Abstract
The aim of this study was the morphological evaluation of root surfaces subjected to manual (curette) and ultrasonic (conventional and diamond tips) scaling. The surface was then polished with a rubber cup and three medium-sized pastes. Ninety teeth were randomly divided into three groups of 30 and subjected to three different root instrumentation: (1) manual instrumentation with a Gracey® curette; (2) ultrasonic instrumentation with a standard steel tip (Universal Perio S-SERIES: USU, Hu-Friedy, Chicago, IL, USA) at a power equal to 50%; and (3) with a diamond tip (Punta Piezo Serie E Scaling, Hu-Friedy, Chicago, IL, USA) at a power of 20%. Each group of the instrumented teeth was then divided in three subgroups of 10 and subjected to 30 s of rubber polishing with three different polishing pastes with medium grain sizes in single-dose cups: (1) Ultrapro Tx cool mint medium®; (2) Stomyprox media®; and (3) Nupro medium orange®. Polyether root surface replicas were then taken from all 90 samples and analyzed by SEM to evaluate surface morphology after scaling and polishing procedures. All scaling techniques caused an alteration of the root surface without statistically significant difference, whereas polishing resulted in maintenance or improvement of the surface texture.
1. Introduction
The goals of periodontal therapy are to eliminate the infectious and inflammatory processes of periodontal tissues. Subgingival plaque and calculus on the root surface in the periodontal pocket are the most important local factors for the occurrence and development of periodontitis [1]. Mechanical removal of plaque and calculus by manual and ultrasonic scaling instrumentation with ultrasound has shown equal effectiveness in in vitro and in vivo studies [1,2]. Manual root planing eliminates “infected cementum”, while ultrasonic instruments will not remove the cementum excessively and are also superior in terms of time saving and labour saving compared to using hand instruments [1]. The amount of tooth substance removed and the surface roughness after instrumentation need to be considered [3]. In this regard, it is important to take into account the involved tissue and investigate the mechanical properties of its components, which includes hydroxylapatite [4,5,6,7,8]. Extensive cement removal can results in an increase in root surface roughness and promote plaque accumulation and dentinal hypersensitivity [2]. On the other hand, while the roughness of the root favors the accumulation of plaque, its role in periodontal healing is still controversial. In fact, this surface favors the adhesion of fibroblasts and periodontal regeneration [9,10].
Polishing can be considered as the final stage of periodontal treatment after scaling and root planing [11]. During polishing, procedures could be selective or widely performed [12,13], such as plaque, biofilm, acquired film and extrinsic pigmentation removal from the tooth surface. Different techniques can determine surface alterations and roughness depending on the time and materials used [11,14]. In clinical situations, supragingival biofilms and subgingival biofilms measuring within 3 mm can be removed mechanically using rotary instruments and a prophylaxis paste [15].
Numerous studies have evaluated the root surface after periodontal instrumentation with different techniques, such as profilometric analysis [2,11], histological evaluation [2,16] and electron and confocal laser scanning microscopy [15,17,18].
Recently the effects of mechanical periodontal instruments on the root surface have been studied through the SEM observation of replicas of the instrumented surface [19]. Replica technique, by means of SEM inspection of polyether replicas of tooth surface, allows the evaluation of the morphological alterations and changes in time, as proposed in several in vitro and in vivo studies [20,21,22].
The first aim of this study was the morphological evaluation of the root surface subjected to manual and ultrasonic scaling with curette and conventional and diamond tips, respectively. The second objective of the study was to evaluate the effects of polishing carried out with a rubber cup and three medium-sized pastes on teeth subjected to manual and ultrasonic scaling. The root surface was examined—after instrumentation and after polishing—to evaluate the effect of the different procedures.
The first null hypothesis of the study was that scaling, with both manual and ultrasonic tips, did not alter the root surface. The second null hypothesis was that polishing with the rubber cup and medium grain size pastes increases the roughness of root surfaces that were previously subjected to periodontal instrumentation.
2. Materials and Methods
For this study, 90 roots of incisors (n = 54) and premolars (n = 36) extracted for periodontal reasons from patients aged 57 to 70 years, free of caries, restorations, and root resorption were used. Before the extraction, the patients were informed of the use of the teeth for research purposes and gave their consent.
After extraction and during all procedures, the teeth were stored in bi-distilled water at 4 °C.
The teeth were stabilized with polyvinyl siloxane (Affinis light body; Coltene, Altstätten, Switzerland) and instrumented on a precision electronic balance (TM 503 Power Module, Tektronix®, Beaverton, OR, USA) to obtain an instrumentation standardized force of 0.1 ± 0.05 N.
