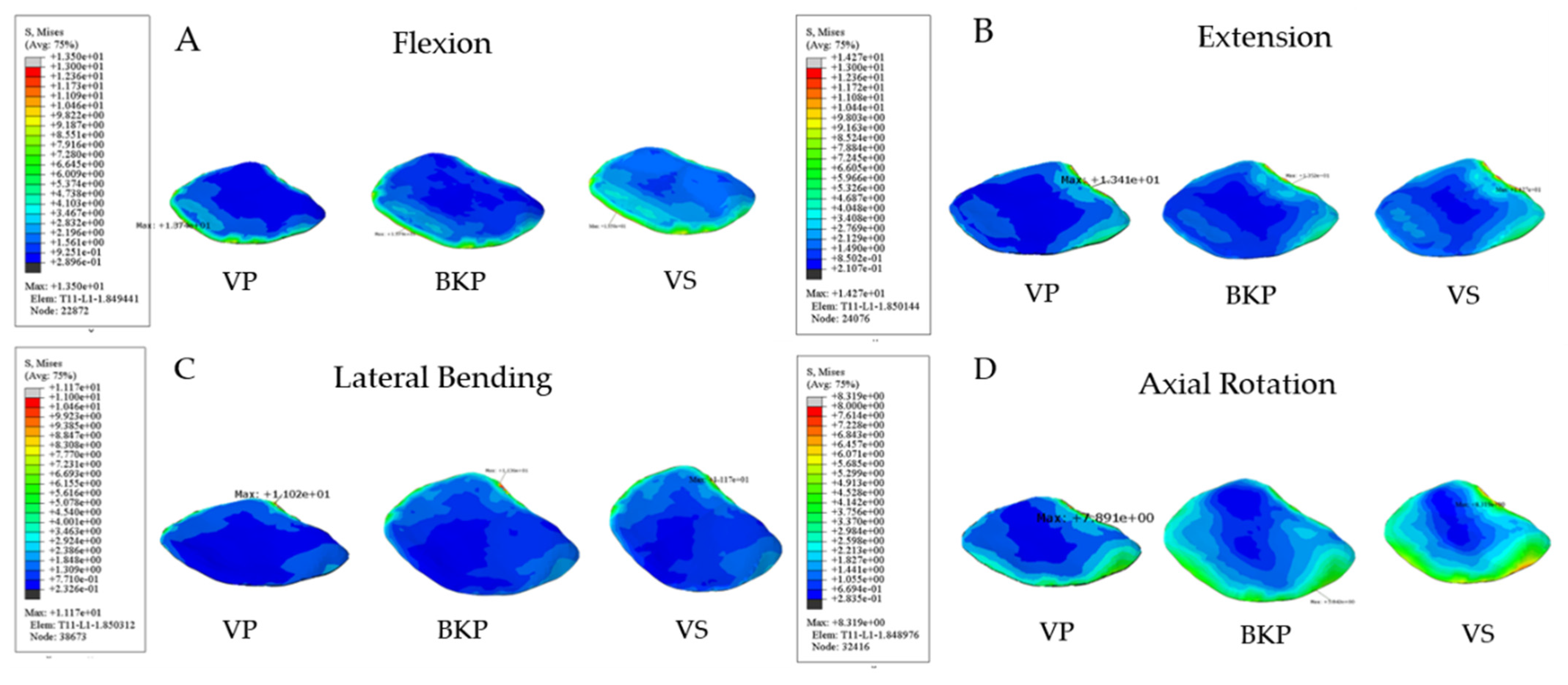
3.2.1. Maximum Interfacial Stress at Lower T11 Endplate
3.2.2. Maximum Interfacial Stress at Upper L1 Endplate
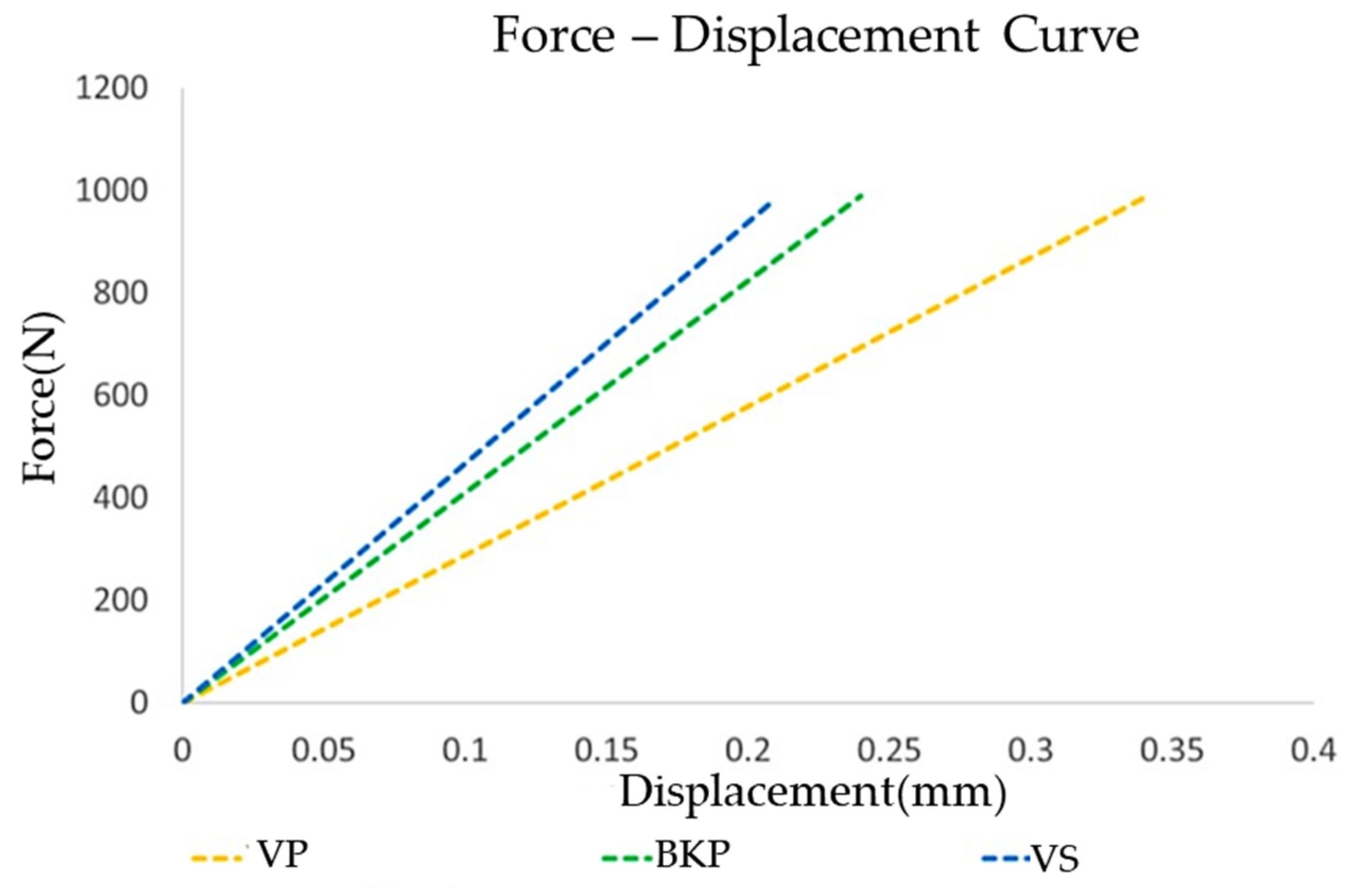
3.2.3. Surgical Segment Vertebra Stiffness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cockerill, W.; Lunt, M.; Silman, A.J.; Cooper, C.; Lips, P.; Bhalla, A.K.; Cannata, J.B.; Eastell, R.; Felsenberg, D.; Gennari, C.; et al. Health-related quality of life and radiographic vertebral fracture. Osteoporos. Int. 2003, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Lee, S.-H.; Yoon, S.-P.; Lee, Y.-T.; Jang, G.; Lim, S.-Y.; Lee, H.-M.; Moon, S.-H.; Song, K.-S. Quality of Life Comparison between Vertebroplasty and Kyphoplasty in Patients with Osteoporotic Vertebral Fractures. Asian Spine J. 2014, 8, 799–803. [Google Scholar] [CrossRef]
- Klazen, C.A.; Lohle, P.N.; de Vries, J.; Jansen, F.H.; Tielbeek, A.V.; Blonk, M.C.; Venmans, A.; van Rooij, W.J.J.; Schoemaker, M.C.; Juttmann, J.R.; et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): An open-label randomised trial. Lancet 2010, 376, 1085–1092. [Google Scholar] [CrossRef]
- Wardlaw, D.; Cummings, S.R.; Van Meirhaeghe, J.; Bastian, L.; Tillman, J.B.; Ranstam, J.; Eastell, R.; Shabe, P.; Talmadge, K.; Boonen, S. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): A randomised controlled trial. Lancet 2009, 373, 1016–1024. [Google Scholar] [CrossRef]
- Noriega, D.C.; Ramajo, R.H.; Lite, I.S.; Toribio, B.; Corredera, R.; Ardura, F.; Krüger, A. Safety and clinical performance of kyphoplasty and SpineJack((R)) procedures in the treatment of osteoporotic vertebral compression fractures: A pilot, monocentric, investigator-initiated study. Osteoporos. Int. 2016, 27, 2047–2055. [Google Scholar] [CrossRef]
- Liu, J.-T.; Li, C.-S.; Chang, C.-S.; Liao, W.-J. Long-term follow-up study of osteoporotic vertebral compression fracture treated using balloon kyphoplasty and vertebroplasty. J. Neurosurg. Spine 2015, 23, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Liao, W.J.; Tan, W.C.; Lee, J.K.; Liu, C.H.; Chen, Y.H.; Lin, T.B. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: A prospective, comparative, and randomized clinical study. Osteoporos. Int. 2010, 21, 359–364. [Google Scholar] [CrossRef]
