Durability of Ternary Cements Based on New Supplementary Cementitious Materials from Industrial Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blended Cements
2.3. Method
2.4. Instrumental Techniques
3. Results
3.1. Mechanical Properties
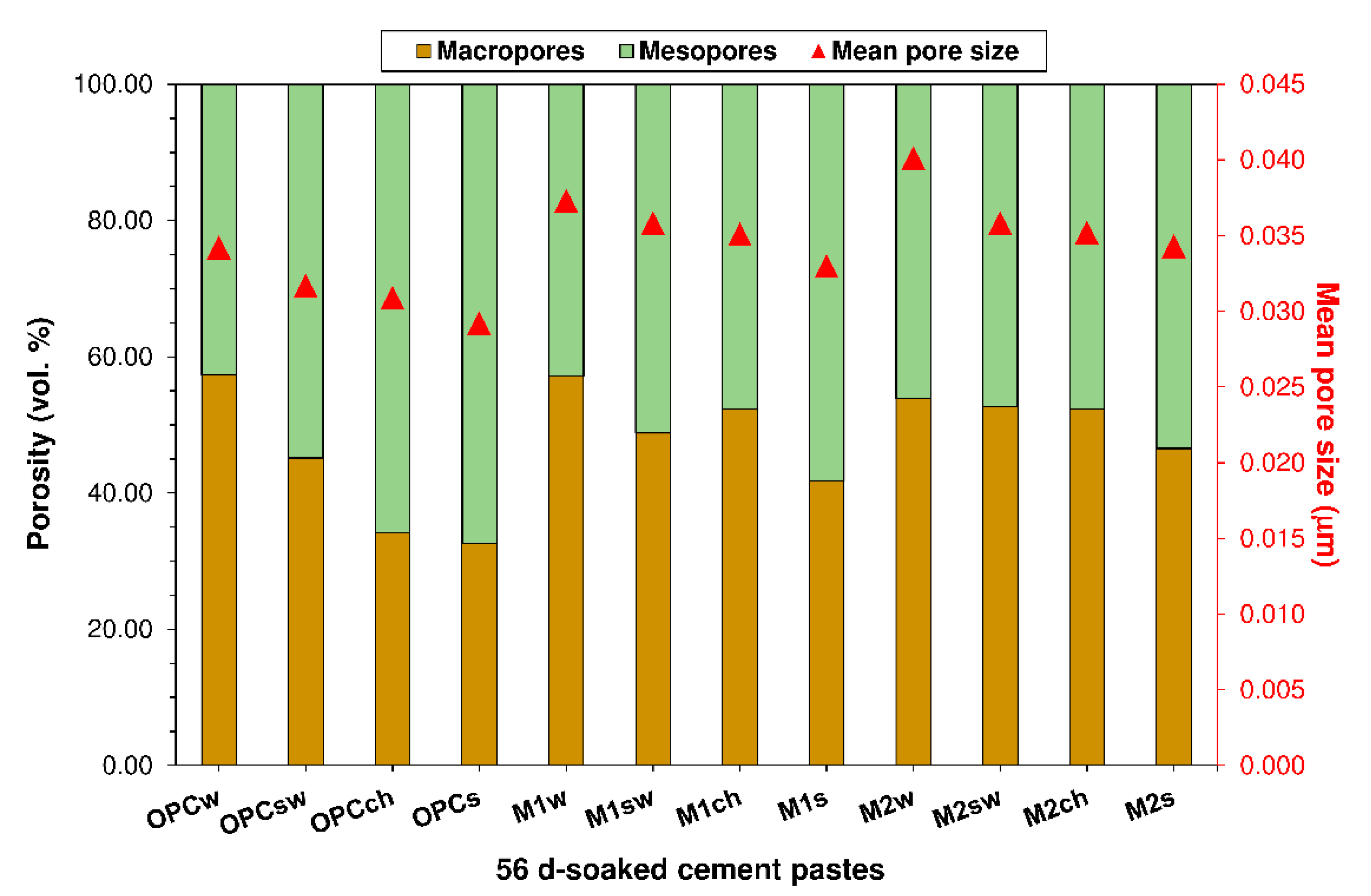
3.2. Porosity
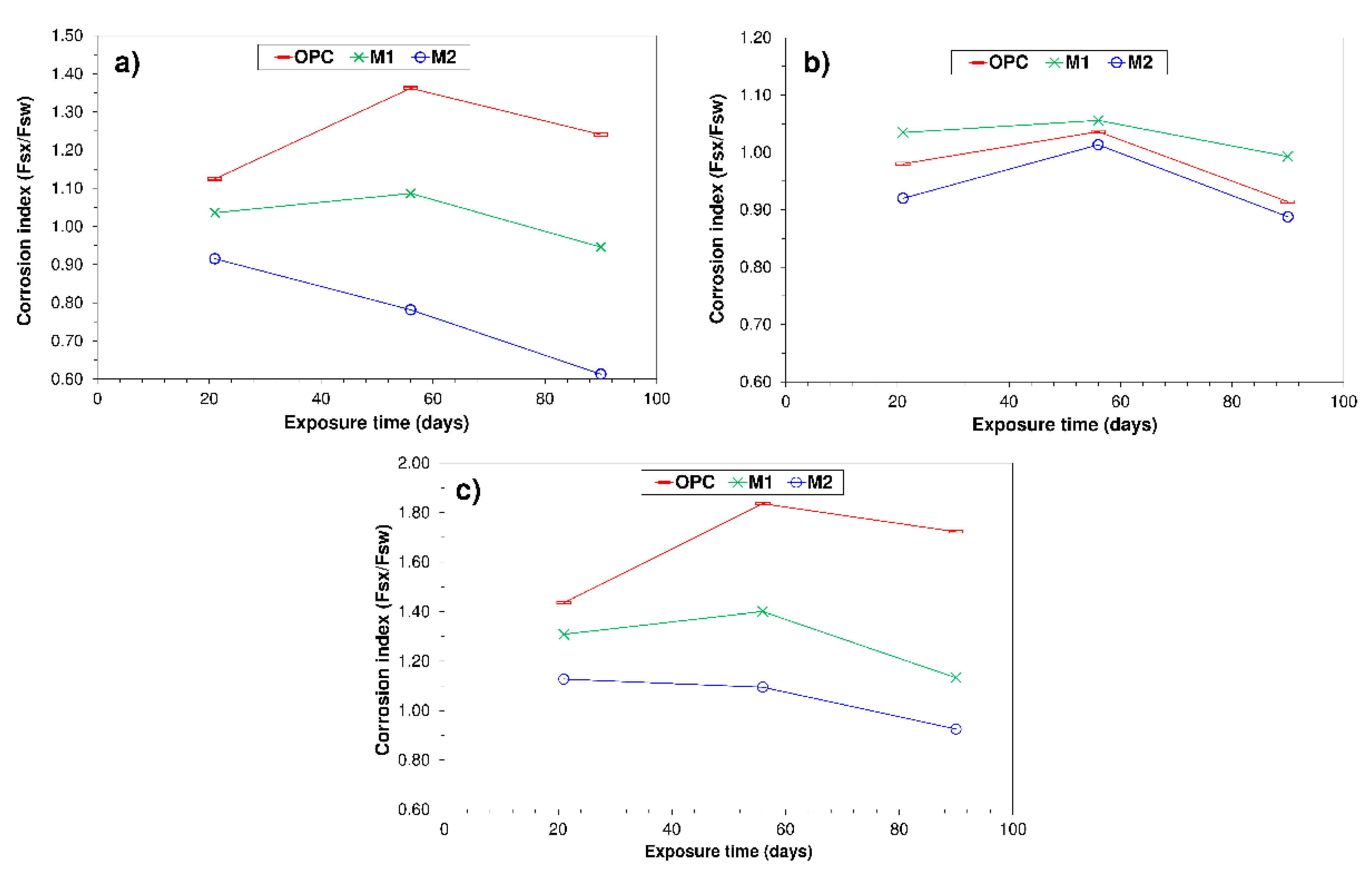