All procedures were performed by the same operator in a single session for 30 s.
The roots were subjected to preliminary instrumentation with a universal curette (M 23, Deppeler, Rolle, Switzerland) and ultrasound with a steel tip for the removal of tartar, stains, and periodontal ligament residues.
Only one surface from each root was selected for the experiment, deliberately excluding extreme grooves, restoration margins, or the cementoenamel junction. A rectangular area of interest was identified and was outlined with a diamond-coated disk (Intensiv, Swiss Dental Diamond, Discoflex, 173 D, Montagnola, Switzerland) in a slow contra angle (Micro Mega, Genève, Acacias, Switzerland) with water cooling. Only this area was subjected to sequential impressions.
Following preliminary instrumentation, each root was brushed with a prophy brush mounted on a micromotor at 4000 rpm for 30 s, then washed for another 30 s and air-dried for 10 s.
Teeth were randomly divided into 3 experimental groups (n = 30) to be then subjected to three treatments:
- Group 1 (G1): manual instrumentation with a Gracey® curette (SG 5/69E2®, Hu-Friedy, Chicago, IL, USA);
- Group 2 (G2): ultrasonic instrumentation (Piezon®, EMS, Geneva, Switzerland) with a standard steel tip (Universal Perio S-SERIES: USU, Hu-Friedy, Chicago, IL, USA) at a power equal to 50%;
- Group 3 (G3): ultrasonic instrumentation (Piezon®, EMS, Geneva, Switzerland) with a diamond tip (Punta Piezo Serie E Scaling, Hu-Friedy, Chicago, IL, USA) at a power of 20%.
After the experimental procedures, the samples were washed for 30 s and dried with air-water spray for 10 s.
The instrumented teeth were then homogeneously distributed in three groups (n = 30) so that the three types of instrumentation were equally represented (10 from each instrumentation group) and subjected to 30 s of polishing with a rubber cup (Prophy Cups, Kerr, Switzerland) on a low-angle contra-angle (2000 rpm), with three different polishing pastes with medium grain size (compositions reported in Table 1) in single-dose cups:

Table 1.
Composition of polishing pastes used in the study.
- Group I (GI): Ultrapro Tx cool mint medium®, Ultradent;
- Group II (GII): Stomyprox media®, Coswell;
- Group III (GIII): Nupro medium orange®, Dentsply Sirona.
2.1. Replica Technique
Root surface impressions were taken before and after instrumentation and polishing procedure with polyvinylsiloxane material (Affinis light body; Coltene, Altstätten, Switzerland) with a setting time of 5 min. After the experimental procedures, each surface was washed for 30 s and air dried for 10 s before taking the impression. Once the impression hardened, it was removed from the root surface and left in the open air for 48 h to degas. Impressions were subsequently replicated with polyether material (Permadyne Garant; 3M_ESPE, St. Paul, MN, USA), as described in the works of Bertacci et al. and Chersoni et al. [20,21].
2.2. SEM Evaluation and Statistical Analysis
After separation, the replicas were gold-sputtered and examined by scanning electron microscopy (SEM Jeol 5400, Tokyo, Japan) in accordance with methodological approaches proposed in previous studies [20,21,23].
After preliminary scaling procedures, each sample was analyzed at 50× magnification by two operators in double blind before scaling (T0) and after scaling (T1) to evaluate the surface regularity and the presence of signs and lesions produced by the instrumentation in the cervical third of the root surfaces. These values were measured by using a modification of the “Modified Loss of Tooth Substance Index” [17]:
- 0.
- Regular root surface, without loss of substance and signs of instrumentation;
- 1.
- Regular root surface with isolated instrumentation marks;
- 2.
- Widespread presence of signs produced by the instrumentation;
- 3.
- Irregular surface and/or presence of lesions produced by the instrumentation.
SEM pictures of replicas of each sample at T1 were then compared by two operators with images of replicas obtained after polishing (T2), both at a magnification of 50×, in double blind, to evaluate the surface roughness with the assignment of a value, according to the variation of the surface morphology and according to a protocol suggested by Tsurumaki et al. [16]:
- Smoother surface;
- Unchanged surface;
- Rougher surface.
Data (scores) were expressed as mean ± standard deviation and group mean differences and were assessed with Student’s t-test. The chosen level of statistical significance was 0.05. For the statistical analysis, the samples collected before any root scaling treatment (T0) are to be considered as a control group to assess the variation of root surface morphology [16].
3. Results
3.1. Statistical Analysis
The statistical analysis, carried out with the paired Student’s t-test, showed statistically significant differences between the experimental times (before T0 and after T1; manual and ultrasonic scaling) for scaling procedures (see the results reported in Table 2).

