Effects of Garden Amendments on Soil Available Lead and Plant Uptake in a Contaminated Calcareous Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
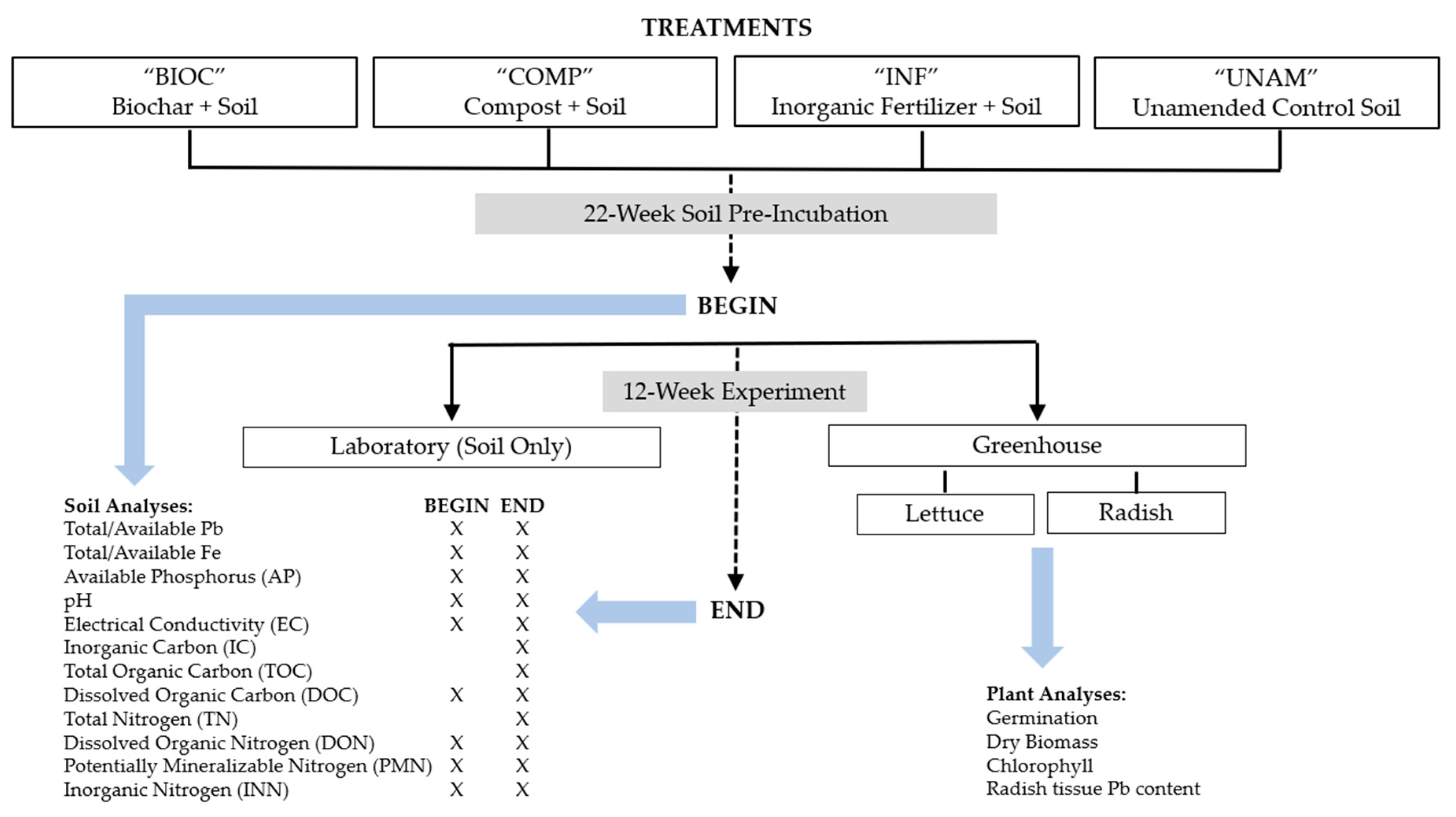
2.2. Soil Pre-Incubation
2.3. Lab Incubation and Greenhouse Experiment
2.4. Soil Analyses
2.5. Plant Analyses
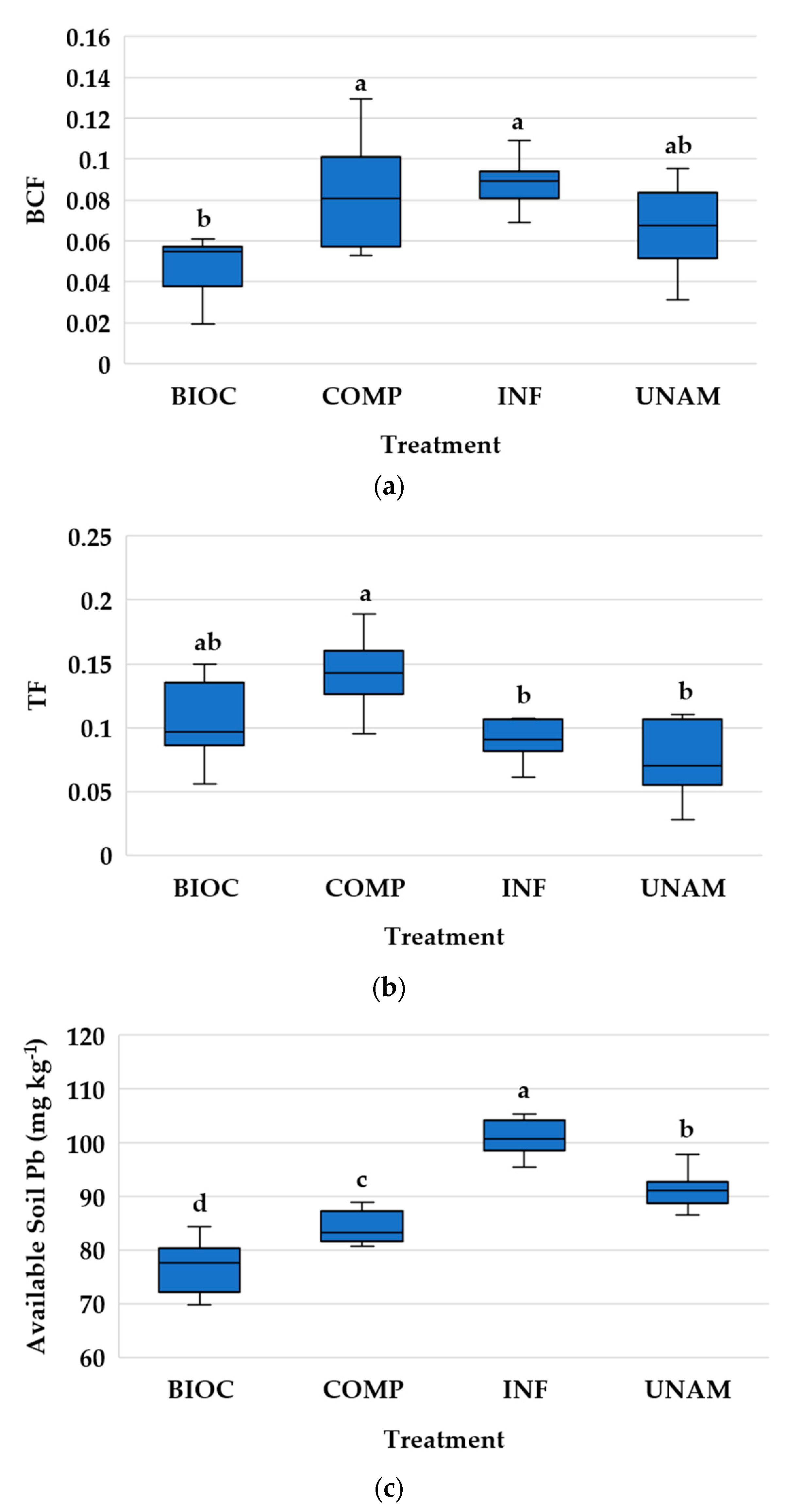
2.6. Plant Lead Uptake Indicators
2.7. Statistical Analyses
3. Results
3.1. Beginning of the Experiment
3.2. Experiment Termination
3.2.1. Soil Physicochemical Properties
3.2.2. Soil Labile Nitrogen and Carbon
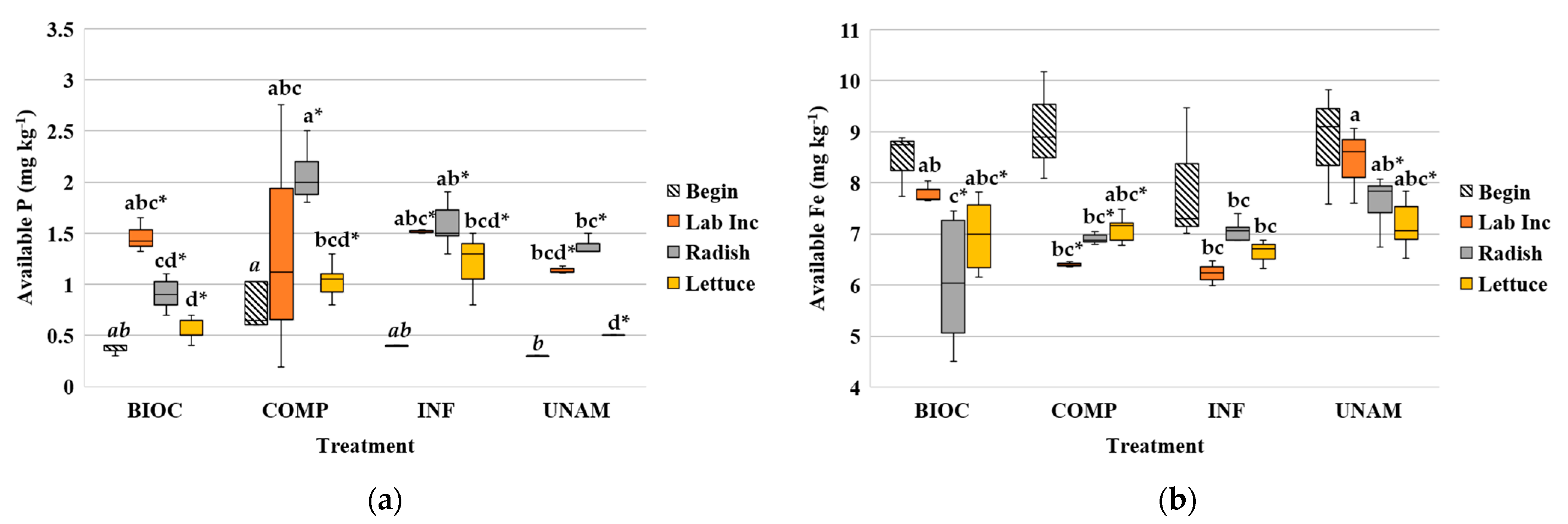
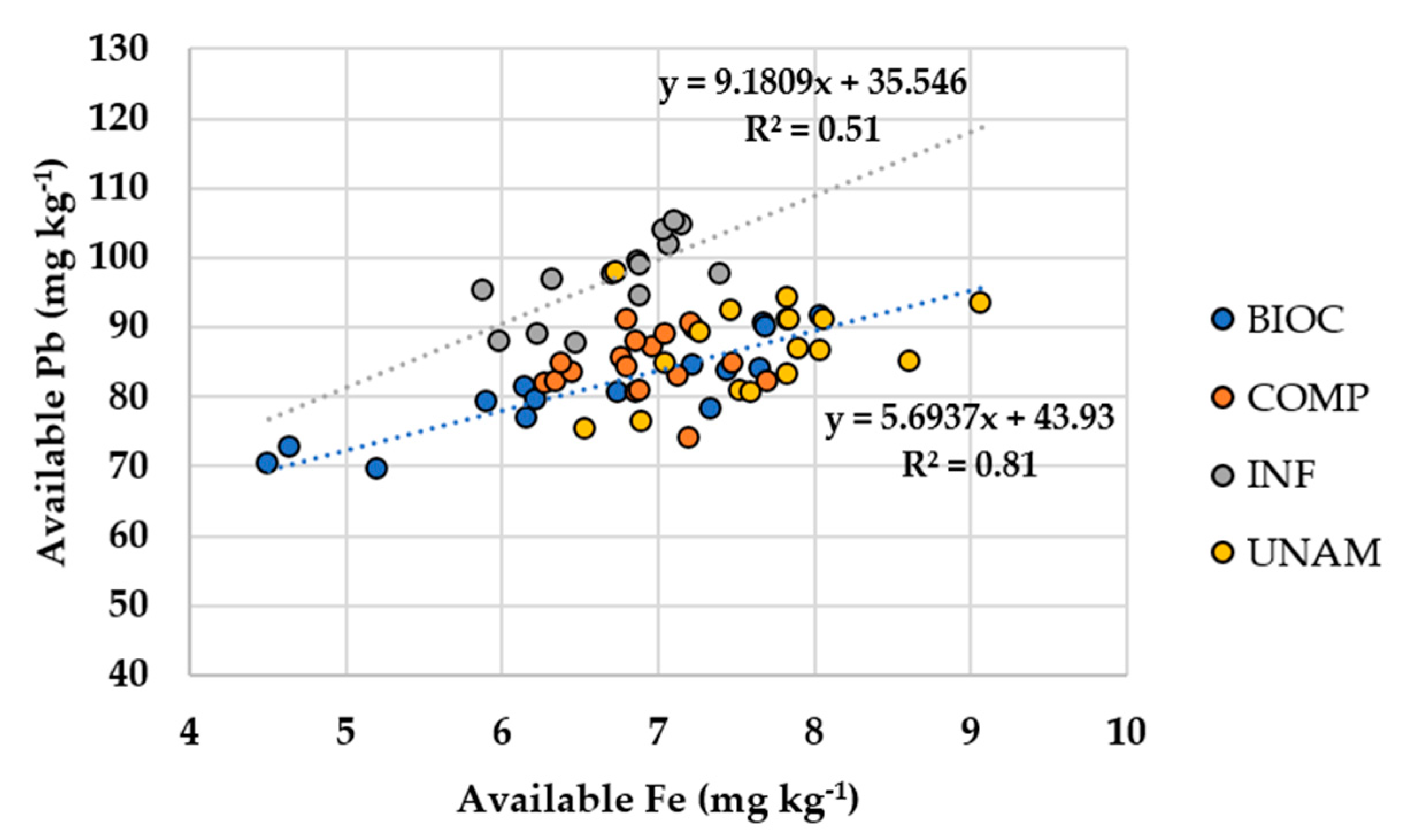
3.2.3. Soil Iron and Phosphorus
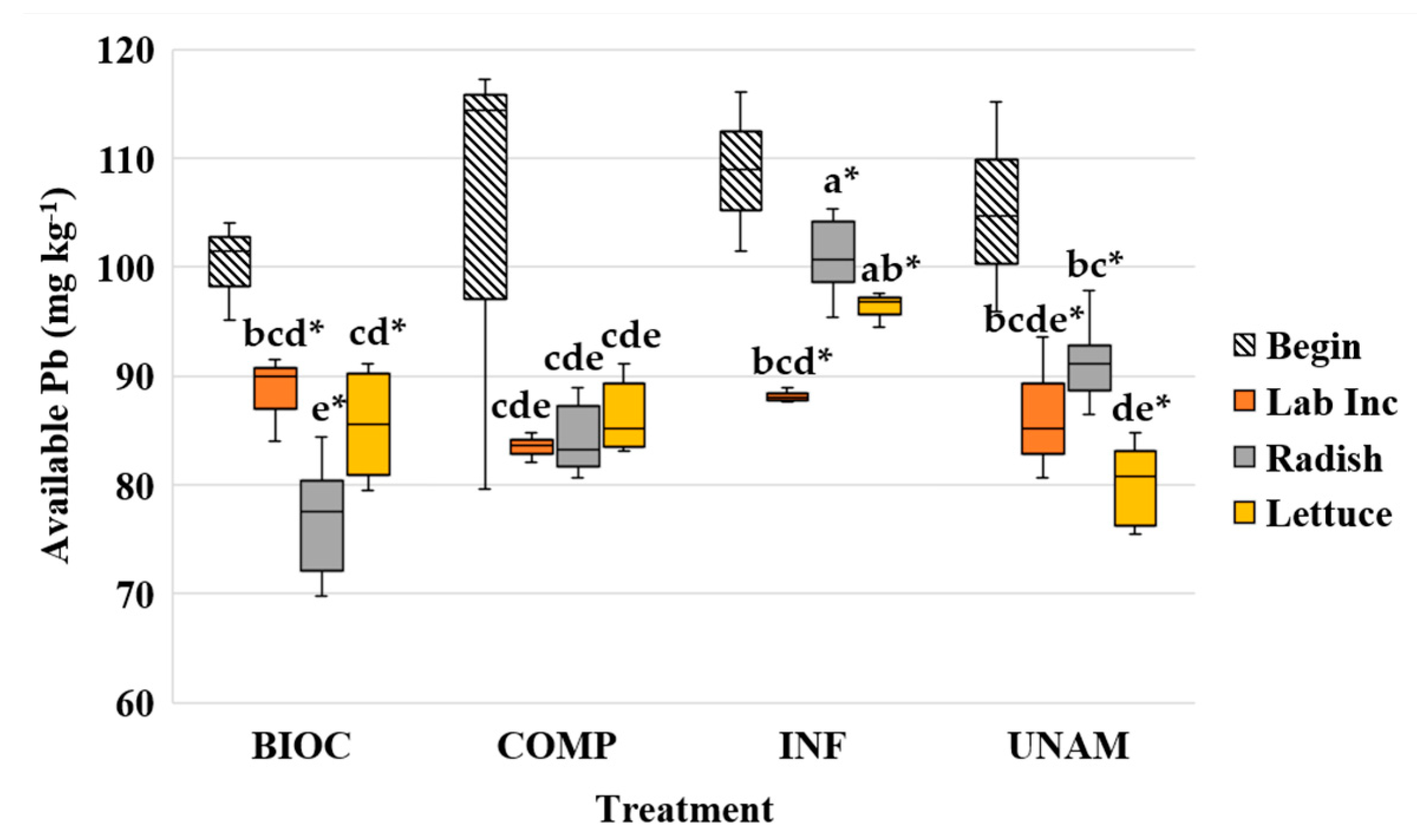
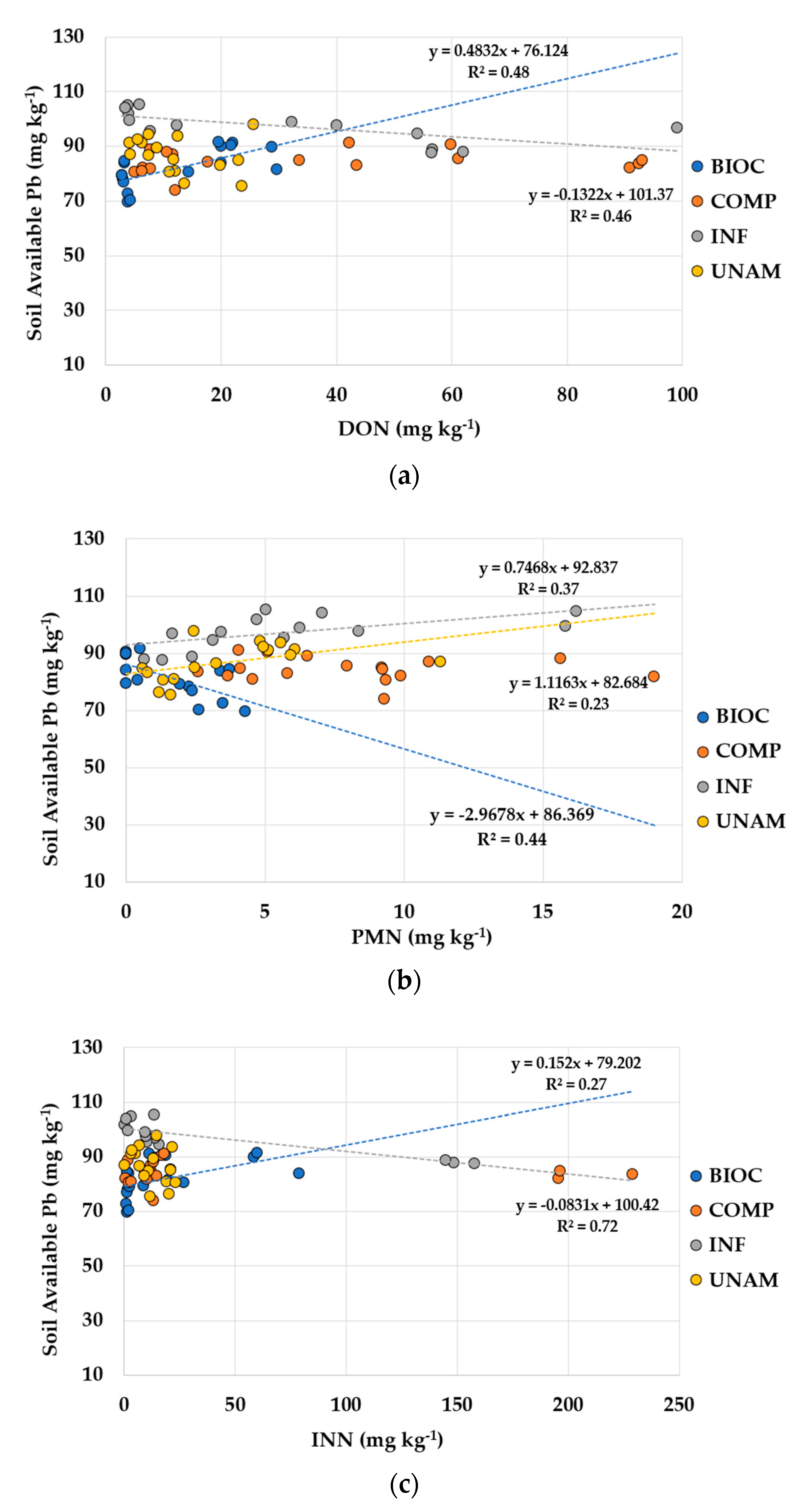
3.2.4. Soil Lead
3.2.5. Plant Growth Parameters
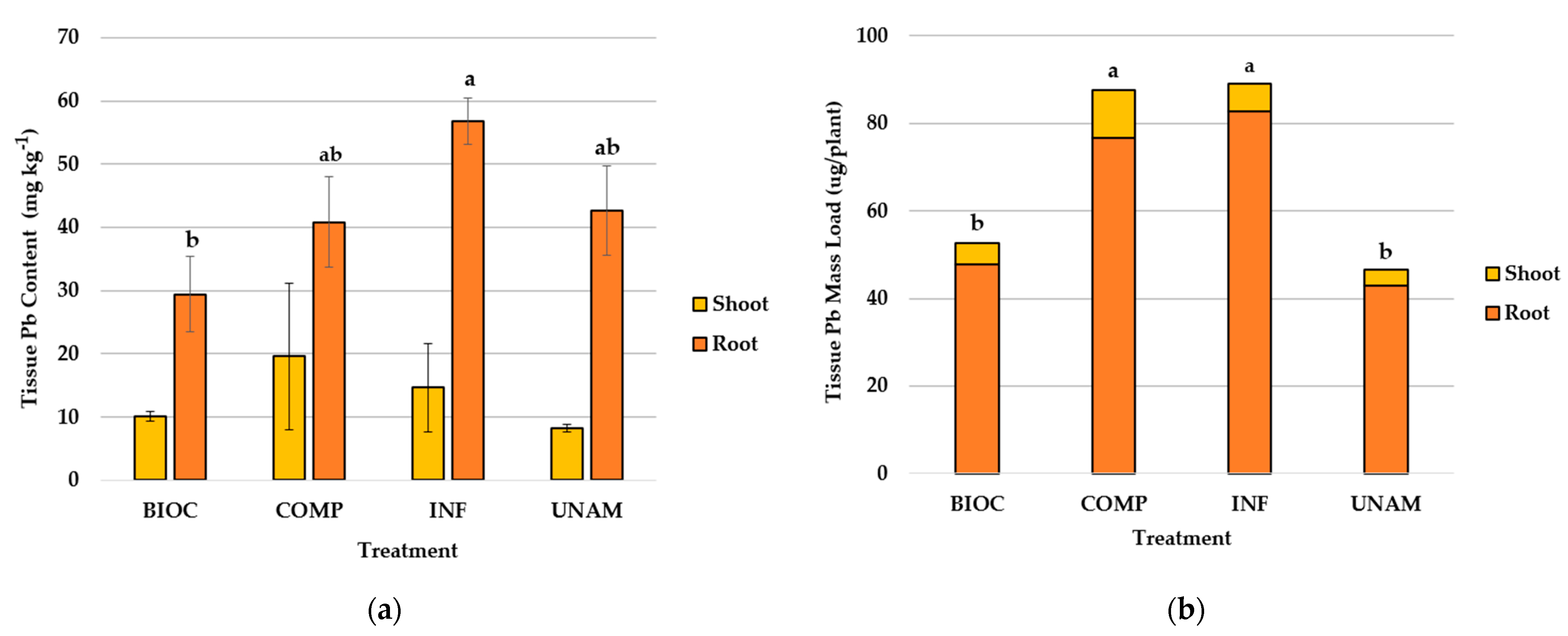
3.2.6. Lead Content and Uptake by Radish
4. Discussion
4.1. Amendments and Soil Properties
4.1.1. Soil Physicochemical Properties
4.1.2. Biogeochemistry in the Absence of Plants
4.1.3. Soil Iron and Phosphorous in Absence of Plants
4.1.4. Soil Biogeochemical Properties after Plant Harvest
4.1.5. Soil Phosphorus and Iron after Plant Harvest
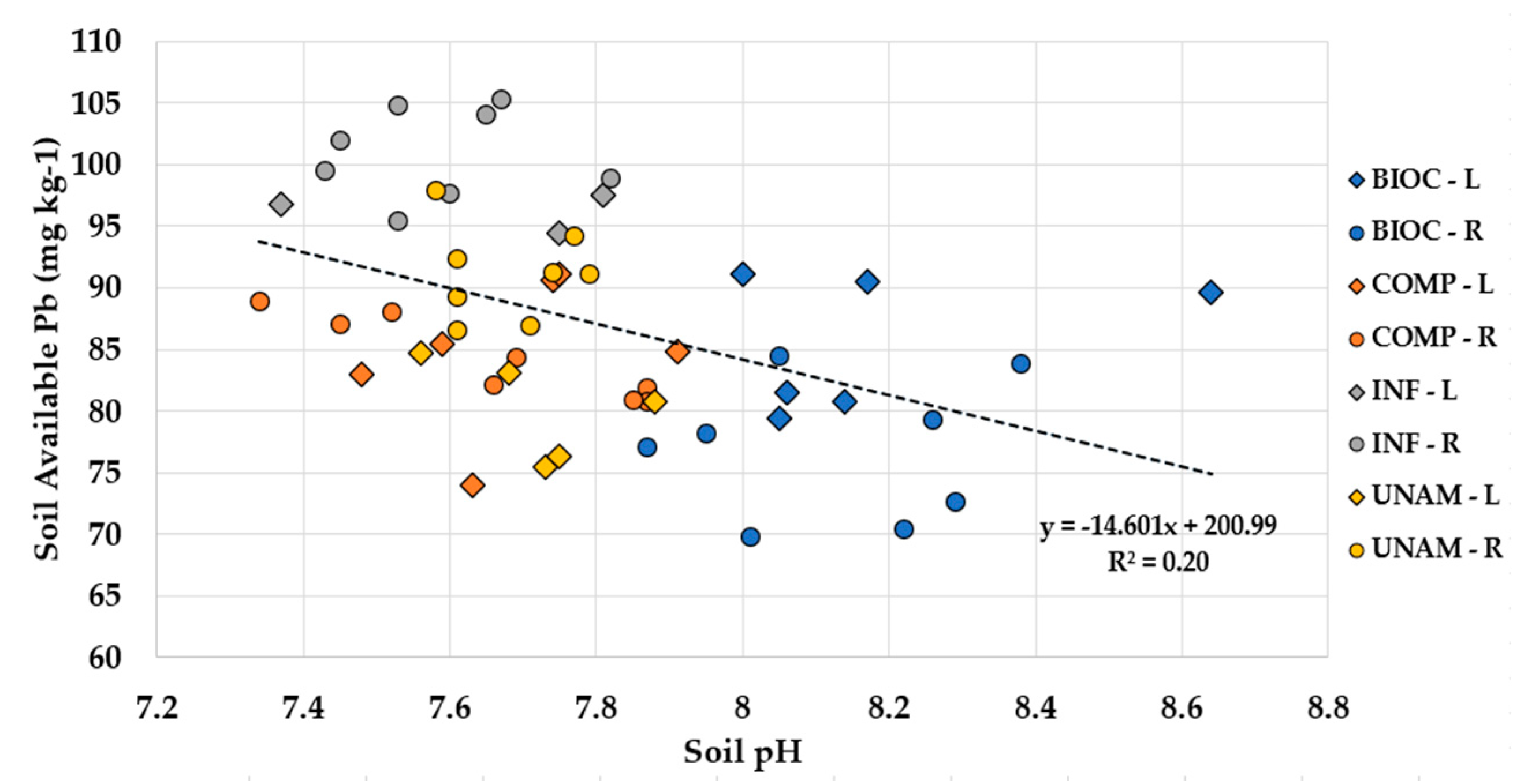
4.2. Soil Lead
4.2.1. Soil Lead Concentrations in Absence of Plant Growth
4.2.2. Soil Lead Concentrations after Plant Harvest
4.2.3. Lead Uptake in Radish
4.3. Limitations and Future Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, D. A survey of community gardens in upstate New York: Implications for health promotion and community development. Health Place 2000, 6, 319–327. [Google Scholar] [CrossRef]
- Twiss, J.; Dickinson, J.; Duma, S.; Kleinman, T.; Paulsen, H.; Rilveria, L. Community Gardens: Lessons Learned from California Healthy Cities and Communities. Am. J. Public Health 2003, 93, 1435–1438. [Google Scholar] [CrossRef]
- Brown, K.H.; Jameton, A.L. Public Health Implications of Urban Agriculture. J. Public Health Policy 2000, 21, 20–39. [Google Scholar] [CrossRef]