3.3. Corrosion Index
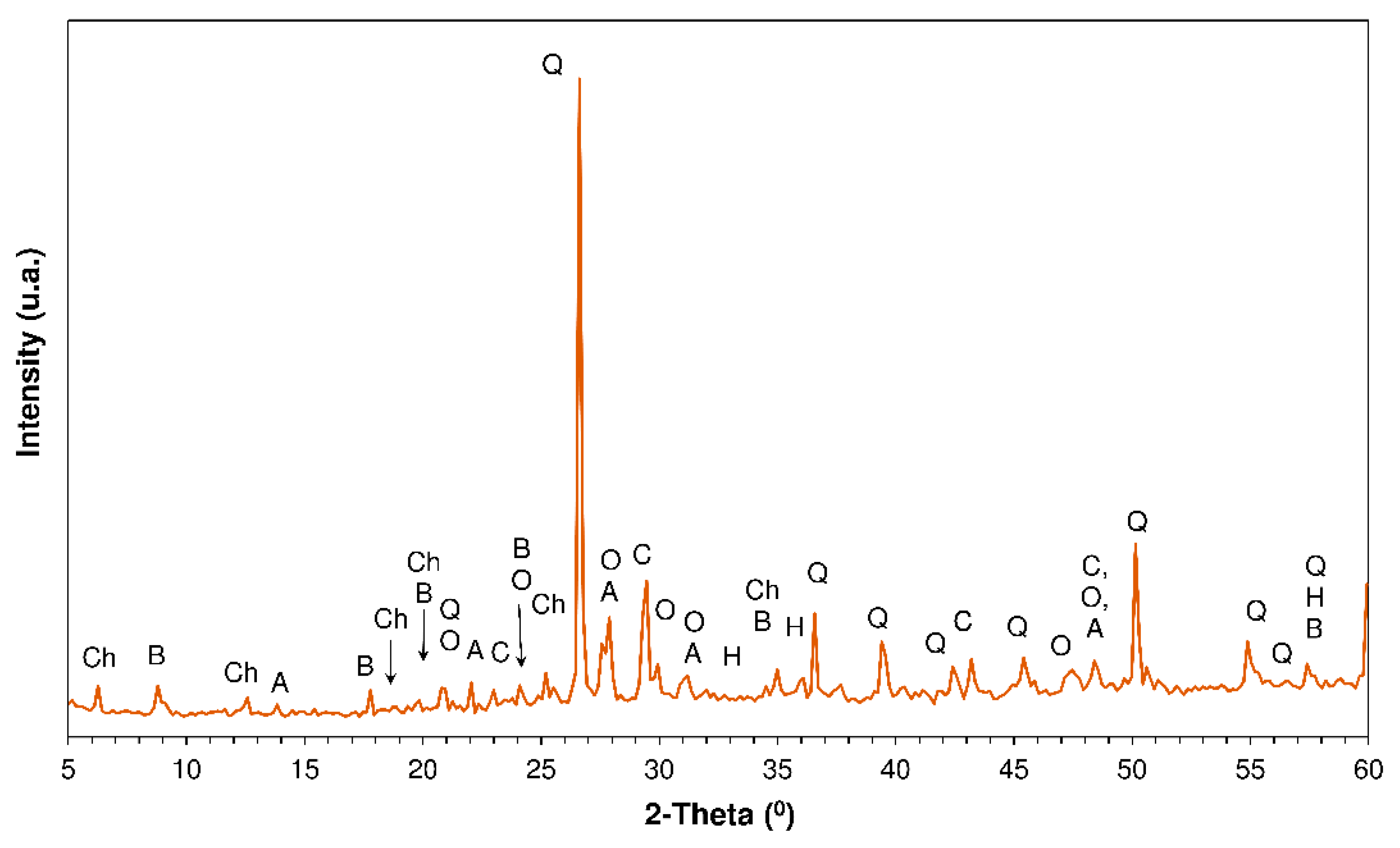
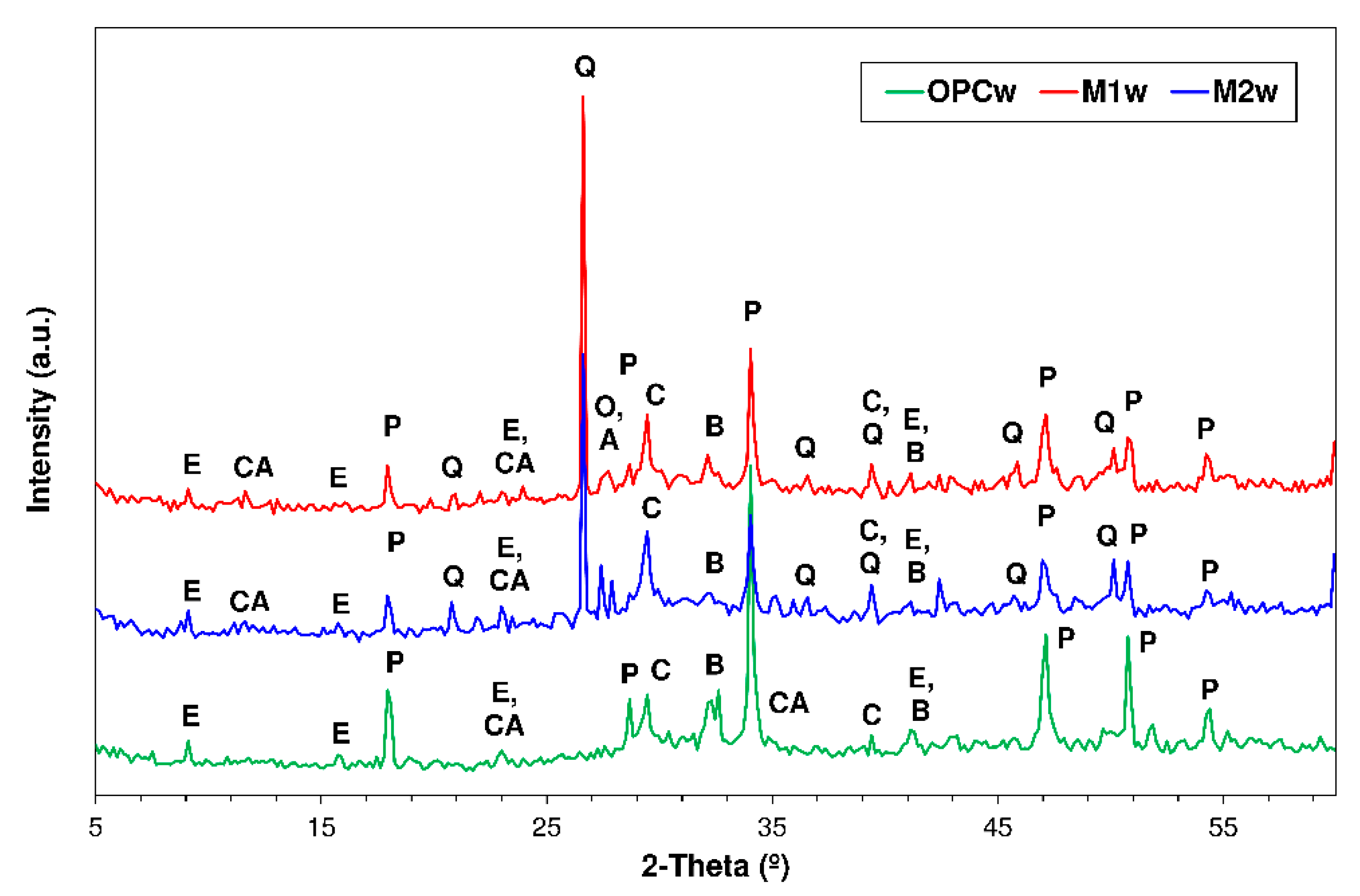
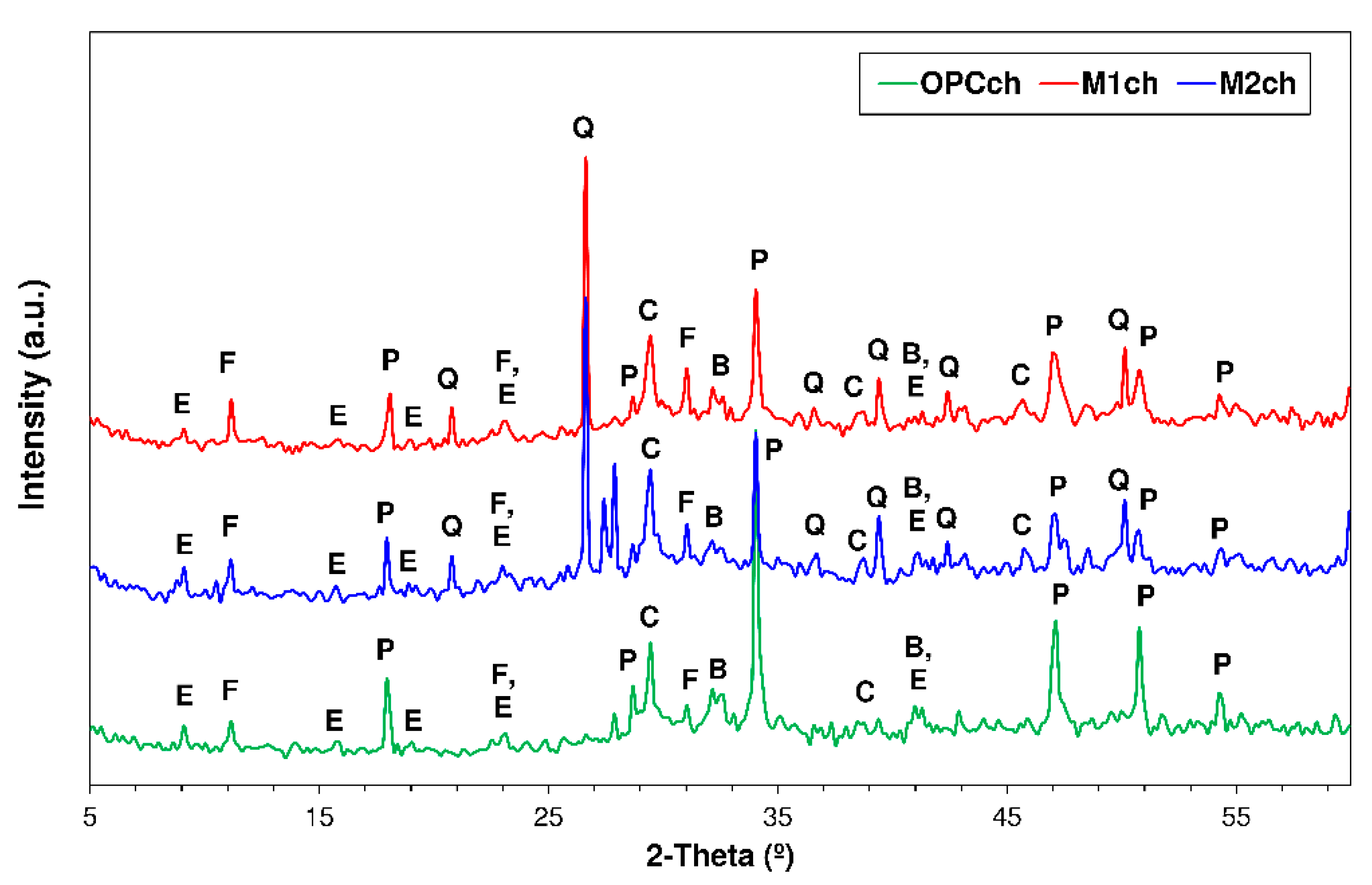
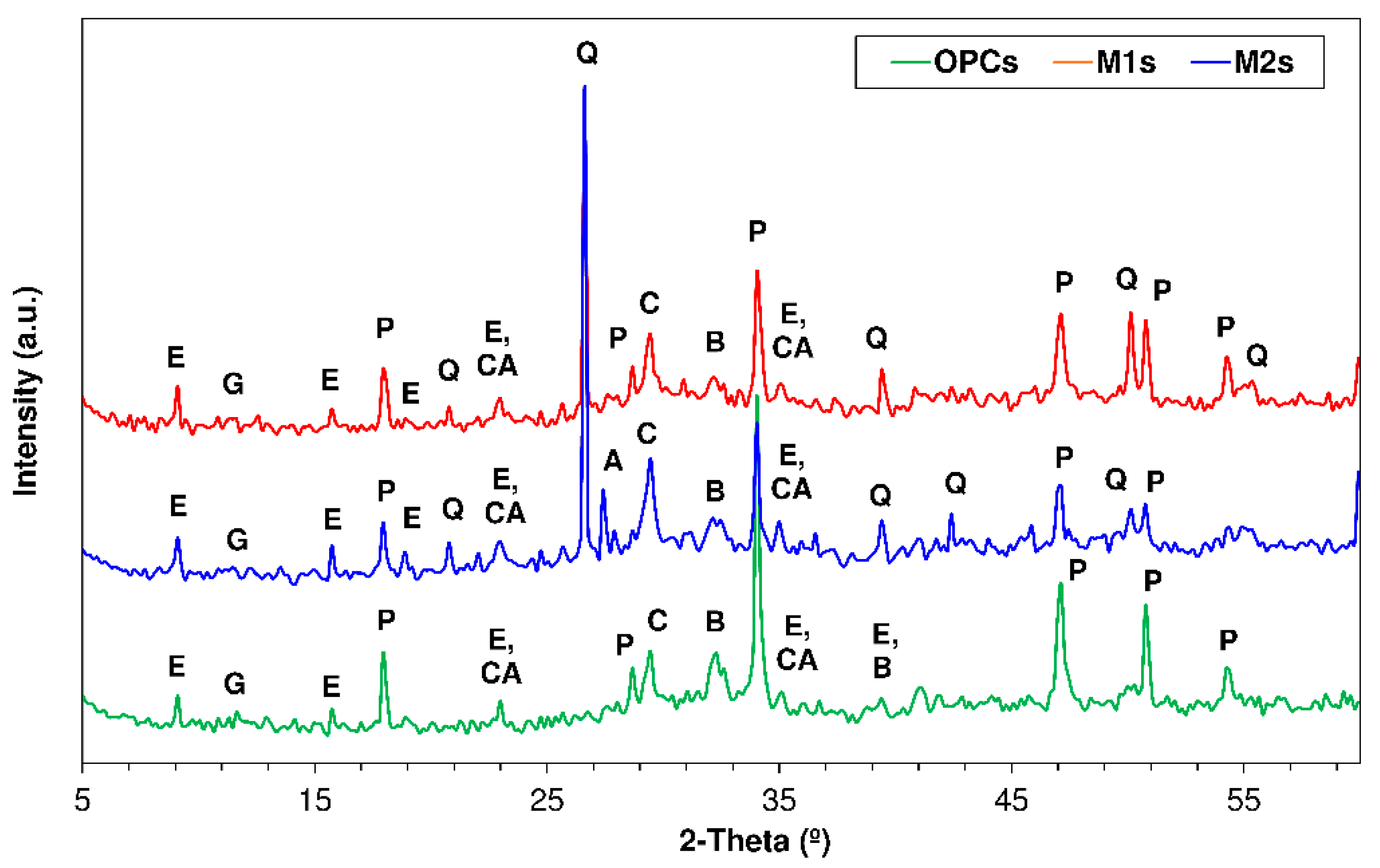
3.4. X-ray Diffration Findings
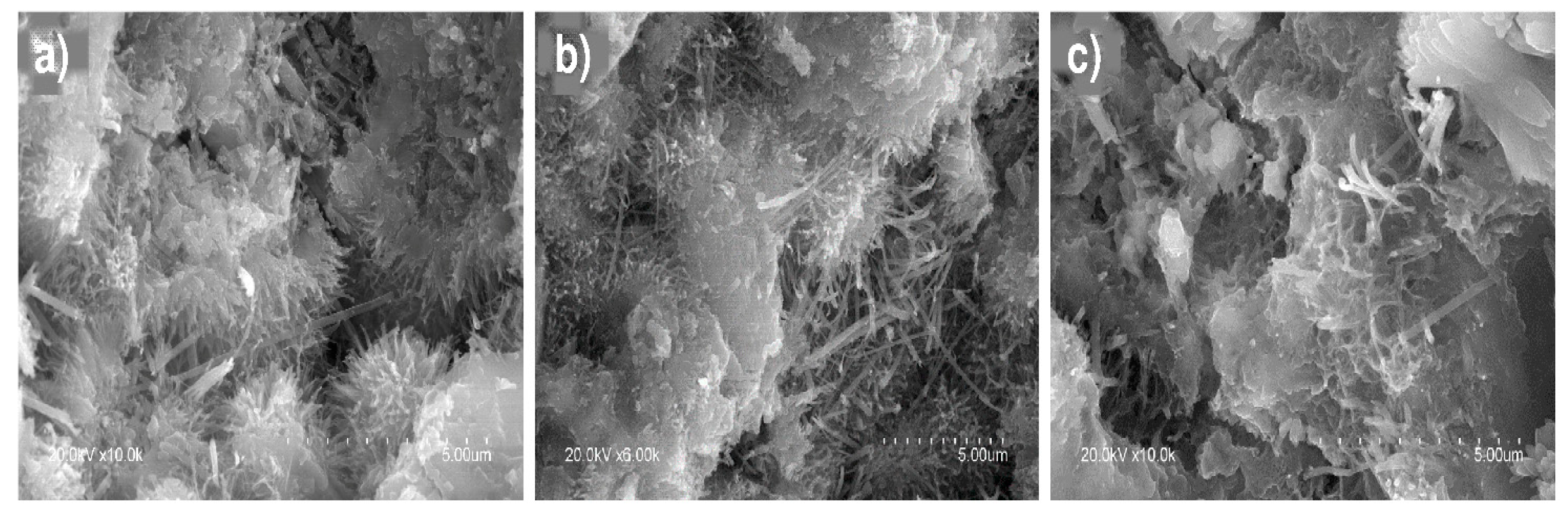
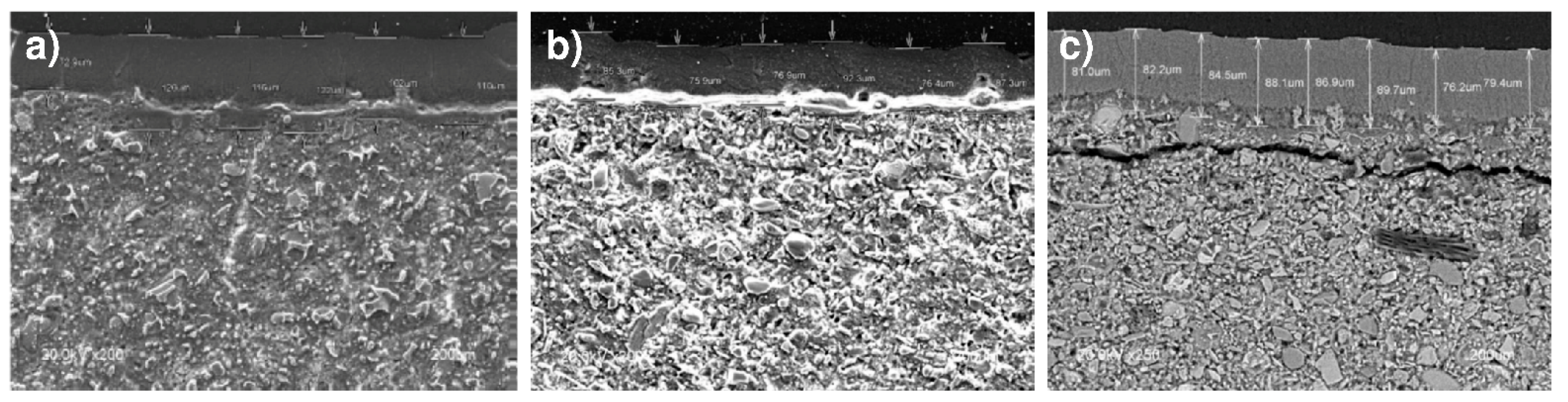
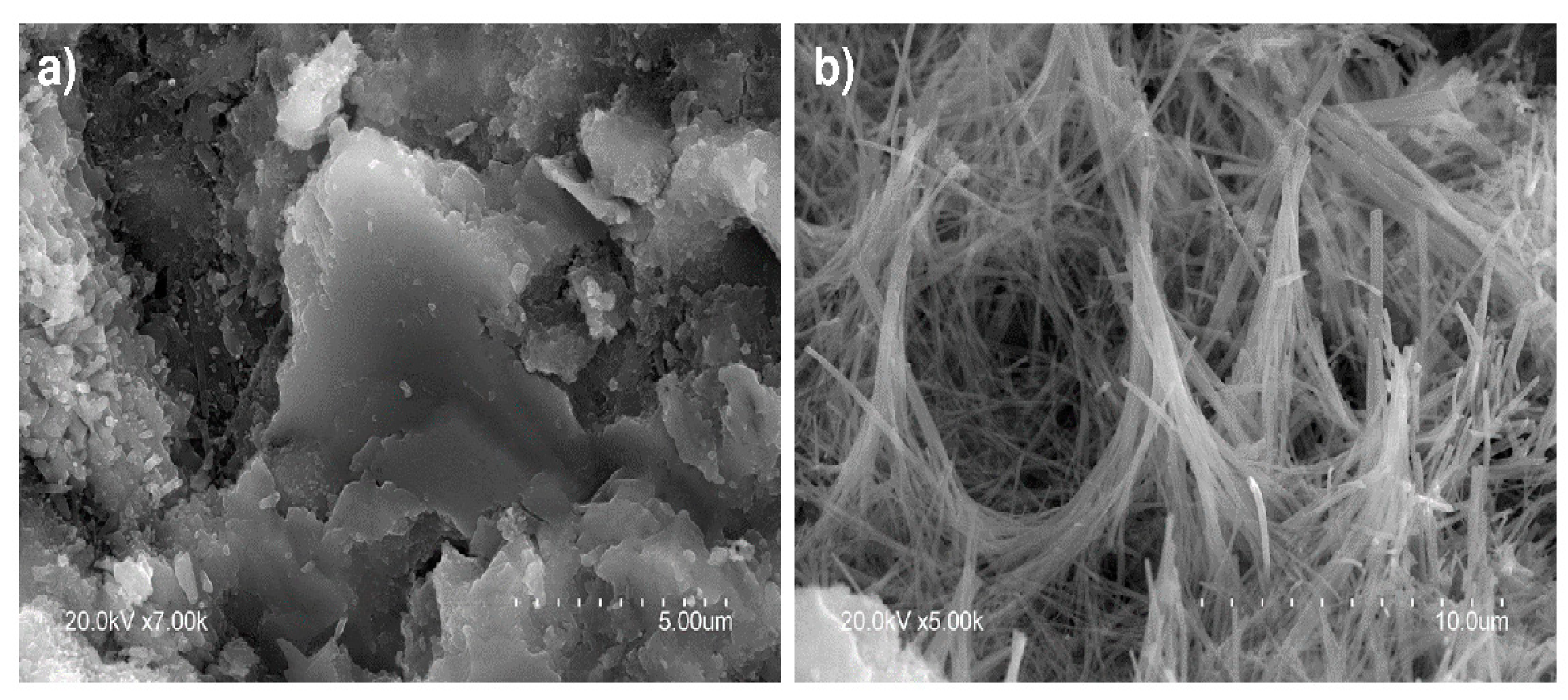
3.5. SEM/EDX Microstructural Analysis
4. Conclusions
- Pastes bearing 30% (20% UC&DW + 10%BA) SCMs exhibit 10% lower flexural strength than OPC and those with 45% (20%UC&DW + 20%BA + 5%SF) supplementary cementitious materials 11% lower strength than the 56-d water-soaked pastes.
- As the pozzolanic reaction between SCMs and portlandite does not suffice to offset the dilution induced by a lower proportion of cement, the new pastes exhibit a larger mean pore size and greater porosity than OPC.
- Including UC&DW + BA or UC&DW + BA + SF yields cements able to resist the chemical attacks analysed, with a 56-d corrosion index >0.7 in all cases.
- Resistance to aggressive media attack is not enhanced by raising the percentage of SCM added, for the cements bearing the binary blend UC&DW + BA proved to be more resistant than those to which silica fume was added.
- Paste M1 bearing 30% SCMs had a higher 56-d corrosion index, at 1.06, for the 0.5 M NaCl solution than both OPC (CI = 1.04) and M2 (CI = 1.02).