- Rohlmann, A.; Zander, T.; Schmidt, H.; Wilke, H.-J.; Bergmann, G. Analysis of the influence of disc degeneration on the mechanical behaviour of a lumbar motion segment using the finite element method. J. Biomech. 2006, 39, 2484–2490. [Google Scholar] [CrossRef]
- Eck, J.C.; Nachtigall, D.; Humphreys, S.C.; Hodges, S.D. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: A meta-analysis of the literature. Spine J. 2008, 8, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Tonetti, J.; Bodin, A.; Vouaillat, H.; Merloz, P.; Assaker, R.; Court, C. Kyphoplasty versus vertebroplasty in osteoporotic thoracolumbar spine fractures. Short-term retrospective review of a multicentre cohort of 127 consecutive patients. Orthop. Traumatol. Surg. Res. 2012, 98 (Suppl. 6), S112–S119. [Google Scholar] [CrossRef]
- Keller, T.S.; Kosmopoulos, V.; Lieberman, I.H. Vertebroplasty and kyphoplasty affect vertebral motion segment stiffness and stress distributions: A microstructural finite-element study. Spine 2005, 30, 1258–1265. [Google Scholar] [CrossRef]
- Takano, H.; Yonezawa, I.; Todo, M.; Mazlan, M.H.; Sato, T.; Kaneko, K. Biomechanical Study of the Effects of Balloon Kyphoplasty on the Adjacent Vertebrae. J. Biomed. Sci. Eng. 2016, 09, 478–487. [Google Scholar] [CrossRef][Green Version]
- Becker, S.; Dabirrahmani, D.; Hogg, M.; Appleyard, R.; Baroud, G.; Gillies, M. Disadvantages of Balloon Kyphoplasty with PMMA—A Clinical and Biomechanical Statement. J. Miner Stoff. Wechs. 2011, 18, 9–12. [Google Scholar]
- Werner, C.M.; Osterhoff, G.; Schlickeiser, J.; Jenni, R.; Wanner, G.A.; Ossendorf, C.; Simmen, H.P. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: A randomized trial. J. Bone Jt. Surg. Am. 2013, 95, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.C.; Rodrίguez-Monsalve, F.; Ramajo, R.; Sánchez-Lite, I.; Toribio, B.; Ardura, F. Long-term safety and clinical performance of kyphoplasty and SpineJack procedures in the treatment of osteoporotic vertebral compression fractures: A pilot, monocentric, investigator-initiated study. Osteoporos. Int. 2019, 30, 637–645. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Marcia, S.; Ryan, A.; Beall, D.P.; Masala, S.; Deschamps, F.; Kelekis, A. New Implant-Based Technologies in the Spine. Cardiovasc. Interv. Radiol. 2018, 41, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Chosa, E.; Goto, K.; Totoribe, K.; Tajima, N. Analysis of the Effect of Lumbar Spine Fusion on the Superior Adjacent Intervertebral Disk in the Presence of Disk Degeneration, Using the Three-Dimensional Finite Element Method. J. Spinal Disord. 2004, 17, 134–139. [Google Scholar] [CrossRef]
- Cho, A.R.; Cho, S.B.; Lee, J.H.; Kim, K.H. Effect of augmentation material stiffness on adjacent vertebrae after osteoporotic vertebro-plasty using finite element analysis with different loading methods. Pain Physician 2015, 18, E1101–E1110. [Google Scholar] [CrossRef]
- Rohlmann, A.; Boustani, H.N.; Bergmann, G.; Zander, T. A probabilistic finite element analysis of the stresses in the augmented vertebral body after vertebroplasty. Eur. Spine J. 2010, 19, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kuh, S.U.; Chin, D.K.; Jin, B.H.; Kim, K.S.; Yoon, Y.S.; Cho, Y.E. Kyphoplasty versus vertebroplasty: Restoration of vertebral body height and correction of kyphotic deformity with special attention to the shape of the fractured vertebrae. J. Spinal Disord. Tech. 2012, 25, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Purcell, P.; Tyndyk, M.; McEvoy, F.; Tiernan, S.; Morris, S. A parametric finite element analysis of the compacted bone-cement interface following balloon kyphoplasty. Proc. Inst. Mech. Eng. H 2014, 228, 89–97. [Google Scholar] [CrossRef]
- Garfin, S.R.; Yuan, H.A.; Reiley, M.A. New technologies in spine: Kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 2001, 26, 1511–1515. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, X.; Li, F. Balloon kyphoplasty versus percutaneous vertebroplasty for treatment of osteoporotic vertebral compression fractures (OVCFs). Osteoporos. Int. 2016, 27, 2823–2834. [Google Scholar] [CrossRef]
- Noriega, D.; Marcia, S.; Theumann, N.; Blondel, B.; Simon, A.; Hassel, F.; Maestretti, G.; Petit, A.; Weidle, P.A.; Mandly, A.G.; et al. A prospective, international, randomized, noninferiority study comparing an implantable titanium vertebral augmentation device versus balloon kyphoplasty in the reduction of vertebral compression fractures (SAKOS study). Spine J. 2019, 19, 1782–1795. [Google Scholar] [CrossRef] [PubMed]