Table 2.
Student’s t-test performed for the experimental groups G1, G2 and G3 before (T0) and after (T1) scaling treatments. A and B represent the analysed groups (G1, G2 or G3) where T is the t-value, df is the degrees of freedom and p is the p-value.
For all the examined groups G1, G2 and G3 (i.e., type of root scaling procedure), statistically significant differences in surface regularity and the presence of instrumentation signs were found between time 0 and time 1 (p < 0.05) within each group, with mean values close to zero (i.e., regular root surface and without the loss of substance and signs of instrumentation) at T0 and close to 1 (i.e., regular root surface with isolated instrumentation marks) at T1. In all cases, therefore, the instrumentation with curette, ultrasonic scaling with conventional and diamond tip altered the texture of the root surfaces examined. On the other hand, the comparison performed as a control test by means of unpaired t-test of the means of the scores at time 0 (i.e., before scaling) between the three groups did not show significance (p > 0.05).
Regarding the relative comparison between the groups at T1 (i.e., after scaling), no statistical significance (at the chosen level of 0.05) was highlighted by unpaired t-test neither between the group treated with the curette (G1) and the one treated with the ultrasonic steel tip (G2), p = 0.068 > 0.05, nor between G1 and the group treated with the ultrasonic diamond tip (G3), p = 0.057 > 0.05, nor between G2 and G3, p = 0.44 > 0.05. However, the p-values for the group treated with the curette (G1) with respect to the groups treated with ultrasonic tips (G2 and G3) are very close to 0.05 due the higher mean score of G1.
The results of the statistical analysis on the data collected after the teeth polishing treatment (T2) with the three different polishing pastes (groups GI, GII and GIII) are reported in Table 3. The data are grouped by type of scaling procedure (manual curette, US steel tip and US diamond tip). All the groups showed mean scores <2, evidencing that the polishing treatments produce smoother surfaces on average. The unpaired t-test did not evidence statistically significative differences (at the chosen level of 0.05) between the use of Ultrapro Tx cool mint medium® (group GI), Stomyprox media® (group GII) and Nupro medium orange® (group GIII). Polishing with Stomyprox media® produced the smoothest surface on average (lower mean score) independently on the employed scaling procedure, even if it was not statistically significant in terms of mean scores with respect to the other pastes at the chosen level of 0.05.

Table 3.
Student’s t-test performed for the experimental groups GI, GII and GIII after the polishing treatments (T2). A and B represent the analysed groups (GI, GII or GIII), T is the t-value, df is the degrees of freedom, and p is the p-value.
3.2. SEM Evaluation
The morphological analysis was performed by scanning electron microscopy in high vacuum (signal of secondary electrons) and at 50× magnification of the replicas and, after scaling some alterations of the root surface were revealed. Conversely, before scaling, examination of the untreated surfaces revealed the widespread presence of smear layers and debris in the absence of visible signs of instrumentation (an example is reported in Figure 1a).
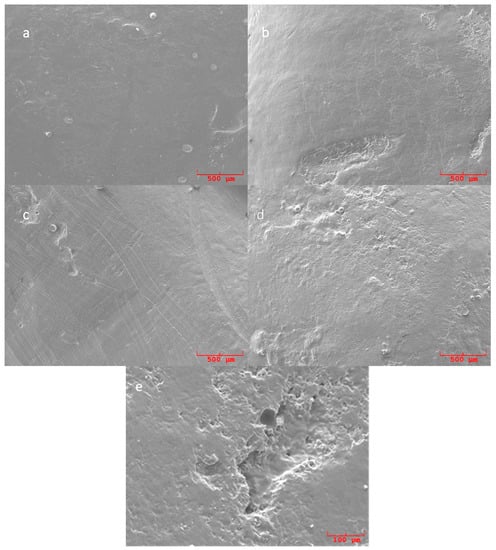
Figure 1.
SEM pictures of replicas of untreated ((a): 50×) and scaled ((b–d): 50×; (e): 200×) root surfaces. (a) Untreated surface showing the presence of smear layers and debris in the absence of visible signs of instrumentation. (b) Replica of root surface treated with ultrasonic conventional tips showing a relatively smooth and homogeneous surface, with the absence of deposits and isolated signs produced by the instrumentation. (c) Replicas of curette treated teeth showing widespread signs of the instrumentation. (d) Replicas of samples treated with ultrasonic diamond tip showing a relatively homogeneous surface with localized signs produced by the instrumentation. (e) SEM pictures at 200× of sample treated with ultrasonic diamond tip showing an isolated area of loss of substance.