- OPC paste resists seawater and 0.3 M Na2SO4 better than M1, which in turn had a higher corrosion index than M2 irrespective of the medium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Dong, Y.; Gu, X. Durability assessment of reinforced concrete structures considering global warming: A performance-based engineering and experimental approach. Constr. Build. Mater. 2020, 233, 117251. [Google Scholar] [CrossRef]
- Islam, R.; Nazifa, T.H.; Mohammed, S.F.; Zishan, M.A.; Yusof, Z.M.; Mong, S.G. Impacts of design deficiencies on maintenance cost of high-rise residential buildings and mitigation measures. J. Build. Eng. 2021, 39, 10221. [Google Scholar]
- Yang, K.-H.; Lim, H.S.; Kwon, S.J.; Kim, J.H. Repair cost estimation techniques for reinforced concrete structures located at the seashore: Considering various probabilistic service life functions and actual mix proportions. Constr. Build. Mater. 2020, 256, 119469. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.I.; Frías, M.; Sabador, E.; Asensio, E.; Rivera, J.; Medina, C. Durability and chromatic behavior in cement pastes containing ceramic industry milling and glazing by-products. J. Am. Ceram. Soc. 2019, 102, 1971–1981. [Google Scholar] [CrossRef]
- Claisse, P.A. Cements and cement replacement materials. In Civil Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2016; pp. 163–176. [Google Scholar]
- Kumar Metha, P.; Monteiro, P.J.M. Concrete: Microstructure, Properties and Materials, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006; p. 659. [Google Scholar]
- UK Quality Ash Association. Fact Sheet 18—Embodied CO2e of UK cement, additions and cementitious materials. Available online: https://cement.mineralproducts.org/documents/Factsheet_18.pdf (accessed on 15 June 2021).
- European Committee for Standardization. EN 197-1. Cement. Composition, Specifications and Conformity Criteria for Common Cements; European Committee for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Villoria-Sáez, P.; Porras-Amores, C.; del Río Merino, M. Estimation of construction and demolition waste. In Advances in Construction and Demolition Waste Recycling; Woodhead Publishing: Sawston, UK, 2020; pp. 13–30. [Google Scholar]
- Velardo, P.; Sáez del Bosque, I.F.; Matías, A.; Sánchez de Rojas, M.I.; Medina, C. Properties of concretes bearing mixed recycled aggregate with polymer-modified surfaces. J. Build. Eng. 2021, 38, 102211. [Google Scholar] [CrossRef]
- Gonzalez-Fonteboa, B.; Seara-Paz, S.; de Brito, J.; Gonzalez-Taboada, I.; Martinez-Abella, F.; Vasco-Silva, R. Recycled concrete with coarse recycled aggregate. An overview and analysis. Mater. Constr. 2018, 68, e151. [Google Scholar] [CrossRef] [Green Version]
- Plaza, P.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Use of recycled coarse and fine aggregates in structural eco-concretes. Physical and mechanical properties and CO2 emissions. Constr. Build. Mater. 2021, 285, 122926. [Google Scholar] [CrossRef]
- Moreno-Juez, J.; Vegas, I.J.; Gebremariam, A.T.; García-Cortés, V.; di Maio, F. Treatment of end-of-life concrete in an innovative heating-air classification system for circular cement-based products. J. Clean Prod. 2020, 263, 121515. [Google Scholar] [CrossRef]
- Letelier, V.; Tarela, E.; Muñoz, P.; Moriconi, G. Combined effects of recycled hydrated cement and recycled aggregates on the mechanical properties of concrete. Constr. Build. Mater. 2017, 132, 365–375. [Google Scholar] [CrossRef]
- Xiao, J.; Xiao, Y.; Liu, Y.; Ding, T. Carbon emission analyses of concretes made with recycled materials considering CO2 uptake through carbonation absorption. Struct. Concr. 2021, 22, E58–E73. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L.; Xiao, J.; Singh, A.; Zeng, J. Compound utilization of construction and industrial waste as cementitious recycled powder in mortar. Resour. Conserv. Recycl. 2021, 170, 105561. [Google Scholar] [CrossRef]
- Cantero, B.; Bravo, M.; de Brito, J.; Sáez del Bosque, I.F.; Medina, C. Assessment of the Permeability to Aggressive Agents of Concrete with Recycled Cement and Mixed Recycled Aggregate. Appl. Sci. 2021, 11, 3856. [Google Scholar] [CrossRef]
- Caneda-Martínez, L.; Monasterio, M.; Moreno-Juez, J.; Martínez-Ramírez, S.; García, R.; Frías, M. Behaviour and Properties of Eco-Cement Pastes Elaborated with Recycled Concrete Powder from Construction and Demolition Wastes. Materials 2021, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Use of clay-based construction and demolition waste as additions in the design of new low and very low heat of hydration cements. Mater. Struct. 2018, 51, 101. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Clay-based construction and demolition waste as a pozzolanic addition in blended cements. Effect on sulfate resistance. Constr. Build. Mater. 2016, 127, 950–958. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Characterization of Ceramic-Based Construction and Demolition Waste: Use as Pozzolan in Cements. J. Am. Ceram. Soc. 2016, 99, 4121–4127. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Fired clay-based construction and demolition waste as pozzolanic addition in cements. Design of new eco-efficient cements. J. Clean Prod. 2020, 265, 121610. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.I.; Marin, F.; Rivera, J.; Frias, M. Morphology and properties in blended cements with ceramic wastes as a pozzolanic material. J. Am. Ceram. Soc. 2006, 89, 3701–3705. [Google Scholar] [CrossRef]
- Medina, C.; Saez del Bosque, I.F.; Asensio, E.; Frias, M.; Sanchez de Rojas, M.I. Mineralogy and Microstructure of Hydrated Phases During the Pozzolanic Reaction in the Sanitary Ware Waste/Ca(OH)2 System. J. Am. Ceram. Soc. 2016, 99, 340–348. [Google Scholar] [CrossRef]
- Medina, J.M.; Sánchez de Rojas, M.I.; Sáez del Bosque, I.F.; Frías, M.; Medina, C. Sulfate Resistance in Cements Bearing Bottom Ash from Biomass-Fired Electric Power Plants. Appl. Sci. 2020, 10, 8982. [Google Scholar] [CrossRef]
