The Potential of Visible and Far-Red to Near-Infrared Light in Glaucoma Neuroprotection
Abstract
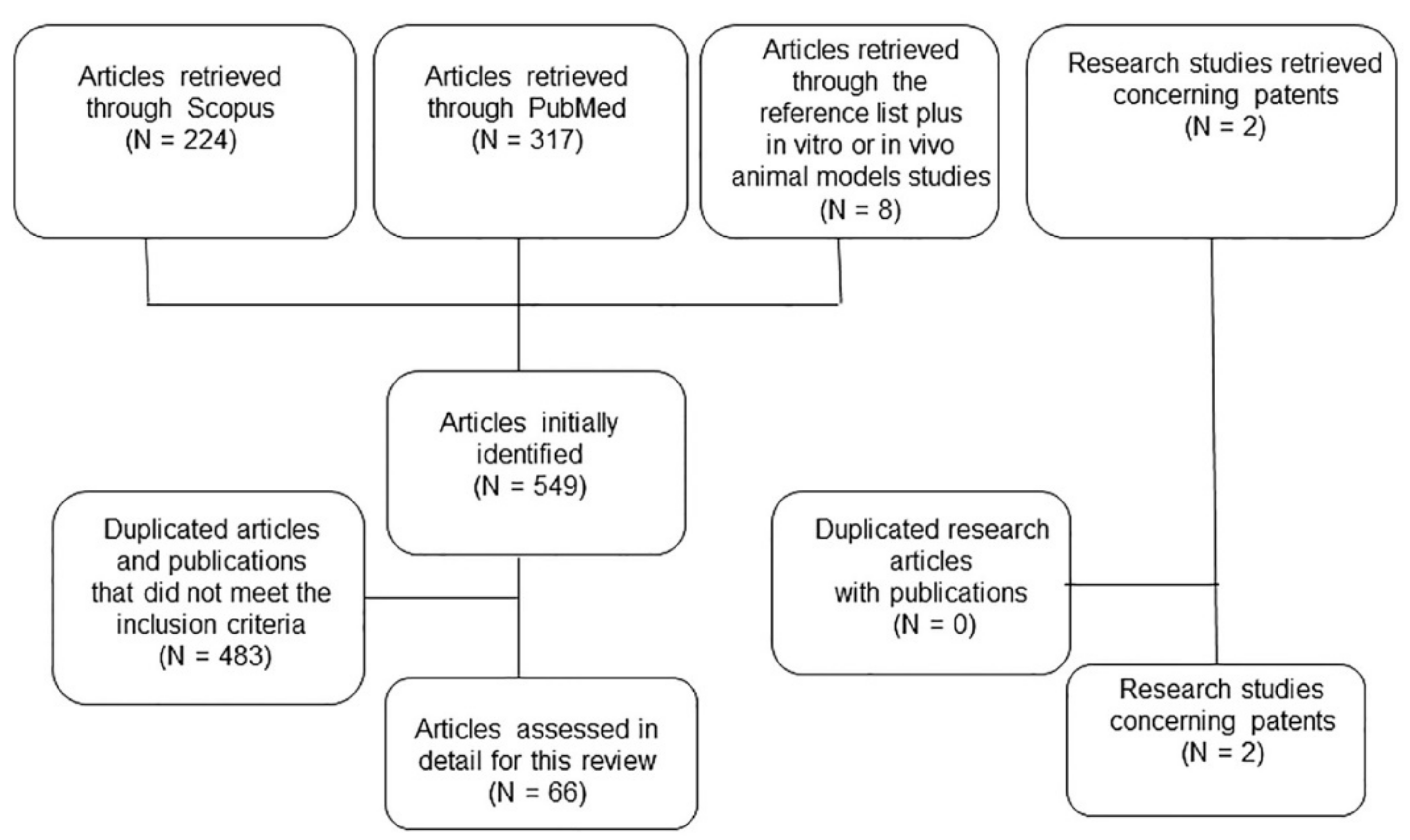
:1. Introduction
2. Photobiomodulation
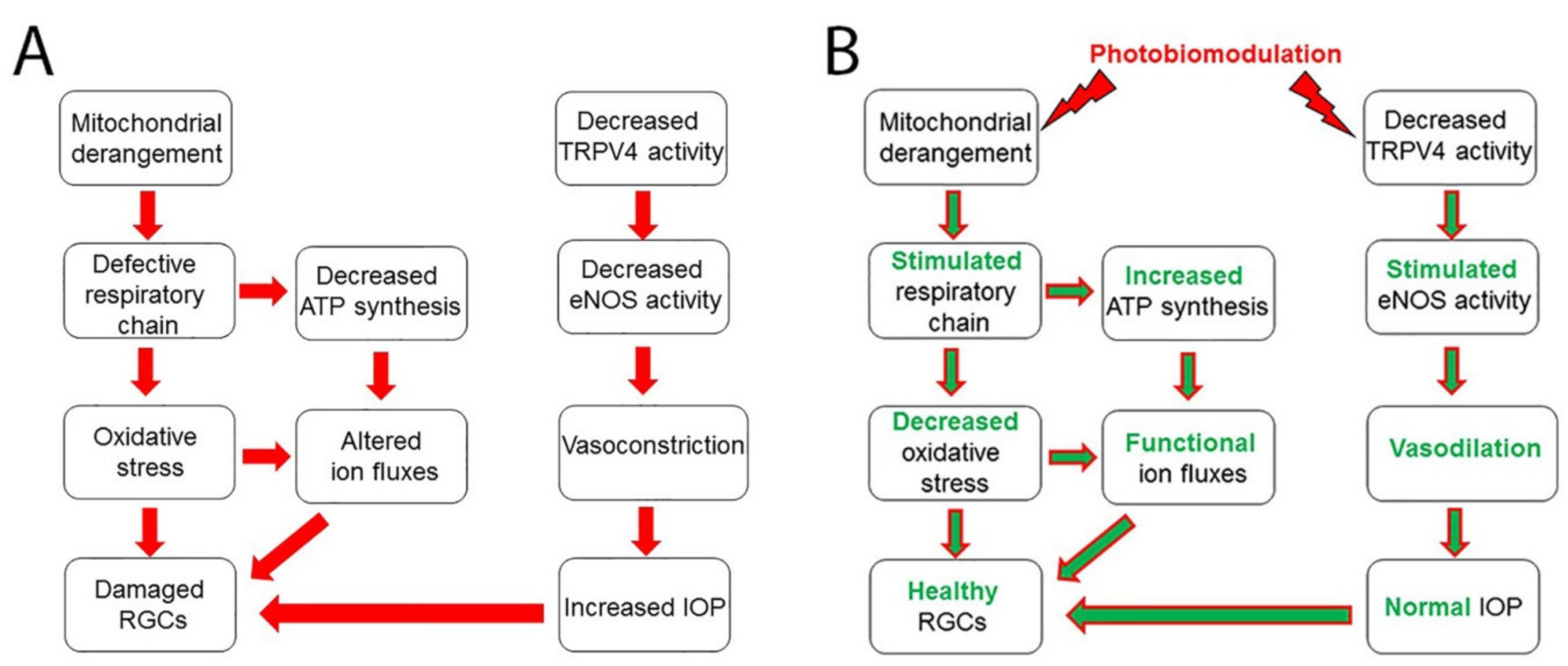
3. Pathophysiology of Glaucoma: Increased IOP, Mitochondrial Dysfunction, and Altered Ion Fluxes
4. Visible and Far-Red to Near-Infrared Photobiomodulation in RGCs in Mitochondrial Protection and Energy Metabolism
5. Visible and Far-Red to Near-Infrared Photobiomodulation in Neuronal Differentiation
6. Current and New Approaches in Glaucoma Treatment Modalities
7. Existing Patents for Ocular Treatments Using Laser Red Light
8. The Future of Clinical Trials for the Treatment of Glaucoma
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kreft, D.; Doblhammer, G.; Guthoff, R.F.; Frech, S. Prevalence, Incidence, and Risk Factors of Primary Open-Angle Glaucoma—A Cohort Study Based on Longitudinal Data from a German Public Health Insurance. BMC Public Health 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Jayanetti, V.; Sandhu, S.; Lusthaus, J.A. The Latest Drugs in Development That Reduce Intraocular Pressure in Ocular Hypertension and Glaucoma. J. Exp. Pharmacol. 2020, 12, 539–548. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinnon, S.J.; Goldberg, L.D.; Peeples, P.; Walt, J.G.; Bramley, T.J. Current Management of Glaucoma and the Need for Complete Therapy. Am. J. Manag. Care 2008, 14, S20–S27. [Google Scholar] [PubMed]
- Gandolfi, S.; Marchini, G.; Caporossi, A.; Scuderi, G.; Tomasso, L.; Brunoro, A. Cytidine 5′-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients 2020, 12, 793. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, A.C.; Liu, J. Neurodegeneration and Neuroprotection in Glaucoma. Yale J. Biol. Med. 2016, 89, 73–79. [Google Scholar] [PubMed]
- Osborne, N.N.; del Olmo-Aguado, S. Maintenance of Retinal Ganglion Cell Mitochondrial Functions as a Neuroprotective Strategy in Glaucoma. Curr. Opin. Pharmacol. 2013, 13, 16–22. [Google Scholar] [CrossRef]
- Del Olmo-Aguado, S.; Núñez-Álvarez, C.; Osborne, N.N. Red Light of the Visual Spectrum Attenuates Cell Death in Culture and Retinal Ganglion Cell Death in Situ. Acta Ophthalmol. 2016, 94, e481–e491. [Google Scholar] [CrossRef] [Green Version]
- Geneva, I.I. Photobiomodulation for the Treatment of Retinal Diseases: A Review. Int. J. Ophthalmol. 2016, 9, 145–152. [Google Scholar] [CrossRef]
