Phase Compensation Technique for Effective Heat Focusing in Microwave Hyperthermia Systems
Abstract
:1. Introduction
2. The Antenna Array System for Microwave Hyperthermia Treatment
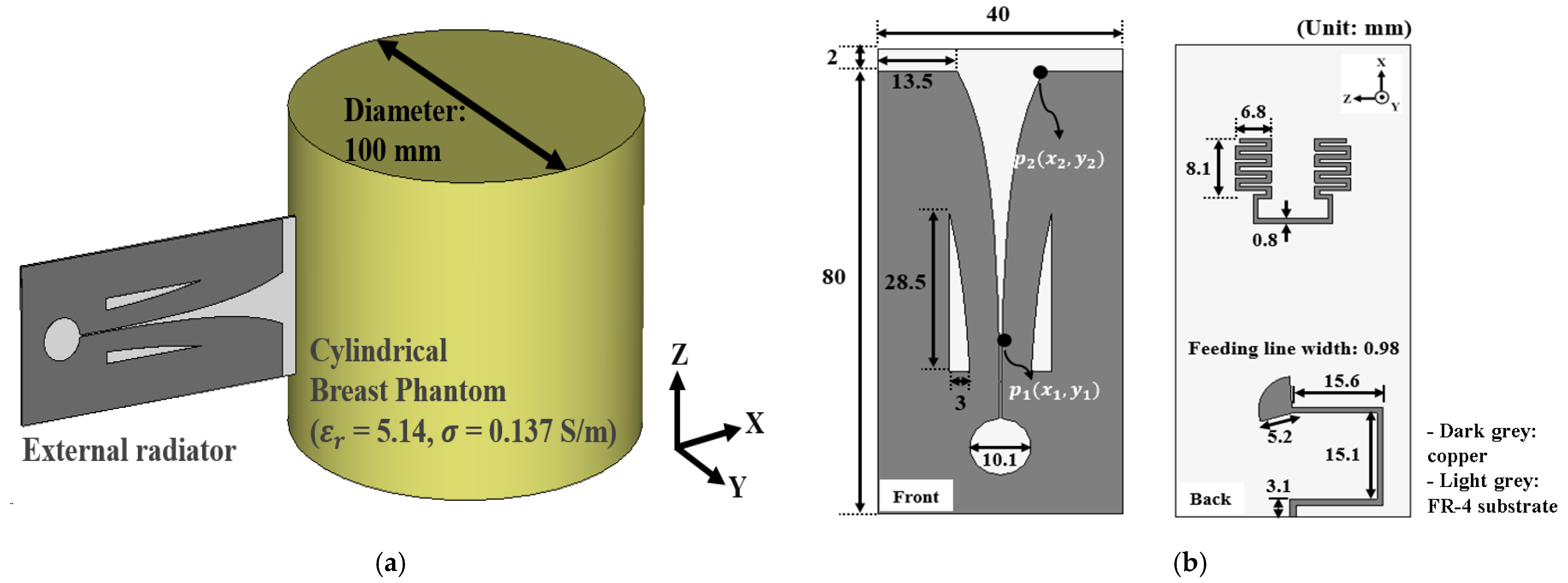
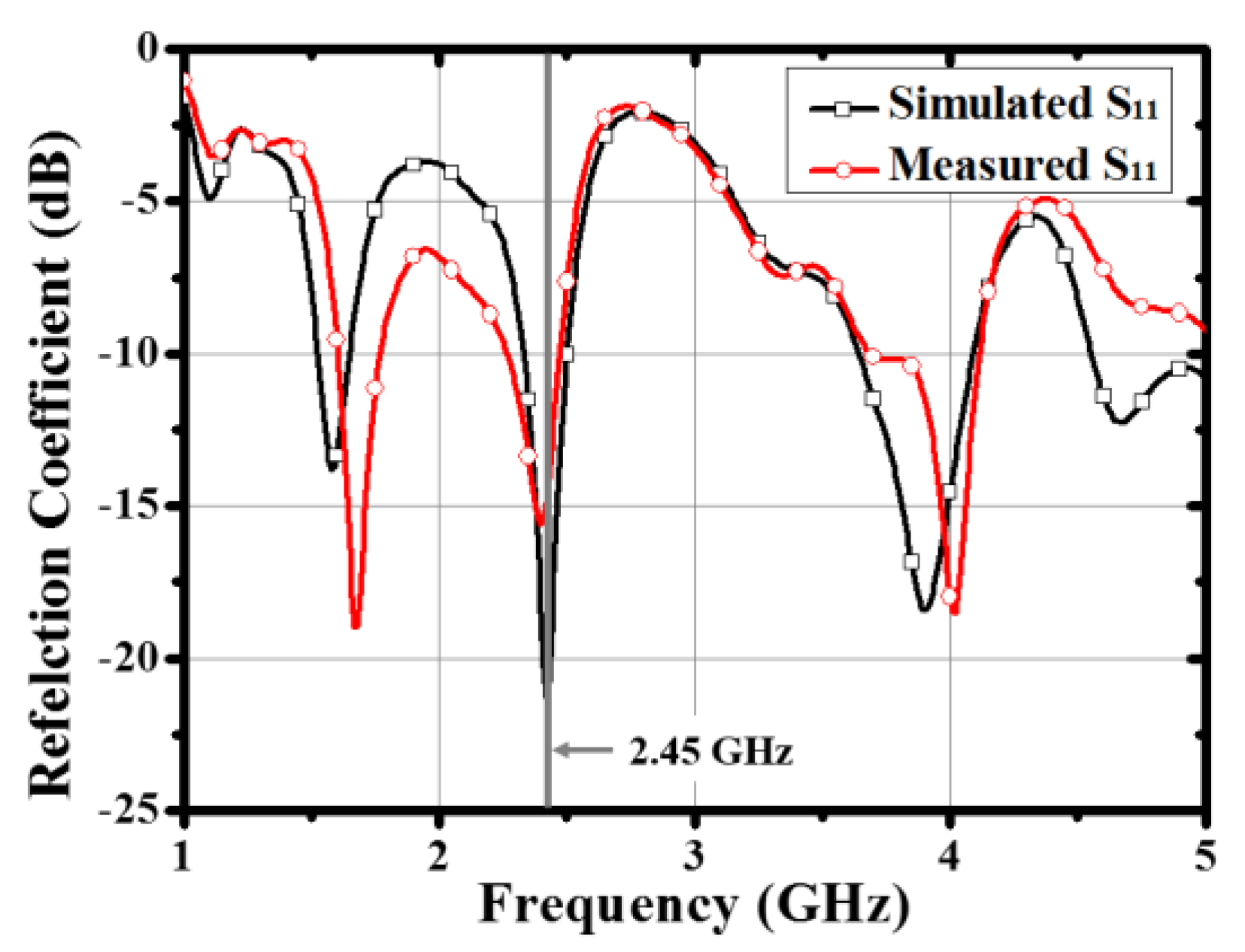
2.1. The Antenna for the Array Configuration
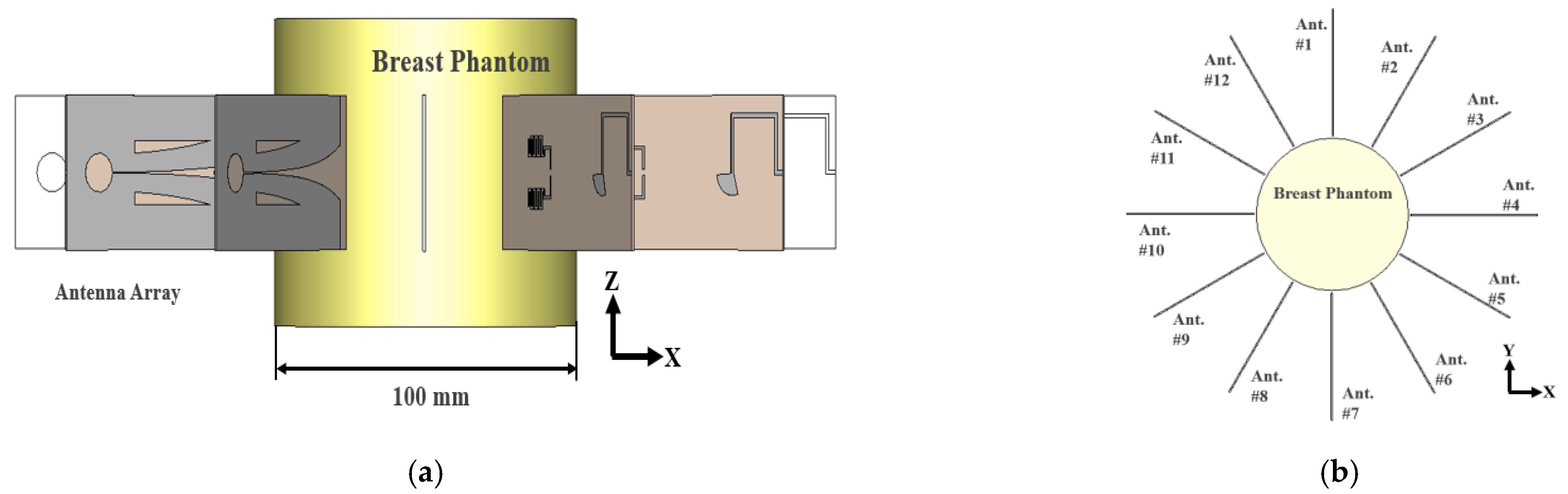
2.2. Antenna Array Configuration for Effective Electric Field Concentration
2.3. The Specific Absorption Rate and Temperature Increase
3. Electromagnetic Focusing for Microwave Hyperthermia
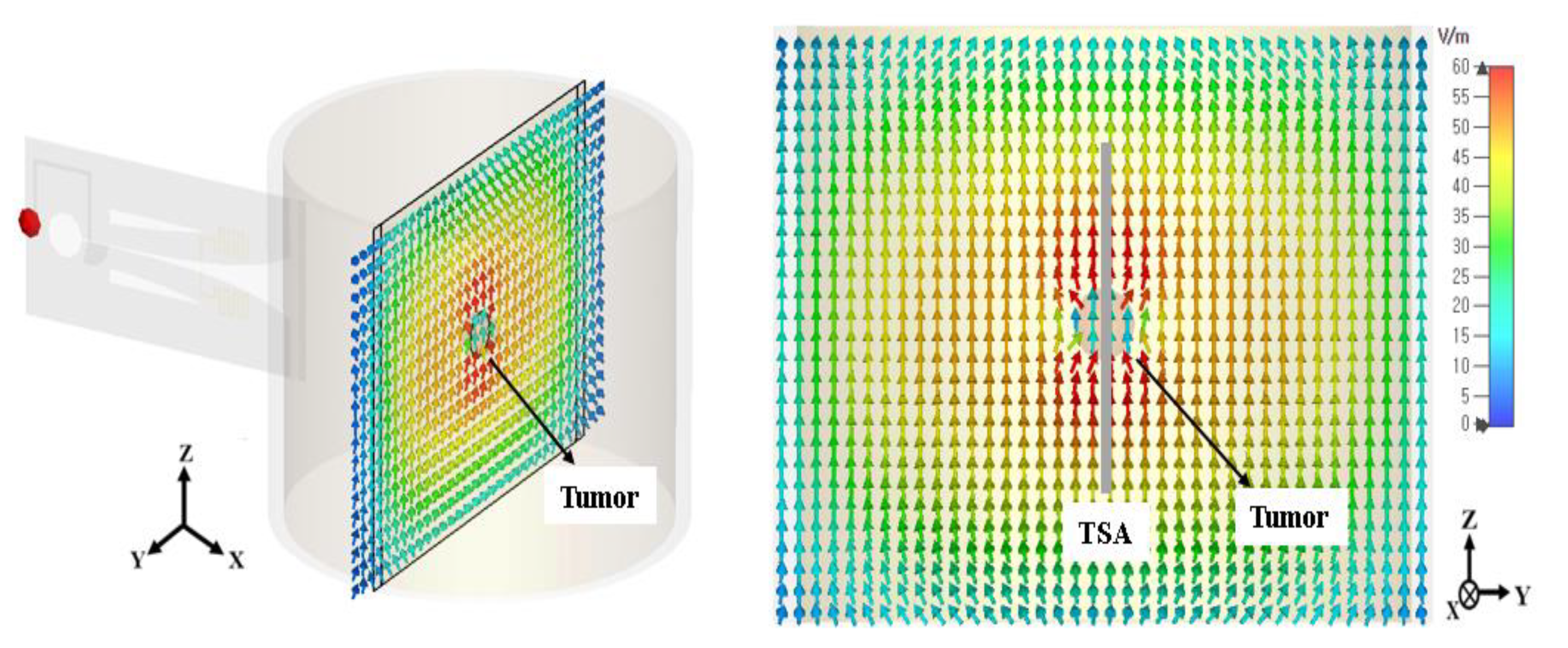
3.1. Electromagnetic Focusing for Unifocal Breast Cancers
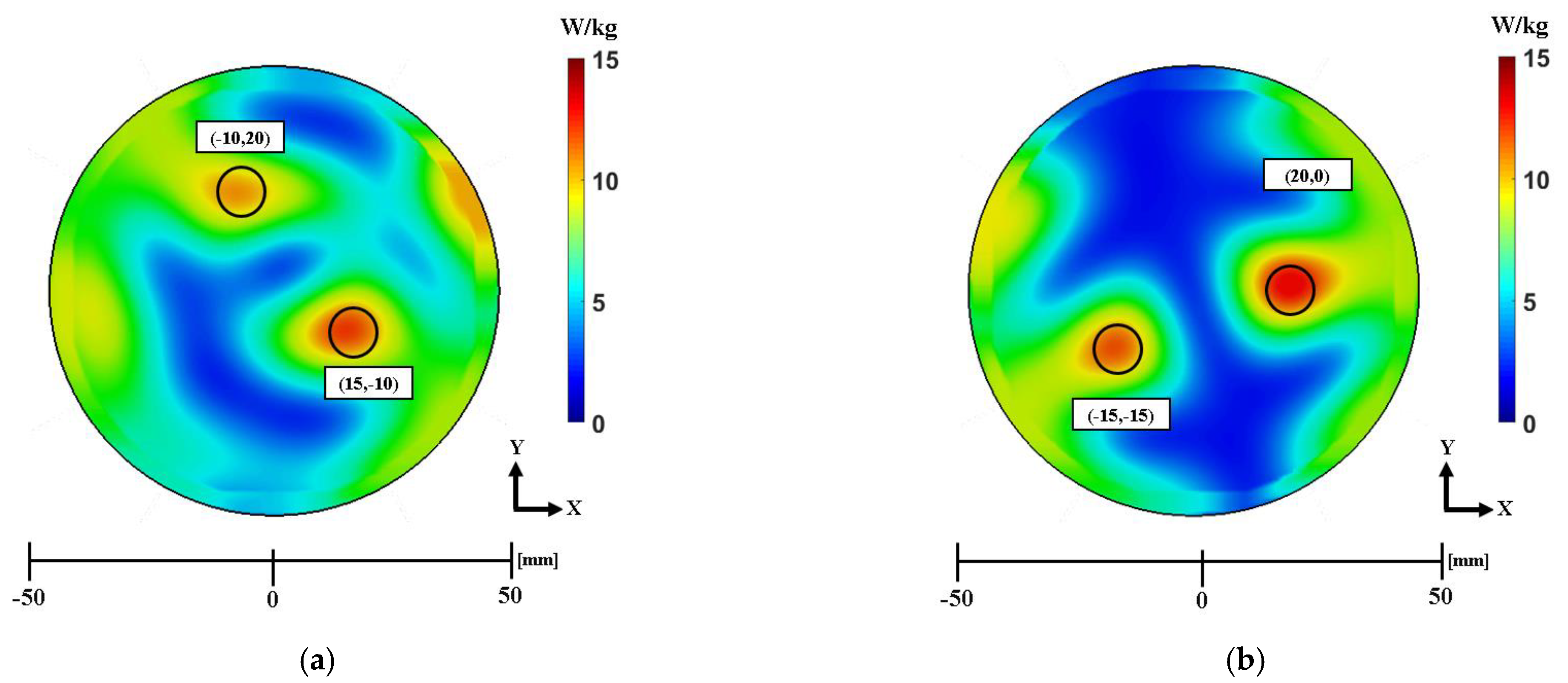
3.2. Electromagnetic Focusing for Multifocal Breast Cancer
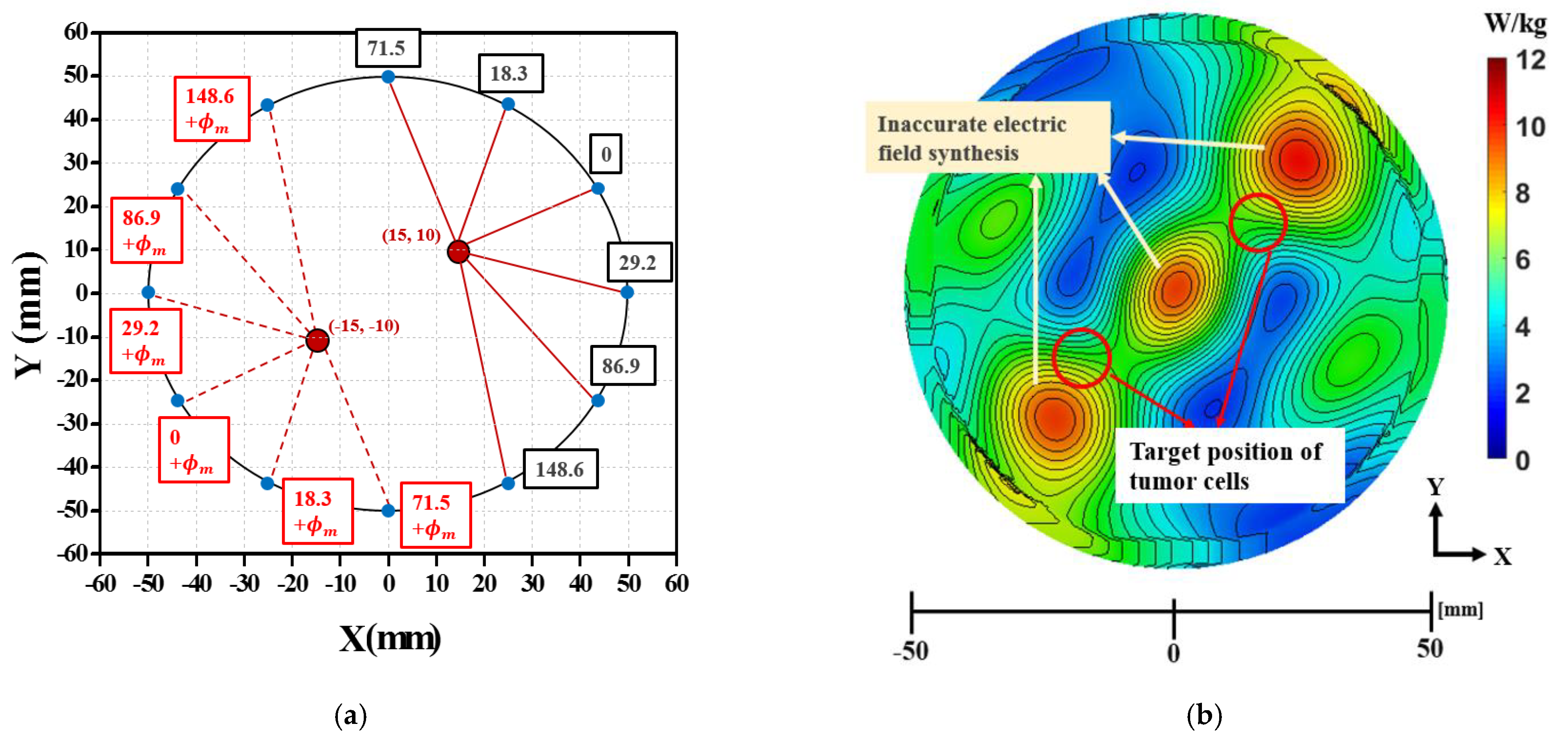
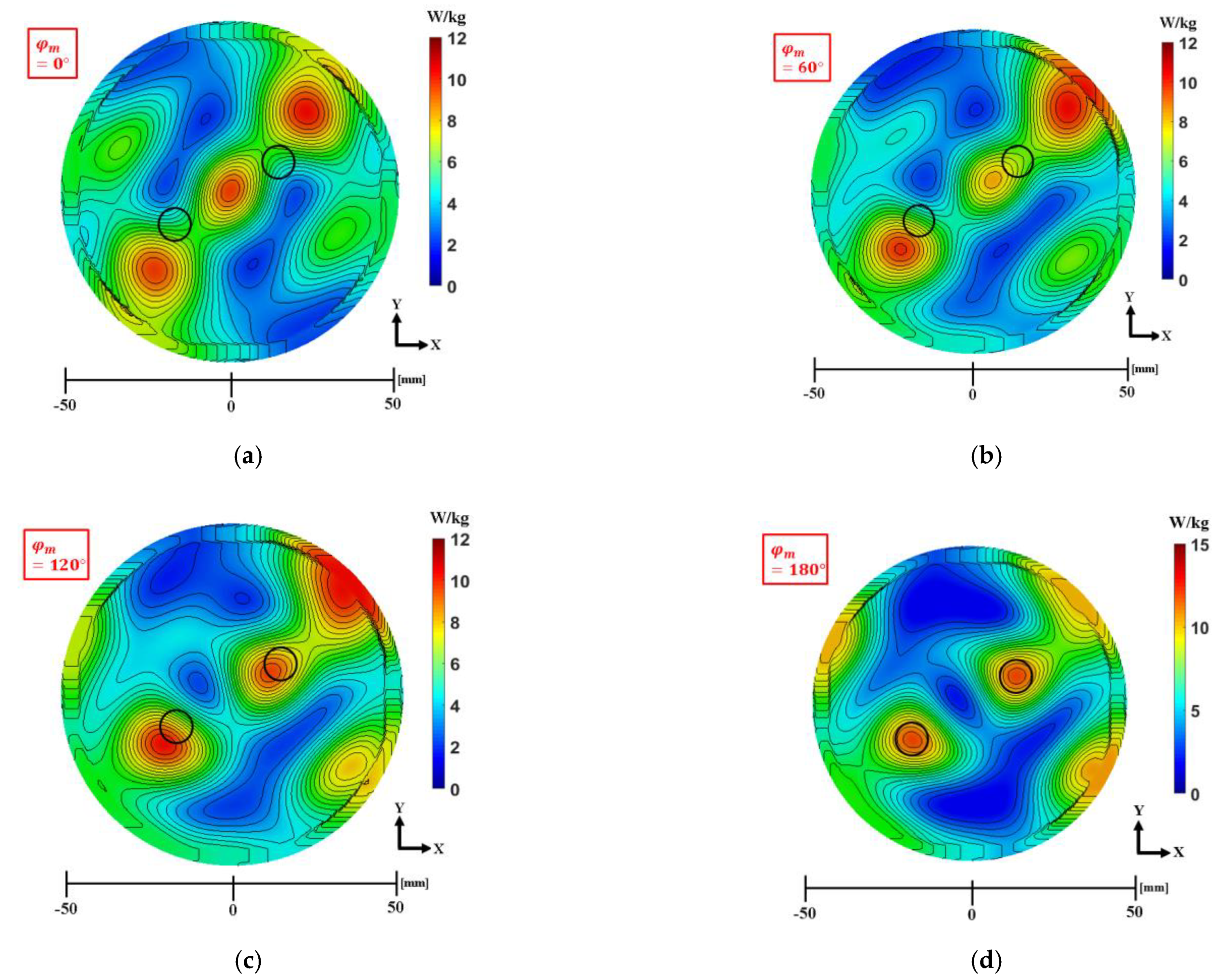
3.3. Phase Compensation Technique for Multiple Focusing
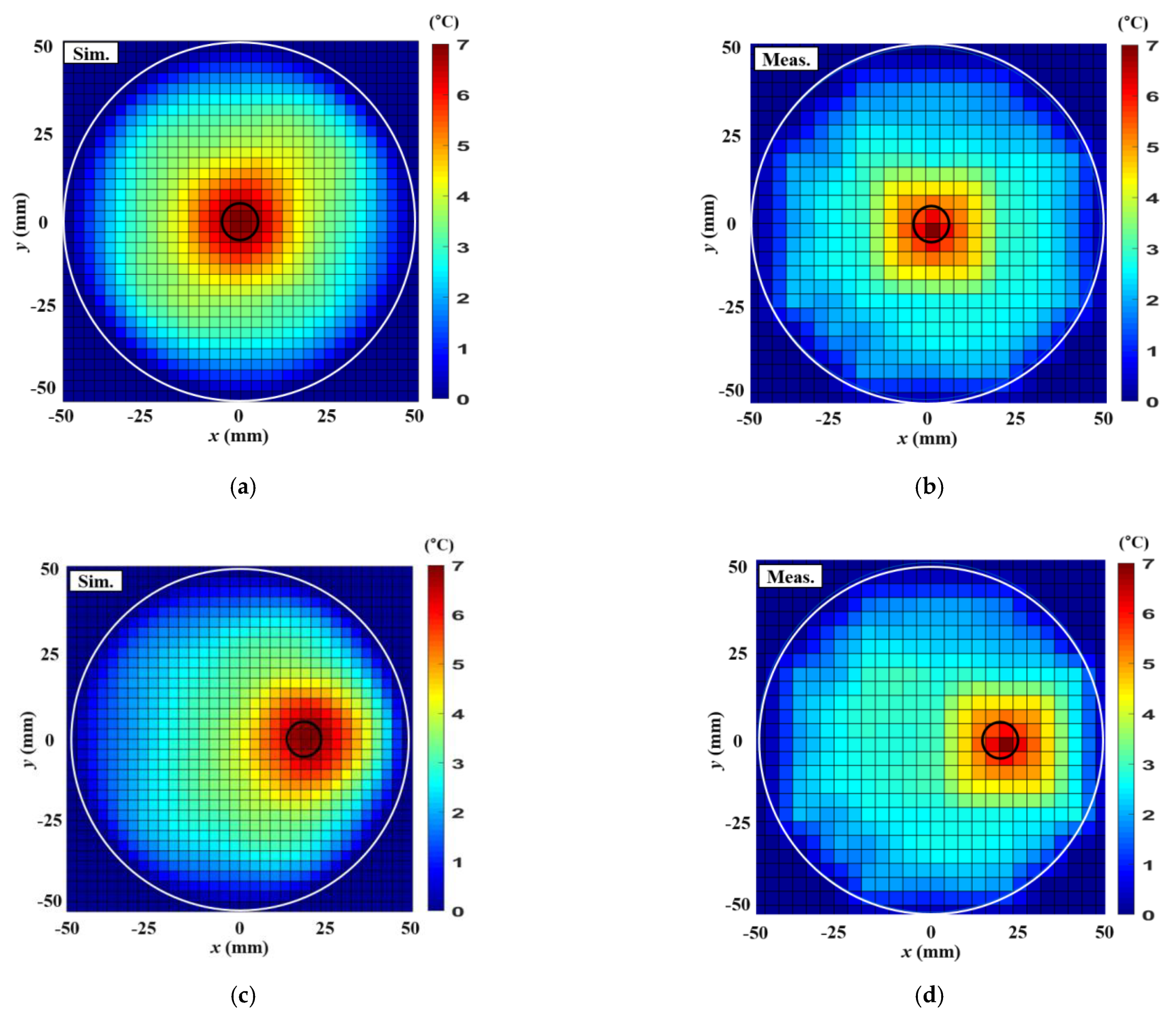
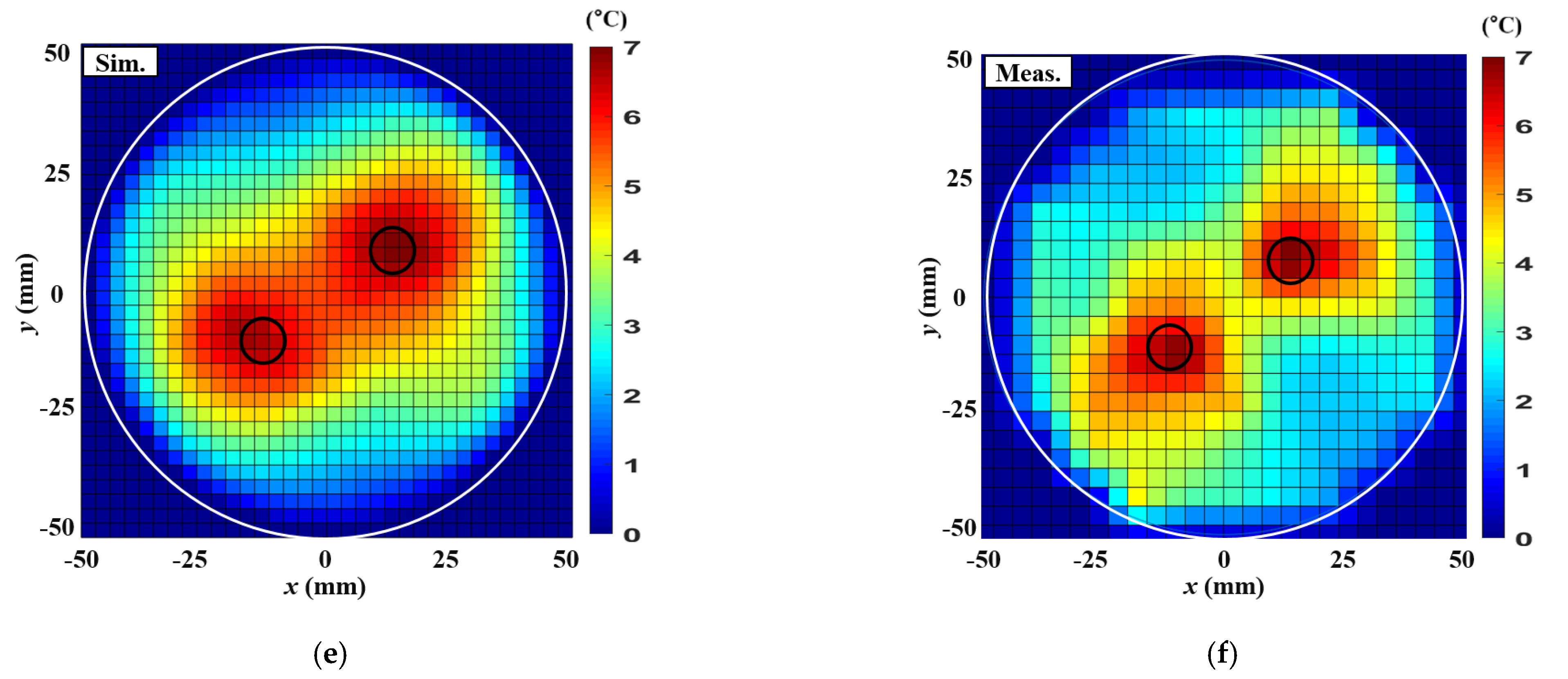
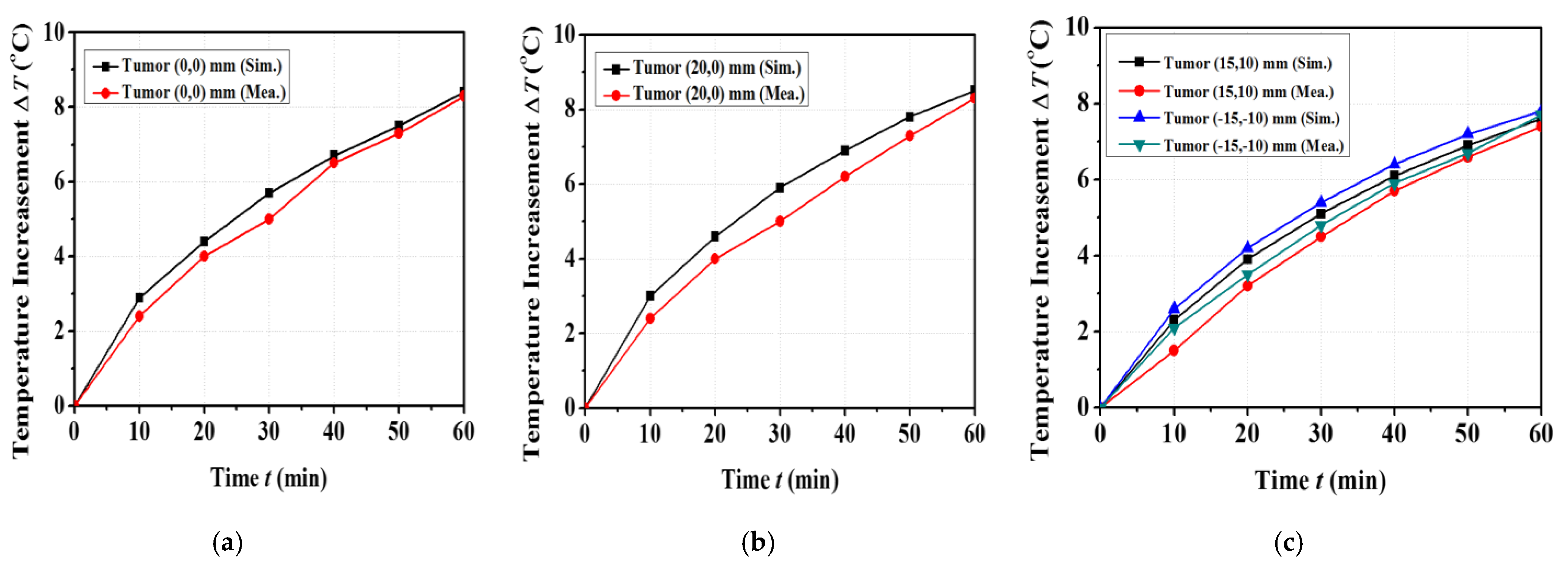
4. Thermal Experiment Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cala, P.; Bienkowski, P. Antenna array for microwave ablation or hyperthermia working in the ISM 2.4 GHz band. Przegl. Elektrotech. 2018, 94, 238–241. [Google Scholar]