- Medina, C.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I. Design and characterisation of ternary cements containing rice husk ash and fly ash. Constr. Build. Mater. 2018, 187, 65–76. [Google Scholar] [CrossRef]
- Frías, M.; Savastano, H.; Villar, E.; Sánchez de Rojas, M.I.; Santos, S. Characterization and properties of blended cement matrices containing activated bamboo leaf wastes. Cem. Concr. Compos. 2012, 34, 1019–1023. [Google Scholar] [CrossRef]
- Gonzalez-Kunz, R.N.; Pineda, P.; Bras, A.; Morillas, L. SPlant biomass ashes in cement-based building materials. Feasibility as eco-efficient structural mortars and grouts. Sust. Cities Soc. 2017, 31, 151–172. [Google Scholar] [CrossRef]
- Rajamma, R.; Senff, L.; Ribeiro, M.J.; Labrincha, J.A.; Ball, R.J.; Allen, G.C.; Ferreira, V.M. Biomass fly ash effect on fresh and hardened state properties of cement based materials. Compos. Pt. B Eng. 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Ohenoja, K.; Wigren, V.; Osterbacka, J.; Illikainen, M. Mechanically Treated Fly Ash from Fluidized Bed Combustion of Peat, Wood, and Wastes in Concrete. Waste Biomass Valorization 2020, 11, 3071–3079. [Google Scholar] [CrossRef] [Green Version]
- Sáez del Bosque, I.F.; Sánchez de Rojas, M.I.; Asensio, E.; Frías, M.; Medina, C. Industrial waste from biomass-fired electric power plants as alternative pozzolanic materials. In Waste and By-Products in Cement-Based Materials; Woodhead Publishing: Sawston, UK, 2021; pp. 243–282. [Google Scholar]
- Medina, J.M.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Characterisation and valorisation of biomass waste as a possible addition in eco-cement design. Mater. Struct. 2017, 50, 207. [Google Scholar] [CrossRef]
- Carevic, I.; Baricevic, A.; Stirmer, N.; Bajto, J.S. Correlation between physical and chemical properties of wood biomass ash and cement composites performances. Constr. Build. Mater. 2020, 256, 119450. [Google Scholar] [CrossRef]
- Medina, J.M.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Design and properties of eco-friendly binary mortars containing ash from biomass-fuelled power plants. Cem. Concr. Compos. 2019, 104, 103372. [Google Scholar] [CrossRef]
- Agrela, F.; Cabrera, M.; Morales, M.M.; Zamorano, M.; Alshaaer, M. Biomass fly ash and biomass bottom ash. In New Trends in Eco-Efficient and Recycled Concrete; Woodhead Publishing: Sawston, UK, 2019; pp. 23–58. [Google Scholar]
- Sáez del Bosque, I.F.; Medina, J.M.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Use of biomass-fired power plant bottom ash as an addition in new blended cements: Effect on the structure of the C-S-H gel formed during hydration. Constr. Build. Mater. 2019, 228, 117081. [Google Scholar] [CrossRef]
- Koch, A.; Steinegger, U. A rapid test for cements for their behaviour under sulphate attack. Zem-Kalk-Gips 1960, 7, 317–324. [Google Scholar]
- Cantero, B.; Sáez del Bosque, I.F.; Matías, A.; Sánchez de Rojas, M.I.; Medina, C. Effect of Recycled Aggregate on Performance of Granular Skeleton. ACI Mater. J. 2020, 117, 113–124. [Google Scholar] [CrossRef]
- American Society for Testing and Material. ASTM D1141. Standard Practice for the Preparation of Substitute Ocean Water; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Medina, G.; Sáez del Bosque, I.F.; Frias, M.; Sánchez de Rojas, M.I.; Medina, C. Sulfate Resistance in Cements Bearing Ornamental Granite Industry Sludge. Materials 2020, 13, 4081. [Google Scholar] [CrossRef]
- Irassar, E.F. Sulfate resistance of blended cement: Prediction and relation with flexural strength. Cem. Concr. Res. 1990, 20, 209–218. [Google Scholar] [CrossRef]
- American Society for Testing and Material. D 4404-84: Test Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry; ASTM: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Medina, G.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Effect of Granite Waste on Binary Cement Hydration and Paste Performance: Statistical Analysis. ACI Mater. J. 2019, 116, 63–72. [Google Scholar] [CrossRef]
- Medina, G.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Mineralogical study of granite waste in a pozzolan/Ca(OH)2 system: Influence of the activation process. Appl. Clay Sci. 2017, 135, 362–371. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Li, G.; Zhang, A.; Song, Z.; Liu, S.; Zhang, J. Ground granulated blast furnace slag effect on the durability of ternary cementitious system exposed to combined attack of chloride and sulfate. Constr. Build. Mater. 2018, 158, 640–648. [Google Scholar] [CrossRef]
- Li, S.; Roy, D.M. Investigation of relations between porosity, pore structure, and C1—Diffusion of fly ash and blended cement pastes. Cem. Concr. Res. 1986, 16, 749–759. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Brouwers, H.J.H. Modelling of chloride binding related to hydration products in slag-blended cements. Constr. Build. Mater. 2014, 64, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Goni, S.; Frias, M.; Vigil de la Villa, R.; Garcia, R. Sodium chloride effect on durability of ternary blended cement. Microstructural characterization and strength. Compos. Pt. B Eng. 2013, 54, 163–168. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Morales, E.V.; Santos, S.F.; Savastano, H.; Frías, M. Pozzolanic behavior of bamboo leaf ash: Characterization and determination of the kinetic parameters. Cem. Concr. Compos. 2011, 33, 68–73. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Dyer, T.D. Pozzolanas and Pozzolanic Materials. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Butterworth-Heinemann: Oxfrod, UK, 2019; pp. 363–467. [Google Scholar]
- Bassuoni, M.T.; Rahman, M.M. Response of concrete to accelerated physical salt attack exposure. Cem. Concr. Res. 2016, 79, 395–408. [Google Scholar] [CrossRef]
- Frias, M.; Goni, S.; Garcia, R.; Vigil, R. Seawater effect on durability of ternary cements. Synergy of chloride and sulphate ions. Compos. Pt. B Eng. 2013, 46, 173–178. [Google Scholar] [CrossRef]