- Okuyama, S.; Nagaya, T.; Ogata, F.; Maruoka, Y.; Sato, K.; Nakamura, Y.; Choyke, P.L.; Kobayashi, H. Avoiding Thermal Injury during Near-Infrared Photoimmunotherapy (NIR-PIT): The Importance of NIR Light Power Density. Oncotarget 2017, 8, 113194–113201. [Google Scholar] [CrossRef] [Green Version]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Hashmi, J.T.; Huang, Y.-Y.; Osmani, B.Z.; Sharma, S.K.; Naeser, M.A.; Hamblin, M.R. Role of Low-Level Laser Therapy in Neurorehabilitation. PM&R 2010, 2, S292–S305. [Google Scholar] [CrossRef] [Green Version]
- Eells, J.T.; Gopalakrishnan, S.; Valter, K. Near-Infrared Photobiomodulation in Retinal Injury and Disease. Adv. Exp. Med. Biol. 2016, 854, 437–441. [Google Scholar] [CrossRef]
- Rojas, J.C.; Gonzalez-Lima, F. Low-Level Light Therapy of the Eye and Brain. Eye Brain 2011, 3, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Sayin, N.; Kara, N.; Pekel, G. Ocular Complications of Diabetes Mellitus. World J. Diabetes 2015, 6, 92–108. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Stroux, A.; Velten, I.; Juenemann, A.; Martus, P.; Budde, W.M. Central Corneal Thickness Correlated with Glaucoma Damage and Rate of Progression. Invest. Ophthalmol. Vis. Sci. 2005, 46, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Dohlman, C.H.; Zhou, C.; Lei, F.; Cade, F.; Regatieri, C.V.; Črnej, A.; Dohlman, J.G.; Shen, L.Q.; Paschalis, E.I. Glaucoma After Corneal Trauma or Surgery—A Rapid, Inflammatory, IOP-Independent Pathway. Cornea 2019, 38, 1589–1594. [Google Scholar] [CrossRef]
- Cioffi, G.A. Ischemic model of optic nerve injury. Trans. Am. Ophthalmol. Soc. 2005, 103, 592–613. [Google Scholar] [CrossRef]
- Chen, H.; Cho, K.-S.; Vu, T.H.K.; Shen, C.-H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal Microflora-Induced T Cell Responses Mediate Progressive Neurodegeneration in Glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Ge, J.; Zhuo, Y.-H. Role of Mitochondria in the Pathogenesis and Treatment of Glaucoma. Chin. Med. J. 2013, 126, 4358–4365. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J.; Pekny, M.; Cooper, M.L.; Benowitz, L.; Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants. The Challenge of Regenerative Therapies for the Optic Nerve in Glaucoma. Exp. Eye Res. 2017, 157, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walland, M.J.; Carassa, R.G.; Goldberg, I.; Grehn, F.; Heuer, D.K.; Khaw, P.T.; Thomas, R.; Parikh, R. Failure of Medical Therapy despite Normal Intraocular Pressure. Clin. Experiment. Ophthalmol. 2006, 34, 827–836. [Google Scholar] [CrossRef]
- Kaden, T.R.; Li, W. Autophagy, mitochondrial dynamics and retinal diseases. Asia Pac. J. Ophthalmol. 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Osborne, N.N.; Núñez-Álvarez, C.; Del Olmo-Aguado, S.; Merrayo-Lloves, J. Visual Light Effects on Mitochondria: The Potential Implications in Relation to Glaucoma. Mitochondrion 2017, 36, 29–35. [Google Scholar] [CrossRef]