The instrumentation with the ultrasonic steel tip produced a relatively smooth and homogeneous surface, which is more regular than that produced by manual instrumentation, with the absence of deposits and isolated signs produced by the instrumentation. Figure 1b shows, as an example of this type of observation, a replica surface of about 2.5 × 2.0 µm2 with the cited instrumentation signs visible in the lower part, extended up to about 1.2 µm here, and a smooth and homogeneous surface in the upper part.
In the samples treated with curettes, the signs of the blade are clear, resulting in a more irregular surface macro-texture compared to that produced with the ultrasonic instrumentation with a steel tip and with ultrasound with a diamond tip. The SEM micrograph of Figure 1c is reported as an example to shows these surface features caused by the use of curettes, which for the full height of the image (about 2 µm).
Samples treated with a diamond ultrasonic tip showed, at a higher magnification, a relatively homogeneous surface with localized signs produced by the instrumentation and isolated areas of loss of substance. The upper part of Figure 1d provides an example of these features acquired at 50× magnification, whereas in Figure 1e an isolated area of loss of substance produced by the diamond ultrasonic tip is well resolved and up to about 150 µm wide and 350 µm long; the measurements are acquired at 200× magnification.
In general, polishing with the different medium-grained prophylactic pastes used in the study (Stomyprox media®, Nupro medium orange® and Ultrapro Tx cool mint medium®) resulted in the maintenance or improvement of the surface texture, without the score 3 (rougher surface) being assigned in any of the examined groups (Figure 2, Figure 3 and Figure 4). Statistical analysis of the data obtained by the evaluation of samples treated with polishing procedures showed no statistically significant difference between the effects on root surface treated with manual and ultrasonic tips of the three prophylactic pastes examined (Stomyprox media®, Nupro medium orange® and Ultrapro Tx cool mint medium®).
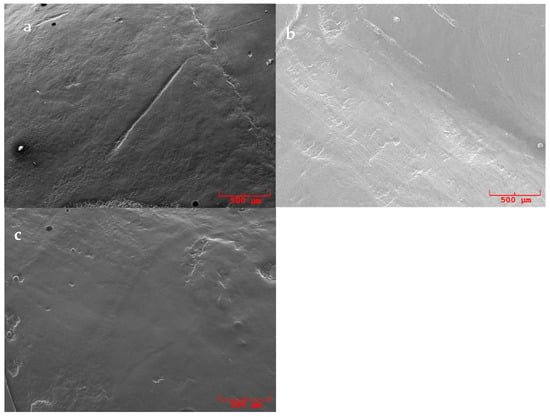
Figure 2.
SEM pictures (50×) of replicas surfaces of samples polished with Ultrapro Tx cool mint medium®: (a) previously treated with an ultrasonic conventional tip, (b) previously treated with a curette and (c) previously treated with an ultrasonic diamond tip.
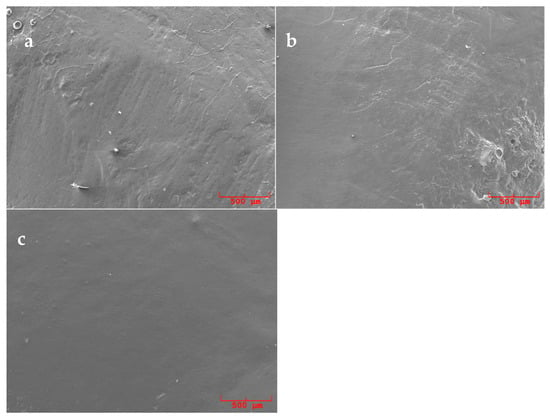
Figure 3.
SEM pictures (50×) of replicas surfaces of samples polished with Nupro medium orange®: (a) previously treated with an ultrasonic conventional tip, (b) previously treated with a curette and (c) previously treated with an ultrasonic diamond tip.
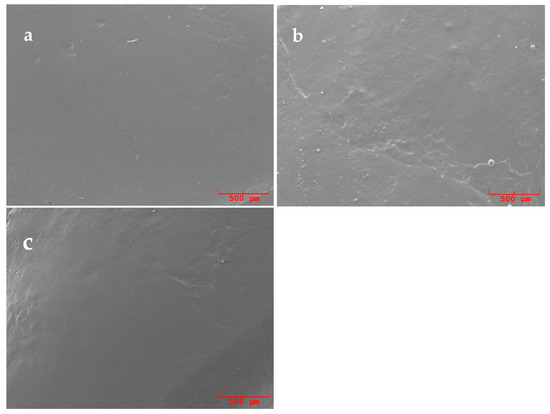
Figure 4.