- Gas, P. Essential Facts on the History of Hyperthermia and their Connections with Electromedicine. Przegl. Elektrotech. 2011, 87, 37–40. [Google Scholar]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating hyperthermia into modern radiation oncology: What evidence is necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refaat, T.; Sachdev, S.; Sathiaseelan, V.; Helenowski, I.; Abdelmoneim, S.; Pierce, M.C.; Woloschak, G.; Small, W.; Mittal, B.; Kiel, K.D. Hyperthermia and radiation therapy for locally advanced or recurrent breast cancer. Breast 2015, 24, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Huang, W.; Seynhaeve, A.L.B.; ten Hagen, T.L.M. Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors. Pharmaceutics 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.C.; Kim, K.J.; Kim, J.; Yoon, Y.J. Compact microwave radiator for improving heating uniformity in hyperthermia system. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 1345–1348. [Google Scholar] [CrossRef]
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef]
- Converse, M.; Bond, E.J.; Van Veen, B.D.; Hagness, S.C. A computational study of ultra-wideband versus narrowband microwave hyperthermia for breast cancer treatment. IEEE Trans. Microwave Theory Tech. 2006, 54, 2169–2180. [Google Scholar] [CrossRef] [Green Version]
- Iero, D.A.M.; Crocco, L.; Isernia, T. Thermal and microwave constrained focusing for patient-specific breast cancer hyperthermia: A robustness assessment. IEEE Trans. Antennas Propag. 2014, 62, 814–821. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Abbosh, A.; Crozier, S. Microwave hyperthermia for breast cancer treatment using electromagnetic and thermal focusing tested on realistic breast models and antenna arrays. IEEE Trans. Antennas Propag. 2015, 63, 4426–4434. [Google Scholar] [CrossRef]
- Bellizzi, G.G.; Sumser, K.; VilasBoas-Ribeiro, I.; Curto, S.; Drizdal, T.; van Rhoon, G.; Franckena, M.; Paulides, M.M. Standardization of patient modeling in hyperthermia simulation studies: Introducing the Erasmus Virtual Patient Repository. Int. J. Hyperth. 2020, 37, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.M.; Ivanov, S.M.; Perelstein, E.A.; Polozov, S.M. Thermometry system development for thermoradiotherapy of deep-seated tumours. J. Phys. Conf. Ser. 2017, 941, 012086. [Google Scholar] [CrossRef]