- Barron, M.J.; Griffiths, P.; Turnbull, D.M.; Bates, D.; Nichols, P. The Distributions of Mitochondria and Sodium Channels Reflect the Specific Energy Requirements and Conduction Properties of the Human Optic Nerve Head. Br. J. Ophthalmol. 2004, 88, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Agostinone, J.; Di Polo, A. Retinal Ganglion Cell Dendrite Pathology and Synapse Loss: Implications for Glaucoma. Prog. Brain Res. 2015, 220, 199–216. [Google Scholar] [CrossRef]
- Núñez-Álvarez, C.; Osborne, N.N. Blue Light Exacerbates and Red Light Counteracts Negative Insults to Retinal Ganglion Cells in Situ and R28 Cells in Vitro. Neurochem. Int. 2019, 125, 187–196. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Weng, C.; Yin, Z. The Changes of Potassium Currents in RCS Rat Müller Cell during Retinal Degeneration. Brain Res. 2012, 1427, 78–87. [Google Scholar] [CrossRef]
- Fischer, R.A.; Roux, A.L.; Wareham, L.K.; Sappington, R.M. Pressure-Dependent Modulation of Inward-Rectifying K+ Channels: Implications for Cation Homeostasis and K+ Dynamics in Glaucoma. Am. J. Physiol. Cell Physiol. 2019, 317, C375–C389. [Google Scholar] [CrossRef]
- Pang, J.-J.; Frankfort, B.J.; Gross, R.L.; Wu, S.M. Elevated Intraocular Pressure Decreases Response Sensitivity of Inner Retinal Neurons in Experimental Glaucoma Mice. Proc. Natl. Acad. Sci. USA 2015, 112, 2593–2598. [Google Scholar] [CrossRef] [Green Version]
- Risner, M.L.; Pasini, S.; Cooper, M.L.; Lambert, W.S.; Calkins, D.J. Axogenic Mechanism Enhances Retinal Ganglion Cell Excitability during Early Progression in Glaucoma. Proc. Natl. Acad. Sci. USA 2018, 115, E2393–E2402. [Google Scholar] [CrossRef] [Green Version]
- Luo, N.; Conwell, M.D.; Chen, X.; Kettenhofen, C.I.; Westlake, C.J.; Cantor, L.B.; Wells, C.D.; Weinreb, R.N.; Corson, T.W.; Spandau, D.F.; et al. Primary Cilia Signaling Mediates Intraocular Pressure Sensation. Proc. Natl. Acad. Sci. USA 2014, 111, 12871–12876. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.D.; Chen, Y.-L.; Kasetti, R.B.; Maddineni, P.; Mayhew, W.; Millar, J.C.; Ellis, D.Z.; Sonkusare, S.K.; Zode, G.S. Impaired TRPV4-ENOS Signaling in Trabecular Meshwork Elevates Intraocular Pressure in Glaucoma. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Frye, A.M.; Phuong, T.T.T.; Yarishkin, O.; Jo, A.O.; Xu, Y.; Lakk, M.; Iuso, A.; Redmon, S.N.; Ambati, B.; et al. TRPV4 Regulates Calcium Homeostasis, Cytoskeletal Remodeling, Conventional Outflow and Intraocular Pressure in the Mammalian Eye. Sci. Rep. 2016, 6, 30583. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.N.; Berry, M.; Logan, A.; Blanch, R.J.; Ahmed, Z. Caspases in Retinal Ganglion Cell Death and Axon Regeneration. Cell Death Discov. 2017, 3, 17032. [Google Scholar] [CrossRef] [Green Version]