- Coelho, F.; Coelho, E.; Egerer, M. Local food: Benefits and failings due to modern agriculture. Sci. Agric. 2018, 75, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Berg, A.E.; Winsum-Westra, M.V.; Vries, S.D.; Dillen, S.M. Allotment gardening and health: A comparative survey among allotment gardeners and their neighbors without an allotment. Environ. Health A Glob. Access Sci. Source 2010, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Blair, D.; Giesecke, C.C.; Sherman, S. A dietary, social and economic evaluation of the Philadelphia urban gardening project. J. Nutr. Educ. 1991, 23, 161–167. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Sci. Plant Nutr. 2018, 156–167. [Google Scholar] [CrossRef]
- Zaccone, C.; Caterina, R.D.; Rotunno, T.; Quinto, M. Soil—Farming System—Food—Health: Effect of conventional and organic fertilizers on heavy metal (Cd, Cr, Cu, Ni, Pb, Zn) content in semolina samples. Soil Tillage Res. 2010, 107, 97–105. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 113–136. [Google Scholar] [CrossRef] [Green Version]
- Angelone, M.; Bini, C. Trace elements concentrations in soils and plants of western Europe. In Biogeochemistry of Trace Metals, 1st ed.; Adriano, D.C., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1992; pp. 19–60. [Google Scholar]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Yunesian, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 2008, 160, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.D.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta- analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Meuser, H. Causes of soil contamination in the urban environment. In Contaminated Urban Soils, 1st ed.; Alloway, B., Trevors, T.J., Eds.; Springer: Heidelberg, Germany, 2010; Volume 18, pp. 29–94. [Google Scholar]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Kim, B.F.; Poulsen, M.N.; Margulies, J.D.; Dix, K.L.; Palmer, A.M.; Nachman, K.E. Urban Community Gardeners’ Knowledge and Perceptions of Soil Contaminant Risks. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.M.; Presley, D.R.; Hettiarachchi, G.M.; Thien, S.J. Assessing the Educational Needs of Urban Gardeners and Farmers on the Subject of Soil Contamination. J. Ext. Educ. 2013, 51, 1FEA10. [Google Scholar]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994; pp. 308–341. [Google Scholar]