- Gaffoglio, R.; Righero, M.; Giordanengo, G.; Zucchi, M.; Vecchi, G. Fast Optimization of Temperature Focusing in Hyperthermia Treatment of Sub-Superficial Tumors. IEEE J. Electromagn. RF Microw. Med. Biol. 2021. [Google Scholar] [CrossRef]
- Bellizzi, G.G.; Paulides, M.M.; Drizdal, T.; van Rhoon, G.C.; Crocco, L.; Isernia, T. Selecting the optimal subset of antennas in hyperthermia treatment planning. IEEE J. Electromagn. RF Microw. Med. Biol. 2019, 3, 240–246. [Google Scholar] [CrossRef]
- Gas, P.; Miaskowski, A. SAR optimization for multi-dipole antenna array with regard to local hyperthermia. Przegl. Elektrotech. 2019, 95, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Curto, S.; Garcia-Miquel, A.; Vidal, N.; Lopez-Villegas, J.M.; Prakash, P. Design and characterisation of a phased antenna array for intact breast hyperthermia. Int. J. Hyperth. 2018, 34, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Fenn, A.J. An adaptive microwave phased array for targeted heating of deep tumours in intact breast: Animal study results. Int. J. Hyperth. 1999, 15, 45–61. [Google Scholar] [CrossRef]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; van Rhoon, G.C.