SEM pictures (50×) of replicas surfaces of samples polished with Stomyprox media®: (a) previously treated with an ultrasonic conventional tip, (b) previously treated with a curette and (c) previously treated with an ultrasonic diamond tip.
The use of Stomyprox media® polishing paste (Figure 4) resulted in the best mean score in terms of surface improvement, with the recording of the highest number of scores 1; however, with p-values and with respect to the other pastes, it is not within the chosen level of statistical significance (0.05).
Figure 2, Figure 3 and Figure 4 present explicative SEM micrographs of the replicas of the treated surfaces, which are acquired at 50x magnification. Residual signs of the root scaling are still discernable independently of the type of executed scaling procedure (see for instance the central groove in Figure 2a, the instrumental signs in the lower right corner of Figure 3b and in the lower half of Figure 4b). Figure 4 a,c provide two examples of replicas of surfaces polished with Stomyprox media® paste after scaling with an ultrasonic conventional tip and diamond tip, respectively, and these presented extended smooth areas.
4. Discussion
Non-surgical periodontal therapy aims to remove bacterial plaque, calculus and contaminated cementum to prevent the progression of periodontal disease [24]. This therapy is based on the techniques of Scaling and Root Planing (SRP) that is carried out using manual and mechanical instruments.
The first purpose of this study was the morphological evaluation of the root surface subjected to scaling by manual curette and ultrasonic devices with conventional tips and diamond tips. The second purpose was to evaluate the effects of polishing on the root surface of teeth subjected to the aforementioned scaling procedures.
Manual instrumentation was once considered more effective than sonic and ultrasonic instrumentation; however, more recently, mechanical instrumentation has been widely accepted as an alternative or an aid to manual instrumentation as it shows the same effects in removing biofilm, subgingival calculus, and endotoxins [1,2]. Manual scaling with curettes allows greater control of the instrument and improves the operator’s tactile perception, while the sonic and ultrasonic instruments allow better access to furcation and deep pockets, as well as reducing operator fatigue and instrumentation time [1,25]. There are various types of inserts for mechanical instruments; the standard steel inserts and diamond inserts were used in this study. The diamond coated inserts have been designed to increase the speed and efficiency of deposit removal [26] and, in fact, the instrumentation time is reduced by 60% when using the diamond coated inserts. Their effectiveness and speed have been proven by several studies [26,27].
The experiments carried out so far agree that ultrasound with diamond inserts removes a large amount of root surface and can damage the root if not used correctly [17,28]. Root debridement with this type of insert should be performed with the utmost caution [3]. The diamond coating of the instrument removes the dental tissue with a “pulverizing” action. The diamond granules provide the tool with a multitude of edges and each cutting surface is a working part of a multi-faceted tool. Each of these diamond granules contact the tooth surface and exerts an impulse so as to remove the harder dental tissue than with other smooth inserts due to microabrasion [3,28].
The roughness produced by sonic, ultrasonic and manual instruments has been examined in several studies, but the findings are often conflicting. Some experiments show that manual instrumentation produces a smoother surface than ultrasonic instrumentation [29]. In contrast, other studies report that mechanical instruments are better for use on the root surface by producing minimal roughness than curettes [3,30].
The effects of scaling procedures on root surface Kocher are still debated. The smear layer often forms following root debridement and causes a barrier between the periodontal tissues and the root surface; this inhibits the formation of a new connective attachment and alters periodontal healing [24]. The roughness produced by scaling could affect bacterial colonization and fibroblast adhesion [24]. A smoother coronal root surface can be very important for avoiding subgingival bacterial colonization, while in the central and apical area it would be optimal to obtain a rougher surface to allow greater adhesion of fibroblasts [31].
Our experimentation revealed statistically significant differences in surface texture and in the presence of instrumentation signs before and after treatment for all groups examined (p < 0.05). The three types of scaling procedures altered the root surface, and the first null hypothesis of the study should be rejected. SEM morphological analysis of the ultrasonic-instrumented root replicas with a standard steel insert showed smoother and more homogeneous surfaces than those instrumented with curettes. However, replicas of ultrasonic-treated samples showed the presence of cracks that are also visible at higher magnification in samples treated with ultrasonic diamond tips and this observation partially agreed with other results showing that ultrasonic instrumentation produces a significantly higher quantity of cracks [24]. A positive correlation between the roughness of the root surface and the power of ultrasonic instruments, likely due to an increase in lateral pressure during instrumentation due to the operator’s poor tactile perception with increasing power, has also been reported [25].