- Oberkircher, L.; Struewer, J.; Bliemel, C.; Buecking, B.; Eschbach, D.A.; Ruchholtz, S.; Krueger, A. Height restoration and preservation in osteoporotic vertebral compression fractures: A biomechanical analysis of standard balloon kyphoplasty versus radiofrequency kyphoplasty in a cadaveric model. J. Spinal Disord. Tech. 2014, 27, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Properties of acrylic bone cement: State of the art review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Ma, X.L.; Xing, D.; Ma, J.X.; Xu, W.G.; Wang, J.; Chen, Y. Balloon kyphoplasty versus percutaneous vertebroplasty in treating osteoporotic vertebral compression fracture: Grading the evidence through a systematic review and meta-analysis. Eur. Spine J. 2012, 21, 1844–1859. [Google Scholar] [CrossRef] [PubMed]
- Rotter, R.; Schmitt, L.; Gierer, P.; Schmitz, K.-P.; Noriega, D.; Mittlmeier, T.; Meeder, P.-J.; Martin, H. Minimum cement volume required in vertebral body augmentation—A biomechanical study comparing the permanent SpineJack device and balloon kyphoplasty in traumatic fracture. Clin. Biomech. 2015, 30, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Hsieh, M.-K.; Liao, J.-C.; Lai, P.-L.; Niu, C.-C.; Fu, T.-S.; Tsai, T.-T.; Chen, W.-J. Repeated percutaneous vertebroplasty for refracture of cemented vertebrae. Arch. Orthop. Trauma Surg. 2011, 131, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, W.F.; Cheney, R. Recurrent fracture after vertebral kyphoplasty. Spine J. 2006, 6, 488–493. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Rhyu, K.-W. Recompression of vertebral body after balloon kyphoplasty for osteoporotic vertebral compression fracture. Eur. Spine J. 2010, 19, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Guo, D.Q.; Zhang, S.C.; Liang Yuan, K.; Mo, G.Y.; Li, D.X.; Guo, H.Z.; Tang, Y.; Luo, P.J. Risk factor analysis for re-collapse of cemented vertebrae after percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP). Int. Orthop. 2018, 42, 2131–2139. [Google Scholar] [CrossRef] [PubMed]