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gas, P.; Miaskowski, A.; Subramanian, M. In Silico Study on Tumor-Size-Dependent Thermal Profiles inside an Anthropomorphic Female Breast Phantom Subjected to Multi-Dipole Antenna Array. Int. J. Mol. Sci. 2020, 21, 8597. [Google Scholar] [CrossRef]
- Stang, J.; Haynes, M.; Carson, P.; Moghaddam, M. A preclinical system prototype for focused microwave thermal therapy of the breast. IEEE Trans. Biomed. Eng. 2012, 59, 2431–2438. [Google Scholar] [CrossRef] [Green Version]
- Cabioglu, N.; Ozmen, V.; Kaya, H.; Tuzlali, S.; Igci, A.; Muslumanoglu, M.; Kecer, M.; Dagoglu, T. Increased lymph node positivity in multifocal and multicentric breast cancer. J. Am. Coll. Surg. 2009, 208, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, S. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 2016, 106, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Nizam-Uddin, N.; Abdulkawi, W.M.; Elshafiey, I.; Sheta, A.F.A. Toward a multi-target multi-channel hyperthermia treatment system: Proof of concept with numerical simulations. Int. J. Heat Mass Transf. 2020, 150, 119257. [Google Scholar] [CrossRef]
- Lim, S.; Yoon, Y.J. Wideband-Narrowband Switchable Tapered Slot Antenna for Breast Cancer Diagnosis and Treatment. Appl. Sci. 2021, 11, 3606. [Google Scholar] [CrossRef]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies; King’s Coll London: London, UK, 1996. [Google Scholar]
- Henin, B.; Abbosh, A.M.; Al Abdulla, W. Electro-biomechanical breast phantom for hybrid breast imaging. In Proceedings of the 2015 International Symposium on Antennas and Propagation (ISAP), Hobart, TAS, Australia, 9–12 November 2015; pp. 1–3. [Google Scholar]
- CST Studio Suite. 2019. Available online: https://www.3ds.com/products-services/simulia/products/cst-studio-suite/ (accessed on 20 August 2018).
- Vander Vorst, A.; Rosen, A.; Kotsuka, Y. RF/Microwave Interaction with Biological Tissues; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Farahat, A.E.; Kahil, H.M.; Hussein, K.F.A. Microwave Diathermy for Deep Heating Therapy of Knee Joint. Prog. Electromagn. Res. C 2020, 99, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Gonzalez, F.J. Non-invasive estimation of the metabolic heat production of breast tumors using digital infrared imaging. Quant. Infrared Thermogr. J. 2011, 8, 139–148. [Google Scholar] [CrossRef]