In relation to the samples treated with curettes, clear signs of instrumentation and surface irregularities are evident. Samples instrumented with diamond ultrasound showed a relatively homogeneous surface with localized signs of instrumentation and loss of substance. This last result is in contrast with previous studies that associate the diamond insert with a more irregular surface and a greater loss of substance than instruments with smooth inserts [3,17,28]. However, in the present study, no statistically significant differences were found between the manual and mechanical scaling procedures at the chosen value of 0.05.
The results of the study can be compared with those of numerous articles in the literature that present partly concordant results [3,30,32]. However, the presence in the literature of studies reporting completely discordant results confirmed that the question is still debated [16]. All these controversies about the effects of manual and ultrasonic instrumentation are probably due to differences in study designs (e.g., in vivo studies and in vitro studies) and the lack of standardization [3].
In current dental practice, polishing agents are predominantly used for the removal of biofilm and extrinsic pigments by using a selective procedure [13,33].
Polishing is a more comfortable, less stressful and less painful act than scaling and root planing and it is commonly well tolerated and requested since it produces immediate benefits that are perceptible by the patient [12]. Extensive removal of the cervical cement, that could be produced by periodontal instrumentation, can result in greater surface roughness favoring plaque retention [34]; however, the literature reports that dentin and cement are highly subject to abrasive phenomena [35] and so polishing maneuvers on these tissues could cause morphological and structural changes [36].
The most common method for dental polishing involves using a rubber cup and pumice as an abrasive agent. Over the years, some studies have suggested the use of other abrasive agents for polishing, such as zinc oxides or kaolinite pastes, but pumice powder and fine glycerin continue to be the most widely used [11].
The effectiveness and safety of polishing with a rubber cup and prophylaxis paste are influenced by rotation speed, abrasiveness of the paste, applied pressure and the duration of treatment [33]. The use of a polishing cup and abrasive agent at excessive speeds and pressure could remove a superficial layer of cement and very thin tissue at the cervical level [36]. For this reason, during polishing with the rubber cup, the handpiece should be equipped with a torque that guarantees a constant speed (from 2000 to 3000 rpm) protected from oscillations [14].
In this study three polishing pastes medium grain size (74–105 μm) in single-dose cups were analyzed: Stomyprox media®, Ultrapro Tx cool mint medium® and Nupro medium orange®.
The results of the statistical and SEM morphological analysis did not show statistically significant changes before and after polishing procedures in all teeth regardless of the type of scaling (manual and ultrasonic). Polishing resulted in the improvement or maintenance of the surface texture, which confirms the effectiveness of polishing in reducing the roughness caused by periodontal instrumentation and its safety in not increasing roughness. In fact, none of the examined groups showed a higher roughness with respect to the unpolished root surface; hence, the second null hypothesis of the study had been rejected.
The best score, in terms of surface improvement, was observed in specimens polished with Stomyprox media®: the root surface appears more homogeneous and freer of irregularities. This can be attributed to the fact that Stomyprox media®, unlike Ultrapro Tx cool mint medium® and Nupro medium orange®, is a paste based on microRepair®, which contains synthetic hydroxyapatite crystals that probably settles on the surface of the tooth.
Although the question of the need to obtain root surfaces as smooth as possible after instrumentation for the creation of a suitable environment for periodontal healing is still controversial [2,9,10], the morphological analysis of the SEM replicates seems to indicate that this procedure is safe even in patients with root surface exposure and, therefore, not only for the removal of coronal extrinsic discolorations. Further studies will be required to investigate the clinical effectiveness and the effects of these procedures repeated over time.
5. Conclusions
The results of the present work showed that all the instrumentation techniques affect root surface, without significant differences between the different investigated methods. The use of the scaling, ultrasonic, manual or combined procedure must be evaluated according to the peculiar advantages of the different techniques towards the patient’s needs.
In addition, the present study suggests that polishing the root surface with a rubber cup and three medium grain size pastes does not produce roughening and hence this procedure could be recommended for patients with areas of root exposure.