- Rosso, M.P.D.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, e4350965. [Google Scholar] [CrossRef] [Green Version]
- Albarracin, R.; Natoli, R.; Rutar, M.; Valter, K.; Provis, J. 670 Nm Light Mitigates Oxygen-Induced Degeneration in C57BL/6J Mouse Retina. BMC Neurosci. 2013, 14, 125. [Google Scholar] [CrossRef] [Green Version]
- Begum, R.; Powner, M.B.; Hudson, N.; Hogg, C.; Jeffery, G. Treatment with 670 Nm Light up Regulates Cytochrome C Oxidase Expression and Reduces Inflammation in an Age-Related Macular Degeneration Model. PLoS ONE 2013, 8, e57828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, N.N.; Núñez-Álvarez, C.; Joglar, B.; del Olmo-Aguado, S. Glaucoma: Focus on Mitochondria in Relation to Pathogenesis and Neuroprotection. Eur. J. Pharmacol. 2016, 787, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, R.; Eells, J.; Valter, K. Photobiomodulation Protects the Retina from Light-Induced Photoreceptor Degeneration. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3582–3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albarracin, R.; Valter, K. 670 Nm Red Light Preconditioning Supports Müller Cell Function: Evidence from the White Light-Induced Damage Model in the Rat Retina. Photochem. Photobiol. 2012, 88, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Hodgetts, S.; Van Den Heuvel, C.; Natoli, R.; Hart, N.S.; Valter, K.; Harvey, A.R.; Vink, R.; Provis, J.; Dunlop, S.A. Red/near-Infrared Irradiation Therapy for Treatment of Central Nervous System Injuries and Disorders. Rev. Neurosci. 2013, 24, 205–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkinopoulos, I.; Colman, A.; Hogg, C.; Heckenlively, J.; Jeffery, G. Age-Related Retinal Inflammation Is Reduced by 670 Nm Light via Increased Mitochondrial Membrane Potential. Neurobiol. Aging 2013, 34, 602–609. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Natoli, R.; Madigan, M.; Fernando, N.; Saxena, K.; Aggio-Bruce, R.; Jiao, H.; Provis, J.; Valter, K. Photobiomodulation with 670 Nm Light Ameliorates Müller Cell-Mediated Activation of Microglia and Macrophages in Retinal Degeneration. Exp. Eye Res. 2017, 165, 78–89. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Fernando, N.; Natoli, R.; Madigan, M.; Valter, K. 670 nm Light Treatment Following Retinal Injury Modulates Müller Cell Gliosis: Evidence from in Vivo and in Vitro Stress Models. Exp. Eye Res. 2018, 169, 1–12. [Google Scholar] [CrossRef]
- Meng, C.; Xia, Q.; Wu, H.; Huang, H.; Liu, H.; Li, Y.; Zhang, F.; Song, W. Photobiomodulation with 630-Nm LED Radiation Inhibits the Proliferation of Human Synoviocyte MH7A Cells Possibly via TRPV4/PI3K/AKT/MTOR Signaling Pathway. Lasers Med. Sci. 2020, 35, 1927–1936. [Google Scholar] [CrossRef]