| Properties | Fat | Tumor |
|---|---|---|
| Dielectric permittivity | 5.14 | 55 |
| Loss tangent | 0.137 | 0.29 |
| Density () | 920 | 920 |
| Thermal conductivity (W/K/m) | 0.42 | 0.42 |
| Heat capacity (J/K/kg) | 3000 | 3000 |
| Position of Single Tumor | Antenna Number | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| (20, 0) mm | 159 | 90.6 | 27.8 | 0 | 27.8 | 90.6 | 159 | 216.3 | 253.7 | 266.7 | 253.7 | 216.3 |
| (15,15) mm | 53.8 | 0 | 0 | 53.8 | 126.6 | 194.2 | 244.6 | 271.3 | 271.3 | 244.6 | 194.2 | 126.6 |
| Modified Phase, | Antenna Number | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 0 | 18.3 | 0 | 29.2 | 86.9 | 148.6 | 71.5 | 18.3 | 0 | 29.2 | 86.9 | 148.6 | |
| 60 | 18.3 | 0 | 29.2 | 86.9 | 148.6 | 131.5 | 78.3 | 60 | 89.2 | 146.9 | 208.6 | |
| 120 | 18.3 | 0 | 29.2 | 86.9 | 148.6 | 191.5 | 138.3 | 120 | 149.2 | 206.9 | 268.6 | |
| 180 | 18.3 | 0 | 29.2 | 86.9 | 148.6 | 251.5 | 198.3 | 180 | 209.2 | 266.9 | 328.6 | |
| Antenna Number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Case 1—(−10,20) mm,(15, −10) mm | 199.5° | 148.6° | 86.8° | 29.2° | 0° | 18.3° | 467.72° | 429.9° | 369.2° | 293.8° | 219.9° | 180° |