Author Contributions
Conceptualization, A.B., G.V., D.M. and G.U.; methodology, A.B. and D.M.; validation, A.B., G.V., D.M. and G.U.; formal analysis, A.B., G.V., D.M. and G.U.; investigation, A.B., G.V., D.M. and G.U.; data curation, A.B., G.V., D.M. and G.U.; writing—original draft preparation, A.B., G.V., D.M. and G.U.; writing—review and editing, A.B., G.V., D.M. and G.U.; supervision, A.B., G.V., D.M. and G.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are reported within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yan, Y.; Zhan, Y.; Wang, X.; Hou, J. Clinical evaluation of ultrasonic subgingival debridement versus ultrasonic subgingival scaling combined with manual root planing in the treatment of periodontitis: Study protocol for a randomized controlled trial. Trials 2020, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.; Martins, O.; Matos, S.; Lopes, P.; Rolo, T.; Baptista, I. Histological and profilometric evaluation of the root surface after instrumentation with a new piezoelectric device—Ex vivo study. Int. J. Dent. Hyg. 2015, 13, 138–144. [Google Scholar] [CrossRef]
- Vastardis, S.; Yukna, R.A.; Rice, D.A.; Mercante, D. Root surface removal and resultant surface texture with diamond-coated ultrasonic inserts: An in vitro and SEM study. J. Clin. Periodontol. 2005, 32, 467–473. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. Hydroxylapatite and related minerals in bone and dental tissues: Structural, spectroscopic and mechanical properties from a computational perspective. Biomolecules 2021, 11, 728. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. First-principles study of structural and surface properties of (001) and (010) surfaces of hydroxylapatite and carbonated hydroxylapatite. J. Appl. Crystallogr. 2016, 49, 1893–1903. [Google Scholar] [CrossRef]
- Ulian, G.; Valdrè, G. Second-order elastic constants of hexagonal hydroxylapatite (P63) from ab initio quantum mechanics: Comparison between DFT functionals and basis sets. Int. J. Quantum Chem. 2018, 118, e25500. [Google Scholar] [CrossRef]
- Ulian, G.; Valdrè, G. Equation of state of hexagonal hydroxylapatite (P63) as obtained from density functional theory simulations. Int. J. Quantum Chem. 2018, 118, e25553. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. Probing the interaction of (001) carbonated hydroxylapatite surfaces with water: A density functional investigation. Micro Nano Lett. 2018, 13, 4–8. [Google Scholar] [CrossRef]
- Adriaens, P.A.; Adriaens, L.M. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontol. 2000 2004, 36, 121–145. [Google Scholar] [CrossRef]
- Hakki, S.S.; Korkusuz, P.; Berk, G.; Dundar, N.; Saglam, M.; Bozkurt, B.; Purali, N. Comparison of Er,Cr:YSGG Laser and Hand Instrumentation on the Attachment of Periodontal Ligament Fibroblasts to Periodontally Diseased Root Surfaces: An In Vitro Study. J. Periodontol. 2010, 81, 1216–1225. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, T.T.; Oztekin, F.; Keklik, E.; Tozum, M.D. Surface roughness of enamel and root surface after scaling, root planning and polishing procedures: An in-vitro study. J. Oral Biol. Craniofacial Res. 2021, 11, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Redford-Badwal, D.A.; Nainar, S.M.H. Assessment of Evidence-Based Dental Prophylaxis Education in Postdoctoral Pediatric Dentistry Programs. J. Dent. Educ. 2002, 66, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Sawai, M.A.; Bhardwaj, A.; Jafri, Z.; Sultan, N.; Daing, A. Tooth polishing: The current status. J. Indian Soc. Periodontol. 2015, 19, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Kimyai, S.; Lotfipour, F.; Pourabbas, R.; Sadr, A.; Nikazar, S.; Milani, M. Effect of two prophylaxis methods on adherence of Streptococcus mutans to microfilled composite resin and giomer surfaces. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e561–e567. [Google Scholar] [CrossRef] [Green Version]
- Amari, Y.; Takamizawa, T.; Kawamoto, R.; Namura, Y.; Murayama, R.; Yokoyama, M.; Tsujimoto, A.; Miyazaki, M. Influence of one-step professional mechanical tooth cleaning pastes on surface roughness and morphological features of tooth substrates and restoratives. J. Oral Sci. 2021, 63. [Google Scholar] [CrossRef] [PubMed]
- Tsurumaki, J.D.N.; Souto, B.H.M.; de Oliveira, G.J.P.L.; Sampaio, J.E.C.; Marcantonio Júnior, E.; Marcantonio, R.A.C. Effect of instrumentation using curettes, piezoelectric ultrasonic scaler and er,cr:Ysgg laser on the morphology and adhesion of blood components on root surfaces—A SEM study. Braz. Dent. J. 2011, 22, 185–192. [Google Scholar] [CrossRef]