| Component | Young’s Modulus (MPa) | Poisson Ratio (υ) | Element Type |
|---|---|---|---|
| Cortical bone | 34 | 0.3 | Tetrahedral |
| Cancellous bone | 34 | 0.3 | Tetrahedral |
| Posterior element | 2345 | 0.25 | Tetrahedral |
| Endplate | 670 | 0.3 | Tetrahedral |
| Annulus substance | 5 | 0.45 | Hexahedral |
| Nucleus | 9 | 0.4 | Hexahedral |
| Annulus fiber | 455 | 0.3 | Surface |
| Bone cement | 3000 | 0.41 | Tetrahedral |
| Vertebrae stent (SpineJack) | 113,800 | 0.34 | Tetrahedral |
| Rigid body | 10 | 0.4 | Pentahedron |
| Flexion | Extension | Lateral Bending | Axial Rotation | |
|---|---|---|---|---|
| Bone cement | ||||
| VP | 3.90 | 2.37 | 2.48 | 2.13 |
| BKP | 3.59 | 3.61 | 2.73 | 1.85 |
| VS | 5.91 | 3.74 | 3.12 | 3.54 |
| Lower T11 endplate | ||||
| VP | 19.52 | 13.41 | 11.61 | 8.74 |
| BKP | 18.46 | 13.99 | 10.91 | 9.13 |
| VS | 20.97 | 12.25 | 12.19 | 8.63 |
| Upper L1 endplate | ||||
| VP | 13.74 | 13.41 | 11.34 | 8.1 |
| BKP | 13.79 | 13.52 | 11.36 | 5.84 |
| VS | 13.50 | 14.27 | 11.17 | 8.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.-C.; Chen, M.J.-W.; Lin, T.-Y.; Chen, W.-P. Biomechanical Comparison of Vertebroplasty, Kyphoplasty, Vertebrae Stent for Osteoporotic Vertebral Compression Fractures—A Finite Element Analysis. Appl. Sci. 2021, 11, 5764. https://doi.org/10.3390/app11135764
Liao J-C, Chen MJ-W, Lin T-Y, Chen W-P. Biomechanical Comparison of Vertebroplasty, Kyphoplasty, Vertebrae Stent for Osteoporotic Vertebral Compression Fractures—A Finite Element Analysis. Applied Sciences. 2021; 11(13):5764. https://doi.org/10.3390/app11135764
Chicago/Turabian StyleLiao, Jen-Chung, Michael Jian-Wen Chen, Tung-Yi Lin, and Weng-Pin Chen. 2021. "Biomechanical Comparison of Vertebroplasty, Kyphoplasty, Vertebrae Stent for Osteoporotic Vertebral Compression Fractures—A Finite Element Analysis" Applied Sciences 11, no. 13: 5764. https://doi.org/10.3390/app11135764
APA StyleLiao, J.-C., Chen, M. J.-W., Lin, T.-Y., & Chen, W.-P. (2021). Biomechanical Comparison of Vertebroplasty, Kyphoplasty, Vertebrae Stent for Osteoporotic Vertebral Compression Fractures—A Finite Element Analysis. Applied Sciences, 11(13), 5764. https://doi.org/10.3390/app11135764