- Pinto, A.; Mota, A.; Varennes, A.D.; Pinto, F. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total Environ. 2004, 326, 239–247. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizoua, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baume, C.; Prasad, M.N.V.; Rinklebeg, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 221–245. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef]
- Al-Heety, E.A.M.S.; Yassin, K.H.; Abd-Alsalaam, S. Health risk assessment of some heavy metals in urban community garden soils of Baghdad City, Iraq. Hum. Ecol. Risk Assess. 2017, 23, 225–240. [Google Scholar] [CrossRef]
- Khan, D.; Frankland, B. Effects of cadmium and lead on radish plants with particular reference to movement of metals through soil profile and plant. Plant Soil 1983, 70, 335–345. [Google Scholar] [CrossRef]
- Abadin, H.; Ashizawa, A.; Stevens, Y.-W.; Llados, F.; Diamond, G.; Sage, G.; Swarts, S.G. Toxicological Profile for Lead. U.S. Department of Health and Human Services. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 21 June 2021).
- Gopal, R.; Botany, A.H. Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 2008, 70, 1539–1544. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. Rev. Environ. Contam. Toxicol. 2019, 249. [Google Scholar] [CrossRef] [Green Version]
- Kizilkaya, R.; Akin, T.; Bayrakli, B.; Sǎlam, M. Microbiological characteristics of soils contaminated with heavy metals. Eur. J. Soil Biol. 2004, 40, 95–102. [Google Scholar] [CrossRef]
- Ussiri, D.A.; Lal, R. Carbon sequestration in reclaimed mine soils. CRC Crit. Rev. Plant Sci. 2005, 24, 151–165. [Google Scholar] [CrossRef]
- Walker, D.; Clemente, R.; Roig, A.; Bernal, M. The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ. Pollut. 2003, 122, 303–312. [Google Scholar] [CrossRef]
- Clemente, R.; Paredes, C.; Bernal, M. A field experiment investigating the effects of olive husk and cow manure on heavy metal availability in a contaminated calcareous soil from Murcia (Spain). Agric. Ecosyst. Environ. 2007, 118, 319–326. [Google Scholar] [CrossRef]
- García-Gil, J.; Ceppi, S.; Velasco, M.; Polo, A.; Senesi, N. Long-term effects of amendment with municipal solid waste compost on the elemental and acidic functional group composition and pH-buffer capacity of soil humic acids. Geoderma 2004, 121, 135–142. [Google Scholar] [CrossRef]