- Jimenez-Puerta, G.J.; Marchal, J.A.; López-Ruiz, E.; Gálvez-Martín, P. Role of Mesenchymal Stromal Cells as Therapeutic Agents: Potential Mechanisms of Action and Implications in Their Clinical Use. J. Clin. Med. 2020, 9, 445. [Google Scholar] [CrossRef] [Green Version]
- Yazdani, S.O.; Golestaneh, A.F.; Shafiee, A.; Hafizi, M.; Omrani, H.-A.G.; Soleimani, M. Effects of Low Level Laser Therapy on Proliferation and Neurotrophic Factor Gene Expression of Human Schwann Cells in Vitro. J. Photochem. Photobiol. B 2012, 107, 9–13. [Google Scholar] [CrossRef]
- Kim, H.B.; Baik, K.Y.; Seonwoo, H.; Jang, K.-J.; Lee, M.C.; Choung, P.-H.; Chung, J.H. Effects of Pulsing of Light on the Dentinogenesis of Dental Pulp Stem Cells in Vitro. Sci. Rep. 2018, 8, 2057. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 Nm) or near-Infrared (810 Nm) Photobiomodulation Stimulates, While Blue (415 Nm), Green (540 Nm) Light Inhibits Proliferation in Human Adipose-Derived Stem Cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Chen, H.; Wu, H.; Yin, H.; Wang, J.; Dong, H.; Chen, Q.; Li, Y. Effect of Photobiomodulation on Neural Differentiation of Human Umbilical Cord Mesenchymal Stem Cells. Lasers Med. Sci. 2019, 34, 667–675. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Liu, Q.; Sun, W.; Jiang, B.; Yang, K.; Li, J.; Gong, Y.; Liu, Q.; Liu, D.; et al. Nongenetic Optical Modulation of Neural Stem Cell Proliferation and Neuronal/Glial Differentiation. Biomaterials 2019, 225, 119539. [Google Scholar] [CrossRef]
- Bernstein, S.L.; Guo, Y.; Kerr, C.; Fawcett, R.J.; Stern, J.H.; Temple, S.; Mehrabian, Z. The Optic Nerve Lamina Region Is a Neural Progenitor Cell Niche. Proc. Natl. Acad. Sci. USA 2020, 117, 19287–19298. [Google Scholar] [CrossRef]
- Nuzzi, R.; Marolo, P.; Nuzzi, A. What Is New in Glaucoma: From Treatment to Biological Perspectives. J. Ophthalmol. 2021, 2021. [Google Scholar] [CrossRef]
- Wilmsmeyer, S.; Philippin, H.; Funk, J. Excimer Laser Trabeculotomy: A New, Minimally Invasive Procedure for Patients with Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 670–676. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Elagouz, M.; McHugh, D.; Shona, O.; Dorin, G. Micropulsed Diode Laser Therapy: Evolution and Clinical Applications. Surv. Ophthalmol. 2010, 55, 516–530. [Google Scholar] [CrossRef]