| Case 2—(20,0) mm, (–15, −15) mm | 159.6° | 90.9° | 27.9° | 0° | 27.9° | 90.9° | 233.9° | 180° | 180° | 233.9° | 307° | 374.9° |
| Parameters | Single Tumor at (0,0) mm | Single Tumor at (20,0) mm | Two Tumors at (15,10) mm, (−15,−10) mm |
|---|---|---|---|
| Input RF power (W) | 7.1 | 6 | 7.1 |
| Maximum SAR in the simulation (W/kg) | 18.1 | 23.1 | 14.1 |
| Maximum temperature increase in simulation () | 8.4 | 8.5 | 7.8 |
| Maximum temperature increase in measurement () | 8.3 | 8.3 | 7.7 |
| ETA in simulation () | 226.9 | 201 | 153.9 |
| ETA in measurement () | 216.3 | 195.9 | 132.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Yoon, Y.J. Phase Compensation Technique for Effective Heat Focusing in Microwave Hyperthermia Systems. Appl. Sci. 2021, 11, 5972. https://doi.org/10.3390/app11135972
Lim S, Yoon YJ. Phase Compensation Technique for Effective Heat Focusing in Microwave Hyperthermia Systems. Applied Sciences. 2021; 11(13):5972. https://doi.org/10.3390/app11135972
Chicago/Turabian StyleLim, Seonho, and Young Joong Yoon. 2021. "Phase Compensation Technique for Effective Heat Focusing in Microwave Hyperthermia Systems" Applied Sciences 11, no. 13: 5972. https://doi.org/10.3390/app11135972