- Vega, F.; Andrade, M.; Covelo, E. Influence of soil properties on the sorption and retention of cadmium, copper and lead, separately and together, by 20 soil horizons: Comparison of linear regression and tree regression analyses. J. Hazard. Mater. 2010, 174, 522–533. [Google Scholar] [CrossRef]
- Dahn, R.; Scheidegger, A.; Manceau, A.; Schlegel, M.; Baeyens, B.; Bradbury, M.; Chateigner, D. Structural evidence for the sorption of Ni(II) atoms on the edges of montmorillonite clay minerals: A polarized X-ray absorption fine structure study. Geochim. Cosmochim. Acta 2003, 67, 1–15. [Google Scholar] [CrossRef]
- Bi, X.; Ren, L.; Gong, M.; He, Y.; Wang, L.; Ma, Z. Transfer of cadmium and lead from soil to mangoes in an uncontaminated area, Hainan Island, China. Geoderma 2010, 155, 115–120. [Google Scholar] [CrossRef]
- Dumat, C.; Quenea, K.; Bermond, A.; Toinen, S.; Benedetti, M. Study of the trace metal ion influence on the turnover of soil organic matter in cultivated contaminated soils. Environ. Pollut. 2006, 142, 521–529. [Google Scholar] [CrossRef]
- Uzu, G.; Sobanska, S.; Aliouane, Y.; Pradere, P.; Dumat, C. Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environ. Pollut. 2009, 157, 1178–1185. [Google Scholar] [CrossRef] [Green Version]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Li, Y.; Gao, W.; Chen, Y.; Pan, W.; Lee, X.; Tang, Y. Effects of biochar on Cd and Pb mobility and microbial community composition in a calcareous soil planted with tobacco. Biol. Fertil. Soils 2018, 54, 373–383. [Google Scholar] [CrossRef]
- Streubel, J.; Collins, H.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Biochar Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Bruun, W.W.; Müller-Stöer, D.; Ambus, P.; Hauggaard-Nielsen, H. Application of biochar to soil and N2o emmisions: Potential effects of blending fast-pyrolysis biochar with anaerobically digested slurry. Eur. J. Soil Sci. 2011, 62, 581–589. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the central Amazon Basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutlidge, H.; Wong, S.; Chia, C.; et al. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 2014, 272, 121–128. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of biochar on availability and plant uptake of heavy metals—A meta-analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. Nature. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Novak, J.M.; Busscher, W.J.; Ahmedna, M.; Rehrah, D.; Watts, D.W. Switchgrass Biochar Affects Two Aridisols. J. Environ. Qual. 2012, 41, 1123–1130. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Gajanan, S.G.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Pennanen, T.; Srivastava, V.; Sillanpaa, M.; Sainio, T. Compost: Potent biosorbent for the removal of heavy metals from industrial and landfill stormwater. J. Clean. Prod. 2020, 273, 122736. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Haroon, B.; Irshad, M.; Hafeez, F.; Pervez, A. Fractionation of heavy metals in contaminated soil after amendment with composted cow manure and poultry litter. Arab. J. Geosci. 2019, 12. [Google Scholar] [CrossRef]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Application of Biochar Changed the Status of Nutrients and Biological Activity in a Calcareous Soil. Soil Sci. Plant Nutr. 2020, 20, 450–459. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, X.; Li, S.; Zhou, W.; Ma, Q.; Zhang, J. Influence of green waste compost on Pb-polluted soil remediation, soil quality improvement, and uptake by Pakchoi cabbage (Brassica campestris L. ssp). Environ. Sci. Pollut. Res. 2020, 27, 7693–7701. [Google Scholar] [CrossRef]
- Sun, L.; Niu, Z.; Sun, T. Effects of amendments of N, P, Fe on phytoextraction of Cd, Pb, Cu, and Zn in soil of Zhangshi by mustard, cabbage, and sugar beet. Environ. Toxicol. 2007, 22, 565–571. [Google Scholar] [CrossRef]
- Lin, C.; Zhu, T.; Liu, L.; Wanga, D. Influences of major nutrient elements on Pb accumulation of two crops from a Pb-contaminated soil. J. Hazard. Mat. 2010, 174, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Agrawala, M.; Marshall, F.M. The role of organic vs. inorganic fertilizers in reducing phytoavailability of heavy metals in a wastewater-irrigated area. Ecol. Eng. 2010, 36, 1733–1740. [Google Scholar] [CrossRef]
- He, W.; Yang, X.; Yang, J.; He, Z. Effect of lead on plant availability of phosphorus and potassium in a vegetable–soil system. Environ. Sci. Pollut. Res. 2018, 25, 34793–34797. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. 2010. Available online: https://www.census.gov/quickfacts/fact/table/laramiecitywyoming/POP060210 (accessed on 1 April 2021).