- Ikumi, T.; Cavalaro, S.H.P.; Segura, I. The role of porosity in external sulphate attack. Cem. Concr. Compos. 2019, 97, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ikumi, T.; Segura, I. Numerical assessment of external sulfate attack in concrete structures. A review. Cem. Concr. Res. 2019, 121, 91–105. [Google Scholar] [CrossRef]
- Liu, K.; Sun, D.; Wang, A.; Zhang, G.; Tang, J. Long-Term Performance of Blended Cement Paste Containing Fly Ash against Sodium Sulfate Attack. J. Mater. Civ. Eng. 2018, 30, 04018309. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Richardson, I.G. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Saez del Bosque, I.F.; van den Heede, P.; de Belie, N.; Sanchez de Rojas, M.I.; Medina, C. Freeze-thaw resistance of concrete containing mixed aggregate and construction and demolition waste-additioned cement in water and de-icing salts. Constr. Build. Mater. 2020, 259, 119772. [Google Scholar] [CrossRef]
- Friedel, P.M. Sur un chloro-aluminate de calcium hydraté se maclant par compression. Bull. Soc. Franç. Minéral. 1987, 19, 122–136. [Google Scholar]
- De Weerdt, K.; Lothenbach, B.; Geiker, M.R. Comparing chloride ingress from seawater and NaCl solution in Portland cement mortar. Cem. Concr. Res. 2019, 115, 80–89. [Google Scholar] [CrossRef]
- Geng, J.; Easterbrook, D.; Li, L.; Mo, L. The stability of bound chlorides in cement paste with sulfate attack. Cem. Concr. Res. 2015, 68, 211–222. [Google Scholar] [CrossRef]
- Ekolu, S.O.; Thomas, M.D.A.; Hooton, R.D. Pessimum effect of externally applied chlorides on expansion due to delayed ettringite formation: Proposed mechanism. Cem. Concr. Res. 2006, 36, 688–696. [Google Scholar] [CrossRef]
- Caneda-Martínez, L.; Kunther, W.; Medina, C.; Sánchez de Rojas, M.I.; Frías, M. Exploring sulphate resistance of coal mining waste blended cements through experiments and thermodynamic modelling. Cem. Concr. Compos. 2021, 121, 104086. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Song, Z.; Chu, H.; Guo, Q. Influences of exposure condition and sulfate salt type on deterioration of paste with and without fly ash. Constr. Build. Mater. 2016, 113, 951–963. [Google Scholar] [CrossRef]
- Komatsu, R.; Mizukoshi, N.; Makida, K.; Tsukamoto, K. In-situ observation of ettringite crystals. J. Cryst. Growth 2009, 311, 1005–1008. [Google Scholar] [CrossRef]
- Hewlett, P.C. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxfrod, UK, 1998; p. 1053. [Google Scholar]
- Neville, A.M. Properties of Concrete, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 844. [Google Scholar]
- Liu, Z.; Deng, D.; de Schutter, G. Does concrete suffer sulfate salt weathering? Constr. Build. Mater. 2014, 66, 692–701. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Famy, C.; Scrivener, K.L. Delayed ettringite formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]


| Composition (wt%) | UC&DW | BA | SF |
|---|---|---|---|
| SiO2 | 50.10 | 52.75 | 97.94 |
| Al2O3 | 11.82 | 3.19 | - |
| Fe2O3 | 4.06 | 3.72 | 0.10 |
| MgO | 2.12 | 1.93 | 0.30 |
| CaO | 13.21 | 14.91 | 0.23 |
| Na2O | 1.41 | 1.85 | 0.21 |
| SO3 | 0.76 | 2.26 | - |
| K2O | 3.58 | 10.13 | 0.50 |
| P2O5 | 0.19 | 1.64 | - |
| Cl- | 0.04 | 1.00 | 0.04 |
| Other oxides | 0.72 | 0.52 | 0.01 |
| LOI | 12.00 | 6.10 | 0.67 |
| Property | Blended Cement | EN 197-1 Requirement | |||||
|---|---|---|---|---|---|---|---|
| OPC | M1 | M2 | Strength Class 42.5 | Strength Class 32.5 | |||
| Standard consistency (mm) | 32 | 34 | 34 | 34 ± 2 | |||
| Physical | Initial setting time (min) | 200 | 170 | 190 | ≥60 | ≥75 | |
| Expansion (mm) | 0 | 0 | 1 | ≤10 | ≤10 | ||
| Mechanical | Compressive strength (MPa) | 7 days | 53.64 | 40.66 | 28.97 | ≥16.00 | ≥12.00 |
| 28 days | 62.77 | 47.32 | 41.10 | ≥42.50 | ≥32.50 | ||
| Medium | Time (days) | Paste | ||
|---|---|---|---|---|
| OPC | M1 | M2 | ||
| Water | 21 | 10.04 ± 0.71 | 8.09 ± 0.85 | 8.87 ± 1.01 |
| 56 | 9.39 ± 1.11 | 8.49 ± 0.93 | 8.33 ± 1.10 | |
| 90 | 10.59 ± 1.180 | 9.79 ± 1.04 | 9.09 ± 1.04 | |
| Seawater | 21 | 11.29 ± 0.83 | 8.38 ± 0.26 | 8.12 ± 0.32 |
| 56 | 12.79 ± 1.13 | 9.17 ± 0.37 | 6.51 ± 0.36 | |
| 90 | 13.14 ± 0.69 | 9.26 ± 0.61 | 5.57 ± 0.55 | |
| 0.5 M NaCl | 21 | 9.84 ± 0.65 | 8.37 ± 0.67 | 8.16 ± 0.42 |
| 56 | 9.73 ± 0.93 | 8.91 ± 0.63 | 8.44 ± 0.48 | |
| 90 | 9.68 ± 1.40 | 9.72 ± 0.58 | 8.36 ± 0.57 | |
| 0.3 M Na2SO4 | 21 | 14.41 ± 1.43 | 11.82 ± 0.98 | 10.00 ± 0.71 |
| 56 | 17.24 ± 1.73 | 10.59 ± 1.21 | 9.12 ± 0.72 | |
| 90 | 18.24 ± 0.50 | 11.09 ± 1.03 | 8.91 ± 0.54 | |
| Medium | Paste | ||
|---|---|---|---|
| OPC | M1 | M2 | |
| Water | 20.79 | 27.47 | 31.00 |
| Seawater | 19.14 | 27.19 | 30.31 |
| 0.5 M NaCl | 19.87 | 27.34 | 30.81 |
| 0.3 M Na2SO4 | 17.99 | 25.47 | 28.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez del Bosque, I.F.; Sánchez de Rojas, M.I.; Medina, G.; Barcala, S.; Medina, C. Durability of Ternary Cements Based on New Supplementary Cementitious Materials from Industrial Waste. Appl. Sci. 2021, 11, 5977. https://doi.org/10.3390/app11135977
Sáez del Bosque IF, Sánchez de Rojas MI, Medina G, Barcala S, Medina C. Durability of Ternary Cements Based on New Supplementary Cementitious Materials from Industrial Waste. Applied Sciences. 2021; 11(13):5977. https://doi.org/10.3390/app11135977
Chicago/Turabian StyleSáez del Bosque, Isabel Fuencisla, María Isabel Sánchez de Rojas, Gabriel Medina, Sara Barcala, and César Medina. 2021. "Durability of Ternary Cements Based on New Supplementary Cementitious Materials from Industrial Waste" Applied Sciences 11, no. 13: 5977. https://doi.org/10.3390/app11135977
APA StyleSáez del Bosque, I. F., Sánchez de Rojas, M. I., Medina, G., Barcala, S., & Medina, C. (2021). Durability of Ternary Cements Based on New Supplementary Cementitious Materials from Industrial Waste. Applied Sciences, 11(13), 5977. https://doi.org/10.3390/app11135977