- Lavespere, J.E.; Yukna, R.A.; Rice, D.A.; LeBlanc, D.M. Root Surface Removal With Diamond-Coated Ultrasonic Instruments: An In Vitro and SEM Study. J. Periodontol. 1996, 67, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Mazzoleni, S.; Fantin, F.; Favero, L.; De Francesco, M.; Stellini, E. Evaluation of three different manual techniques of sharpening curettes through a scanning electron microscope: A randomized controlled experimental study. Int. J. Dent. Hyg. 2015, 13, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Bless, K.L.; Sener, B.; Dual, J.; Attin, T.; Schmidlin, P.R. Cleaning ability and induced dentin loss of a magnetostrictive ultrasonic instrument at different power settings. Clin. Oral Investig. 2011, 15, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertacci, A.; Chersoni, S.; Davidson, C.L.; Prati, C. In vivo enamel fluid movement. Eur. J. Oral Sci. 2007, 115, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Chersoni, S.; Bertacci, A.; Pashley, D.H.; Tay, F.R.; Montebugnoli, L.; Prati, C. In vivo effects of fluoride on enamel permeability. Clin. Oral Investig. 2011, 15, 443–449. [Google Scholar] [CrossRef]
- Bertacci, A.; Lucchese, A.; Taddei, P.; Gherlone, E.F.; Chersoni, S. Enamel structural changes induced by hydrochloric and phosphoric acid treatment. J. Appl. Biomater. Funct. Mater. 2014, 12, 240–247. [Google Scholar] [CrossRef]
- Valdrè, G.; Moro, D.; Ulian, G. Monte Carlo simulation of the effect of shape and thickness on SEM-EDS microanalysis of asbestos fibres and bundles: The case of anthophyllite, tremolite and actinolite. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Nanjing, China, 17–19 August 2018; Volume 304. [Google Scholar]
- Arora, S.; Lamba, A.K.; Faraz, F.; Tandon, S.; Ahad, A. Evaluation of the effects of Er,Cr: YSGG laser, ultrasonic scaler and curette on root surface profile using surface analyser and scanning electron microscope: An in vitro study. J. Lasers Med. Sci. 2016, 7, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Das, S.J.; Sonowal, S.T.; Chawla, J. Comparison of root surface roughness produced by hand instruments and ultrasonic scalers: An invitro study. J. Clin. Diagnostic Res. 2015, 9, ZC56–ZC60. [Google Scholar] [CrossRef]
- Yukna, R.A.; Vastardis, S.; Mayer, E.T. Calculus Removal With Diamond-Coated Ultrasonic Inserts In Vitro. J. Periodontol. 2007, 78, 122–126. [Google Scholar] [CrossRef]
- Scott, J.B.; Steed-Veilands, A.M.; Yukna, R.A. Improved Efficacy of Calculus Removal in Furcations Using Ultrasonic Diamond-Coated Inserts. Int. J. Periodontics Restorative Dent. 1999, 19. [Google Scholar] [CrossRef]
- Kocher, T.; Fanghänel, J.; Sawaf, H.; Litz, R. Substance loss caused by scaling with different sonic scaler inserts—An in vitro study. J. Clin. Periodontol. 2001, 28, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cross-Poline, G.N.; Stach, D.J.; Newman, S.M. Effects of curet and ultrasonics on root surfaces. Am. J. Dent. 1995, 8, 8. [Google Scholar]
- Walmsley, A.D.; Lea, S.C.; Landini, G.; Moses, A.J. Advances in power driven pocket/root instrumentation. J. Clin. Periodontol. 2008, 35, 22–28. [Google Scholar] [CrossRef]
- De Mendonça, A.C.; Máximo, M.B.; Rodrigues, J.A.; Arrais, C.A.G.; De Freitas, P.M.; Duarte, P.M. Er:YAG laser, ultrasonic system, and curette produce different profiles on dentine root surfaces: An in vitro study. Photomed. Laser Surg. 2008, 26, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R. Ultra-morphology of root surface subsequent to periodontal instrumentation: A scanning electron microscope study. J. Indian Soc. Periodontol. 2012, 16, 96–100. [Google Scholar] [CrossRef]
- Petersilka, G.J.; Ehmke, B.; Flemmig, T.F. Antimicrobial effects of mechanical debridement. Periodontol. 2000 2002, 28, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Marda, P.; Prakash, S.; Devaraj, C.G.; Vastardis, S. A comparison of root surface instrumentation using manual, ultrasonic and rotary instruments: An in vitro study using scanning electron microscopy. Indian J. Dent. Res. 2012, 23, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.H.; Gröger, C.; Bizhang, M.; Naumova, E.A. Dentin abrasivity of various desensitizing toothpastes. Head Face Med. 2016, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bains, V.; Madan, C.; Bains, R. Tooth polishing: Relevance in present day periodontal practice. J. Indian Soc. Periodontol. 2009, 13, 58. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).