- 2017 Census of Agriculture, County Profile. USDA National Agricultural Statistics Service. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/County_Profiles/Wyoming/cp56001.pdf (accessed on 10 June 2021).
- Western Regional Climate Center. Laramie AP, Wyoming. Available online: https://wrcc.dri.edu/cgi-bin/cliMAIN.pl?wy5415 (accessed on 25 May 2021).
- U.S. Department of Agriculture, Natural Resources Conservation Service. ALCOVA Series. 2001. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/A/ALCOVA.html (accessed on 25 May 2021).
- Knight, J. An Eyesore? The U.S. Might Have Needed It—Laramie’s Aluminum Plant a WWII Reminder. Albany County Historical Society. 2019. Available online: https://www.wyoachs.com/larmies-living-history-businesses/2019/3/23/an-eyesore-the-us-might-have-needed-it-laramies-aluminum-plant-a-wwii-reminder (accessed on 25 May 2021).
- Tippin, C. DEQ Overseeing Cleanup of Site South of Laramie. Laramie Boomerang. 2017. Available online: https://www.laramieboomerang.com/news/deq-overseeing-cleanup-of-site-south-of-laramie/article_be2471d4-f9c9-593f-8521-f689bb215f45.html (accessed on 25 May 2021).
- Bremner, J. Nitrogen Total. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1122. [Google Scholar]
- Doane, T.; Horwath, W. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 39, 2713–2722. [Google Scholar] [CrossRef]
- Hart, S.; Stark, J.M.; Davidson, E. Nitrogen mineralization, immobilization, and nitrification. In Methods of Soil Analysis. Part 2: Microbiological and Biochemical Properties, 5.2; Weaver, R.W., Angle, S., Bottomly, P., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 985–1018. [Google Scholar]
- Newcomb, J.; Carrilo, Y. Shimadzu Carbon and Nitrogen Analyzer Protocol; University of Wyoming: Laramie, WY, USA, 2011; unpublished work. [Google Scholar]
- Sherrod, L.A.; Dunn, G.; Peterson, G.A.; Kolberg, R.L. Inorganic carbon analysis by modified pressure-calcimeter method. Soil Sci. Soc. Am. J. 2002, 66, 299–305. [Google Scholar] [CrossRef]
- Olsen, S.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. In United States Department of Agriculture Circular No. 939; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Lindsay, W.; Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Soon, Y.K. Determination of Cadmium, Chromium, Cobalt, Lead and Nickel in Plant Tissue. In Handbook of Reference Methods for Plant Analysis, 1st ed.; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 193–198. [Google Scholar]
- Antonangeli, J.A.; Zhang, H. The Use of Biochar as a Soil Amendment to Reduce Potentially Toxic Metals (PTMs) Phytoavailability. In Applications of Biochar for Environmental Safety; Abdelhafez, A.A., Abbas, M.H., Eds.; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef]
- Self, J. Soil Test Explanation—0.502. Colorado State University Extension. 2010. Available online: https://extension.colostate.edu/topic-areas/agriculture/soil-test-explanation-0-502/ (accessed on 25 May 2021).
- Swift, C. Vegetable Gardening—Nitrogen Recommendations—7.247. Colorado State University Extension. 2020. Available online: https://extension.colostate.edu/topic-areas/yard-garden/vegetable-gardening-nitrogen-recommendations-7-247/ (accessed on 25 May 2021).
- Awan, S.; Ippolito, J.A.; Ullman, J.L.; Ansari, K.; Cui, L.; Siyal, A.A. Biochars reduce irrigation water sodium adsorption ratio. Biochar 2021, 3, 77–87. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhu, L.; Cui, H.; Jia, L.; Xie, X.; Li, J.; Wei, Z. Assessing the use of composts from multiple sources based on the characteristics of carbon mineralization in soil. Waste Manag. 2017, 70, 30–36. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; Adani, F. The Effects of Short-Term Compost Application On Soil Chemical Properties and on Nutritional Status of Maize Plant. Compost. Sci. Util. 2007, 15, 176–183. [Google Scholar] [CrossRef]
- Whalen, J.K.; Benslim, H.; Jiao, Y.; Sey, B.K. Soil organic carbon and nitrogen pools as affected by compost applications to a sandy-loam soil in Québec. Can. J. Soil Sci. 2008, 88, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Reynolds, W.D.; Drury, C.F.; Fleming, R.; Tan, C.S.; Denholm, K.; Yang, J. Organic carbon and nitrogen stocks in a clay loam soil 10 years after a single compost application. Can. J. Soil Sci. 2014, 94, 357–363. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Shiono, T.; Shinogi, Y. Influence of Sugarcane Bagasse-derived Biochar Application on Nitrate Leaching in Calcaric Dark Red Soil. J. Environ. Qual. 2012, 41, 1131–1137. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Cowie, A.; Smernik, R. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef]
- Fiorentinoa, N.; Sánchez-Monedero, M.; Lehmann, J.; Enders, A.; Fagnano, M.; Cayuelab, M. Interactive priming of soil N transformations from combining biochar and urea inputs: A 15N isotope tracer study. Soil Biol. Biochem. 2019, 131, 166–175. [Google Scholar] [CrossRef]
- United Nations Food and Agriculture Organization (FAO). Management of Calcareous Soils. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/calcareous-soils/en/#:~:text=Phosphorous%20is%20often%20lacking%20in,be%20in%20water%20soluble%20form (accessed on 25 May 2021).
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, 8th ed.; Pearson, Inc.: Boston, MA, USA, 2013; pp. 261–269. [Google Scholar]
- Prasad, R.; Chakraborty, D. Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil. Alabama A&M and Auburn Universities. Available online: https://www.aces.edu/wp-content/uploads/2019/04/ANR-2535-Phosphorus-Basics_041719L.pdf (accessed on 24 May 2021).
- Weng, L.; Vega, F.A.; Van Riemsdijk, W.H. Competitive and synergistic effects in pH dependent phosphate adsorption in soils: LCD modeling. Environ. Sci. Technol. 2011, 45, 8420–8428. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.; Ahamadou, B.; Li, M.; Gong, C.; Cai, P.; Liang, W.; Huang, Q. Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J. Soils Sediments 2010, 10, 973–982. [Google Scholar] [CrossRef]
- Beegle, D.B.; Durst, P.T. Managing Phosphorus for Crop Production. Pennsylvania State University. 2002. Available online: https://extension.psu.edu/programs/nutrient-management/educational/soil-fertility/managing-phosphorus-for-crop-production/extension_publication_file (accessed on 25 May 2021).