- Santana-Blank, L.; Rodríguez-Santana, E. Photobiomodulation in Light of Our Biological Clock’s Inner Workings. Photomed. Laser Surg. 2018, 36, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Santana-Blank, L.; Rodríguez-Santana, E.; Santana-Rodríguez, K.E.; Reyes, H. “Quantum Leap” in Photobiomodulation Therapy Ushers in a New Generation of Light-Based Treatments for Cancer and Other Complex Diseases: Perspective and Mini-Review. Photomed. Laser Surg. 2016, 34, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, V.; Arjmand, B.; Rezaei Tavirani, M.; Razzaghi, M.; Rostami-Nejad, M.; Hamdieh, M. Evaluation of Efficacy of Low-Level Laser Therapy. J. Lasers Med. Sci. 2020, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T.; Wong-Riley, M.T.T.; Whelan, H.T. Red to Near-Infrared Photobiomodulation Treatment of the Visual System in Visual System Disease or Injury. U.S. Patent 7,354,432, 8 April 2008. [Google Scholar]
- Clark, E.T.; Scott, D.; Scott, B. Devices and Methods for Non-Invasive Multi-Wavelength Photobiomodulation for Ocular Treatments. U.S. Patent 10596037-B2, 24 March 2020. [Google Scholar]
- Wu, Z.; Crabb, D.P.; Chauhan, B.C.; Crowston, J.G.; Medeiros, F.A. Improving the Feasibility of Glaucoma Clinical Trials Using Trend-Based Visual Field Progression Endpoints. Ophthalmol. Glaucoma 2019, 2, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Clinical Trials for Glaucoma Neuroprotection Are Not Impossible. Curr. Opin. Ophthalmol. 2012, 23, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U.; Grisolia, G. Thermal Physics and Glaucoma: From Thermodynamic to Biophysical Considerations to Designing Future Therapies. Appl. Sci. 2020, 10, 7071. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation or Low-Level Laser Therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergandi, L.; Silvagno, F.; Grisolia, G.; Ponzetto, A.; Rapetti, E.; Astori, M.; Vercesi, A.; Lucia, U. The Potential of Visible and Far-Red to Near-Infrared Light in Glaucoma Neuroprotection. Appl. Sci. 2021, 11, 5872. https://doi.org/10.3390/app11135872
Bergandi L, Silvagno F, Grisolia G, Ponzetto A, Rapetti E, Astori M, Vercesi A, Lucia U. The Potential of Visible and Far-Red to Near-Infrared Light in Glaucoma Neuroprotection. Applied Sciences. 2021; 11(13):5872. https://doi.org/10.3390/app11135872
Chicago/Turabian StyleBergandi, Loredana, Francesca Silvagno, Giulia Grisolia, Antonio Ponzetto, Emilio Rapetti, Mariarosa Astori, Antonio Vercesi, and Umberto Lucia. 2021. "The Potential of Visible and Far-Red to Near-Infrared Light in Glaucoma Neuroprotection" Applied Sciences 11, no. 13: 5872. https://doi.org/10.3390/app11135872
APA StyleBergandi, L., Silvagno, F., Grisolia, G., Ponzetto, A., Rapetti, E., Astori, M., Vercesi, A., & Lucia, U. (2021). The Potential of Visible and Far-Red to Near-Infrared Light in Glaucoma Neuroprotection. Applied Sciences, 11(13), 5872. https://doi.org/10.3390/app11135872