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood Biochar Influences Calcareous Soil Physicochemical and Microbiological Status. J. Environ. Qual. 2014, 43, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.A.; Bending, G.D.; White, P.J. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Fekih, I.B.; Ditta, A.; Xing, S. Role of biochar and plant growth promoting rhizobacteria to enhance soil carbon sequestration—A review. Environ. Monit. Assess. 2019, 191. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil phosphorus availability. Cienc. Agrotecnol. 2016, 40, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus Sorption and Availability from Biochars and Soil/Biochar Mixtures. Clean 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid behaviour in a calcareous soil implication for rhizosphere nutrient cycling. Soil Biol. Biochem. 2005, 37, 2046–2054. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Berry, C.M.; Strawn, D.G.; Novak, J.M.; Levine, J.; Harley, A. Biochars Reduce Mine Land Soil Bioavailable Metals. J. Environ. Qual. 2017, 46, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.E.; Zheng, J.; Botte, J.; Cotrufo, M.F. Co-generated fast pyrolysis biochar mitigates greenhouse gas emissions and increases carbon sequestration. Glob. Chang. Biol. Bioenergy 2013, 5, 153–164. [Google Scholar] [CrossRef]
- Shahkolaie, S.S.; Baranimotlagh, M.; Dordipour, E.; Khormali, F. Cadmium and lead immobilization in a calcareous contaminated soil using the cost-effective amendments. Arab. J. Geosci. 2019, 12. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, L.; Rhue, R. Relation of enhanced Pb solubility to Fe partitioning in soils. Environ. Pollut. 2000, 110, 515–522. [Google Scholar] [CrossRef]
- Information Registered Fertilizer, Ag Mineral, Ag Amendment, and Lime Products. Oregon Department of Agriculture. Available online: http://oda.state.or.us/dbs/heavy_metal/detail.lasso?-op=eq&product_id=2462 (accessed on 18 February 2021).
- Sherene, T. Effect of Dissolved Organic Carbon (DOC) on Heavy Metal Mobility in Soils. Nat. Environ. Pollut. Technol. 2009, 8, 817–821. [Google Scholar]
- Guan, W. Effects of Nitrogen Fertilizers on Soil pH. Purdue University, Vegetable Crops Hotline. 2016. Available online: https://vegcropshotline.org/article/effects-of-nitrogen-fertilizers-on-soil-ph/ (accessed on 25 February 2021).
- U.S. Food & Drug Administration. Lead in Food, Foodwares, and Dietary Supplements. 2020. Available online: https://www.fda.gov/food/metals-and-your-food/lead-food-foodwares-and-dietary-supplement. (accessed on 25 May 2021).
- State of the Biochar Industry 2014. International Biochar Initiative. Available online: https://biochar-international.org/state-of-the-biochar-industry-2014/ (accessed on 15 June 2021).
- Grand View Research. U.S. Biochar Market Size, Share & Trend Analysis Report by Technology (Gasification, Pyrolysis), By Application (Agriculture, Animal Feed, Health & Beauty Products), by States, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/us-biochar-market (accessed on 15 June 2021).

| Soil Metrics | Soil Measurement |
|---|---|
| Texture | Sandy Clay Loam |
| Moisture (g g−1) | 0.04 (0.01) |
| pH (1:2 soil: water) | 7.4 (0.1) |
| EC (μS cm−1) | 553 (3) |
| Total Organic Carbon (TOC) (mg kg−1) | 36,793 (1638) |
| Inorganic Carbon (IC) (mg kg−1) | 17,573 (248) |
| Total Nitrogen (mg kg−1) | 2123 (15) |
| Inorganic Nitrogen (INN) (mg kg−1) | 9.90 (0.4) |
| Potentially Mineralizable Nitrogen (PMN) (mg kg−1) | 1.53 (0.29) |
| Dissolved Organic Carbon (DOC) (mg kg−1) | 282 (12) |
| Dissolved Organic Nitrogen (DON) (mg kg−1) | 2.09 (0.55) |
| Available Phosphorus (AP) (mg kg−1) | 0.27 (0.01) |
| Total Pb (mg kg−1) | 733 (29) |
| Available Pb (mg kg−1) | 111 (8) |
| Total Fe (mg kg−1) | 20,703 (386) |
| Available Fe (mg kg−1) | 8.87 (0.48) |
| Chemical Composition | Biochar | Compost | Inorganic Fertilizer |
|---|---|---|---|
| Carbon and Nitrogen: | ---%--- | ||
| Total Nitrogen | 0.42 | 1.24 | n/a |
| Total Carbon | 65.10 | 8.57 | n/a |
| C-to-N ratio | 154 | 7 | n/a |
| Macronutrients: | ---%--- | ||
| Organic N | n/a | 1.15 | n/a |
| Ammonium | n/a | 0.004 | 3.500 |
| Nitrate | n/a | 0.086 | 20.50 |
| Available Phosphorus | 0.11 | 0.85 | 8.00 |
| Potassium | n/a | 2.13 | 16.00 |
| Calcium | 3.21 | 3.93 | n/a |
| Magnesium | 0.39 | 0.68 | n/a |
| Sulfur | 0.06 | 0.41 | n/a |
| Micronutrients: | --ppm-- | ---%--- | |
| Boron | 29.2 | 28.4 | 0.02 |
| Copper | n/a | 23.3 | 0.07 |
| Iron | 181 | 8933 | 0.15 |
| Manganese | 51 | 241.6 | 0.05 |
| Molybdenum | 0.07 | n/a | 0.0005 |
| Zinc | 53 | 95.7 | 0.06 |
| Trace Elements: | ---%--- | ||
| Sodium | n/a | 0.33 | n/a |
| Soil Physicochemical Properties | BIOC | COMP | INF | UNAM | F-Stat | p-Value |
|---|---|---|---|---|---|---|
| pH (1:2 soil: water) | ||||||
| Begin | 8.4 (0.10) a | 7.8 (0.00) b | 7.7 (0.10) b | 7.9 (0.00) b | 26.38 | ≤0.001 |
| End (All) | 8.2 (0.05) a | 7.7 (0.04) b | 7.6 (0.04) b | 7.7 (0.03) b | 37.23 | ≤0.001 |
| EC (μS cm−1) | ||||||
| Begin | 689 (23) bc | 1209 (84) a | 956 (49) ab | 537 (20) c | 22.67 | ≤0.001 |
| End Radish | 464 (14) b | 615 (32) a | 434 (12) b | 472 (27) b | 8.86 | ≤0.001 |
| End Lettuce | 929 (93) ab | 1057 (110) a | 908 (65) ab | 597 (32) b | 3.97 | ≤0.05 |
| IC (mg kg−1) | ||||||
| End (All) | 17,457 (714) | 15,971 (701) | 16,774 (813) | 17,007 (559) | 0.79 | ns |
| TOC (mg kg−1) | ||||||
| End (All) | 78,043 (1778) a | 35,465 (1422) b | 30,887 (1252) b | 33,977 (1247) b | 32.08 | ≤0.001 |
| TN (mg kg−1) | ||||||
| End Lab Inc | 2330 (40) b | 2820 (45) a | 1927 (53) c | 1947 (33) c | 62.15 | ≤0.001 |
| End Radish | 2221 (29) ab | 2279 (67) a | 2064 (37) b | 2134 (32) ab | 4.12 | ≤0.05 |
| End Lettuce | 2220 (35) b | 2528 (53) a | 2130 (64) b | 2022 (23) c | 21.2 | ≤0.001 |
| Soil C:N Ratio | ||||||
| End (All) | 42.30 (0.79) a | 21.11 (0.36) b | 23.41 (0.32) b | 25.04 (0.31) b | 40.80 | ≤0.001 |
| Soil Labile C and N Properties | BIOC | COMP | INF | UNAM | F-Stat | p-Value |
|---|---|---|---|---|---|---|
| DOC (mg kg−1) | ||||||
| Begin | 398.85 (58.12) a | 270.69 (14.38) ab | 163.03 (13.5) b | 212.50 (11.91) b | 7.06 | ≤0.05 |
| End Lab Inc | 298.97 (23.06) ab | 389.10 (17.38) a * | 243.16 (30.09) b | 188.20 (27.27) b | 7.89 | ≤0.01 |
| End Radish | 48.40 (12.10) c * | 132.68 (11.83) b * | 198.24 (16.28) a | 112.20 (9.62) b * | 17.76 | ≤0.001 |
| End Lettuce | 126.05 (13.99) b * | 213.44 (18.12) a | 111.81 (6.02) b * | 147.80 (14.43) ab * | 6.86 | ≤0.01 |
| DON (mg kg−1) | ||||||
| Begin | 12.45 (0.25) c | 75.48 (10.88) ab | 101.50 (15.57) a | 28.33 (3.23) bc | 12.21 | ≤0.01 |
| End Lab Inc | 19.83 (0.11) ab * | 92.08 (0.51) a | 58.38 (1.47) ab | 11.80 (0.35) b * | 10.39 | ≤0.05 |
| End Radish | 3.41 (0.17) b * | 9.13 (1.36) a * | 9.21 (3.24) a * | 8.70 (2.31) a * | 12.31 | ≤0.001 |
| End Lettuce | 19.91 (3.73) b | 42.06 (6.78) ab * | 64.40(14.53) a | 18.44 (2.13) b | 4.65 | ≤0.05 |
| PMN (mg kg−1) | ||||||
| Begin | 1.64 (0.96) | 5.69 (1.36) | <d.l. | 4.23 (0.61) | 5.52 | ≤0.05 |
| End Lab Inc | 0.36 (0.08) b | 3.45 (0.36) a | 1.45 (0.41) ab | 3.13 (1.03) ab | 4.54 | ≤0.05 |
| End Radish | 3.02 (0.27) c | 10.63 (1.55) a | 8.64 (1.55) ab | 5.49 (0.88) b | 7.03 | ≤0.01 |
| End Lettuce | <d.l. | 6.89 (0.83) a | 2.74 (0.45) b | 1.18 (0.20) b * | 61.10 | ≤0.01 |
| INN (mg kg−1) | ||||||
| Begin | 15.2 (1.22) b | 195.16 (17.54) a | 217.12 (25.10) a | 137.76 (21.14) a | 15.75 | ≤0.01 |
| End Lab Inc | 65.73 (5.36) c * | 207.11 (9.01) a | 150.51 (3.13) b | 22.07 (0.59) d * | 153.70 | ≤0.001 |
| End Radish | 1.77 (0.18) * | 6.68 (1.70) * | 6.20 (1.71) * | 6.79 (1.62) * | 1.91 | ns |
| End Lettuce | 16.70 (2.48) | 16.04 (1.25) * | 14.33 (0.66) * | 14.28 (2.06) * | 0.30 | ns |
| Soil Parameter | Soil Available Pb | |
|---|---|---|
| p-Value | Corr Coeff | |
| pH (1:2, soil:H2O) | ≤0.001 | −0.45 |
| TOC (mg kg−1) | ≤0.001 | −0.46 |
| C:N Ratio | ≤0.01 | −0.36 |
| Available Fe (mg kg−1) | ≤0.01 | 0.36 |
| Soil Parameter Correlate | BIOC | COMP | INF | UNAM | ||||
|---|---|---|---|---|---|---|---|---|
| p-Value | Corr Coeff | p-Value | Corr Coeff | p-Value | Corr Coeff | p-Value | Corr Coeff | |
| All | (n = 17) | (n = 17) | (n = 14) | (n = 16) | ||||
| EC (μS cm−1) | ≤0.05 | 0.57 | - | - | ≤0.05 | −0.62 | - | - |
| PMN (mg kg−1) | ≤0.01 | −0.67 | - | - | ≤0.05 | 0.61 | ≤0.10 | 0.48 |
| INN (mg kg−1) | ≤0.05 | 0.52 | - | - | ≤0.001 | −0.85 | - | - |
| DOC (mg kg−1) | ≤0.05 | 0.51 | - | - | - | - | - | - |
| DON (mg kg−1) | ≤0.01 | 0.69 | - | - | ≤0.01 | −0.68 | - | - |
| AP (mg kg−1) | - | - | - | - | - | ≤0.001 | 0.78 | |
| C:N Ratio | - | - | - | - | - | ≤0.05 | −0.52 | |
| Available Fe (mg kg−1) | ≤0.001 | 0.90 | - | - | ≤0.01 | 0.72 | - | - |
| Radish | (n = 8) | (n = 8) | (n = 8) | (n = 8) | ||||
| DOC (mg kg−1) | ≤0.05 | −0.77 | - | - | - | - | - | - |
| TOC (mg kg−1) | ≤0.01 | 0.94 | ≤0.01 | 0.84 | - | - | - | - |
| C:N Ratio | ≤0.05 | 0.83 | - | - | - | - | - | - |
| pH (1:2, soil:H20) | - | - | ≤0.001 | −0.94 | - | - | - | - |
| DON (mg kg−1) | ≤0.05 | −0.72 | - | - | - | - | - | - |
| Available Fe (mg kg−1) | ≤0.01 | 0.88 | - | - | - | - | - | - |
| Lettuce | (n = 6) | (n = 6) | (n = 3) | (n = 5) | ||||
| Available Fe (mg kg−1) | ≤0.01 | 0.93 | - | - | - | - | - | - |
| Plant Growth Parameters | BIOC | COMP | INF | UNAM | F-Stat | p-Value |
|---|---|---|---|---|---|---|
| Radish | ||||||
| No. Germinated | 8 | 8 | 8 | 8 | ||
| Dry Root Biomass (g) | 1.62 (0.10) a | 1.88 (0.13) a | 1.46 (0.14) ab | 1.01 (0.13) b | 7.41 | ≤0.001 |
| Dry Shoot Biomass (g) | 0.49 (0.04) | 0.55 (0.05) | 0.44 (0.08) | 0.45 (0.04) | 0.77 | ns |
| SPAD (SPAD units) | 29.38 (0.87) c | 32.88 (0.39) b | 33 (0.84) b | 36.97 (1.07) a | 12.1 | ≤0.001 |
| Root:Shoot | 3.59 (0.42) a | 3.54 (0.29) a | 4.17 (0.76) a | 2.22 (0.15) b | 5.56 | ≤0.01 |
| Lettuce | ||||||
| No. Germinated | 6 | 6 | 3 | 5 | ||
| Dry Root Biomass (g) | 0.10 (0.04) | 0.19 (0.06) | 0.17 (0.03) | 0.06 (0.03) | 3.23 | ns |
| Dry Shoot Biomass (g) | 0.09 (0.03) | 0.26 (0.05) | 0.24 (0.03) | 0.18 (0.10) | 2.77 | ns |
| SPAD (SPAD units) | 19.70 (3.49) b | 30.83 (2.36) ab | 36.30 (2.59) a | 26.40 (1.85) ab | 4.38 | ≤0.05 |
| Shoot:Root | 1.23 (0.15) b | 1.37 (0.90) ab | 1.46 (1.05) ab | 3.17 (3.04) a | 4.25 | ≤0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiger, T.; Norton, U. Effects of Garden Amendments on Soil Available Lead and Plant Uptake in a Contaminated Calcareous Soil. Appl. Sci. 2021, 11, 5777. https://doi.org/10.3390/app11135777
Geiger T, Norton U. Effects of Garden Amendments on Soil Available Lead and Plant Uptake in a Contaminated Calcareous Soil. Applied Sciences. 2021; 11(13):5777. https://doi.org/10.3390/app11135777
Chicago/Turabian StyleGeiger, Tara, and Urszula Norton. 2021. "Effects of Garden Amendments on Soil Available Lead and Plant Uptake in a Contaminated Calcareous Soil" Applied Sciences 11, no. 13: 5777. https://doi.org/10.3390/app11135777
APA StyleGeiger, T., & Norton, U. (2021). Effects of Garden Amendments on Soil Available Lead and Plant Uptake in a Contaminated Calcareous Soil. Applied Sciences, 11(13), 5777. https://doi.org/10.3390/app11135777






